Abstract
Objectives
Patients with functional single ventricles (FSV) following the Fontan procedure have abnormal cardiac mechanics. We sought to determine factors that influence diastolic function and to describe associations of diastolic function with current clinical status.
Methods
Echocardiograms were obtained as part of the Pediatric Heart Network Fontan Cross-Sectional Study. Diastolic function grade (DFG) was assessed as normal (grade 0), impaired relaxation (grade 1), pseudonormalization (grade 2), restrictive (grade 3). Studies were also classified dichotomously (restrictive pattern present or absent). Relationships between DFG and pre-Fontan variables (e.g., ventricular morphology, age at Fontan, history of volume-unloading surgery), and current status (e.g., systolic function, valvar regurgitation, exercise performance) were explored.
Results
DFG was calculable in 326/546 subjects (60%); mean age = 11.7±3.3 years. Overall, 32% of patients had grade 0, 9% grade 1, 37% grade 2, and 22% grade 3. Although there was no association between ventricular morphology and DFG, there was an association between ventricular morphology and E’, which was lowest in those with right ventricular morphology (p<.001); this association remained significant when using z-scores adjusted for age (p=<.001). DFG was associated with achieving maximal effort on exercise testing (p=.004); the majority (64%) of those not achieving maximal effort had DFG 2 or 3.No additional significant associations of DFG with laboratory or clinical measures were identified.
Conclusion
Assessment of diastolic function by current algorithms results in a high percentage of patients with abnormal DFG, but we found few clinically or statistically significant associations. This may imply a lack of impact of abnormal diastolic function upon clinical outcome in this cohort, or may indicate that the methodology may not be applicable to pediatric FSV patients.
INTRODUCTION
Although surgical palliation of patients with functional single ventricles by the Fontan procedure has resulted in improved survival during childhood, long-term morbidity and mortality remain major concerns. In particular, the relationships of pre-Fontan variables to diastolic function and of diastolic function to long term morbidity and mortality in this cohort are still largely unknown. In the NHLBI-sponsored Pediatric Heart Network Fontan Cross-Sectional Study of 546 Fontan survivors, the majority of patients (73%) had normal ejection fractions by echocardiography, but only 28% had normal indices of diastolic function (1). While patients with single ventricle physiology have been described as having diastolic dysfunction with both reduced compliance and impaired relaxation (2), associations with anatomic, clinical and historical factors have not been described. Additionally, because an acutely increased mass-to-volume ratio immediately following a Fontan operation has been postulated by some to be detrimental to diastolic function (3, 4), we sought to assess correlations between diastolic function and current mass-to-volume ratio, and determine whether mass-to-volume ratio correlates with clinical characteristics.
METHODS
The NHLBI-sponsored Pediatric Heart Network Fontan Cross-Sectional Study characterized the health status of 546 Fontan survivors aged 6 to 18 years, enrolled by seven clinical centers in North America. Prospective data collection included two-dimensional (2D) and Doppler echocardiography, exercise testing, health status questionnaires, and laboratory tests. Full details of the study have been published (1, 5, 6). Written informed consent and assent were obtained according to local guidelines, and the study protocol was approved by the Institutional Review Board/Research Ethics Board of each center.
Echocardiograms were obtained according to a predetermined study protocol. None were performed under sedation. The echocardiograms were submitted to the data coordinating center for initial quality control, and then forwarded to the echocardiographic core lab for analysis. Ventricular morphology was characterized as left ventricular (LV) dominant (e.g., tricuspid atresia), right ventricular (RV) dominant (e.g., hypoplastic left heart syndrome) or mixed (e.g., unbalanced atrioventricular canal defect). A single representative beat was selected for all systolic and diastolic measures. To determine ventricular size and ejection fraction, the ventricle was analyzed from the apical transverse plane (ventricular long-axis) and the parasternal short-axis views. End-diastolic (EDV) and end-systolic volumes (ESV) were calculated using the biplane modified Simpson algorithm (7). Percent ejection fraction (EF %) was then determined as ([EDV-ESV]/EDV) x 100. Ventricular mass was calculated as myocardial end-diastolic volume (epicardial volume - endocardial volume) x myocardial density (1.05 g/ml).
Assessment of diastolic function typically involves a multi-parameter approach, predominantly utilizing spectral Doppler velocities and tissue Doppler velocities. Lester et al described a practical approach to diastolic function assessment (8) which utilized assessments of mitral inflow characteristics, tissue Doppler early diastolic velocities, atrial size, pulmonary vein flow, isovolumic relaxation time and mitral inflow propagation velocity (Vfp). Some of these parameters were not assessable in single ventricle anatomic variants, particularly atrial size, due to the markedly abnormal and variable geometries of the atrial chambers in the various iterations of single ventricle circulations.
Therefore, our diastolic assessment was based on the following measurements: Doppler parameters of atrioventricular valve (AVV) inflow [ratio of early mitral inflow velocity (E) to atrial wave inflow (A) velocity (E/A ratio) and deceleration time (DT) of early inflow], tissue Doppler assessment of peak AVV annulus velocities in early diastole (E’), and Vfp using M-mode color Doppler across the AVV inflow. For E’ values, we used the average of the velocities of the two walls bounding the systemic ventricle (septum and AVV annulus for single ventricles, right and left lateral walls of unbalanced atrioventricular canal systemic ventricle). Two grading systems were used. First, a diastolic function grade (DFG) was assigned to each study based on an algorithm proposed by others (8, 9). For application to a pediatric cohort, an E/E’ z-score of 3 was used, the equivalent of an absolute value E/E’ of 10 in adults :
-
0.
Normal = [(1<E/A≤ 2) and (DT ≥ 140 msec) and (E/E’ z ≤ 3)]
-
1.
Impaired relaxation = [E/A ≤ 1]
-
2.
Pseudonormalization = [(1<E/A≤2) and [(DT < 140 msec) or (E/E’z >3) or (Vfp < 55 cm/sec)]]
-
3.
Restrictive = [E/A > 2]
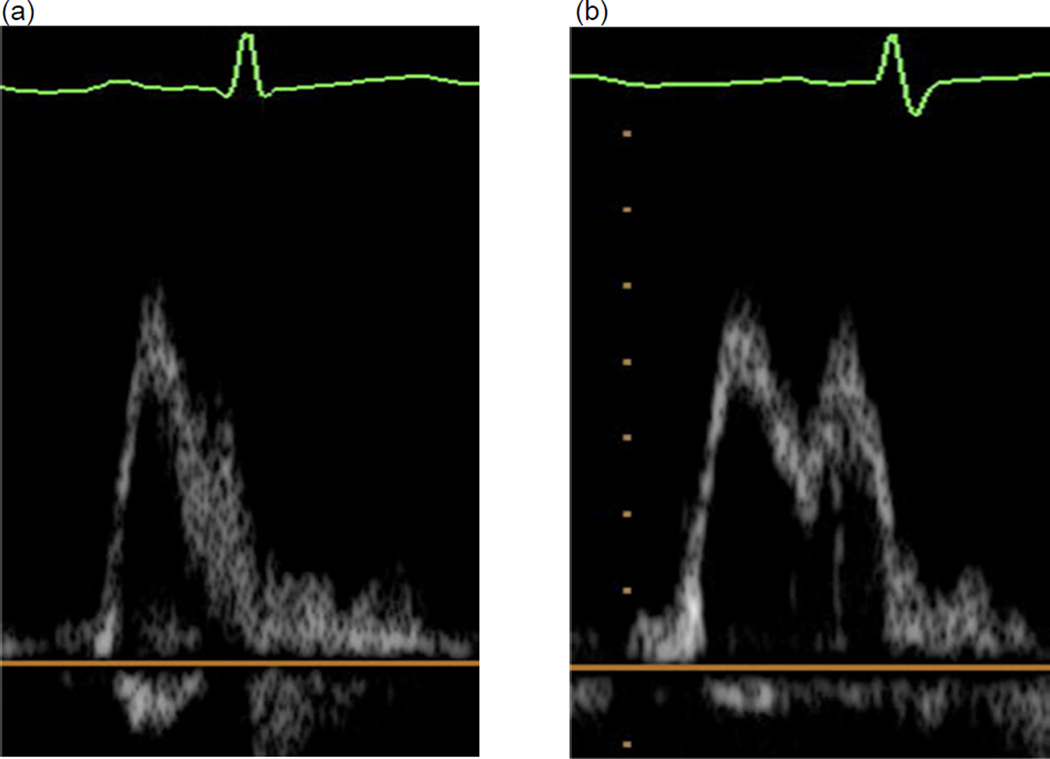
Second, studies were classified as having restrictive pattern present or absent; restrictive pattern was considered to be present if E/A>2 or if 1<E/A<2 and DT<140 msec. Because determination of both the DFG and presence/absence of a restrictive pattern required E/A ratios, 84 studies with E and A wave fusion (either partially or fully, as demonstrated in Figure 1) were excluded from analysis.
Figure 1. Mitral Valve E/A and TDI E’/A’ partial-complete fusion.
Examples of mitral valve inflow E/A complete (a) and partial (b) fusion and TDI E’/A’ complete (c) and partial (d) fusion.
Historical factors were obtained from medical chart review, including pre-Fontan catheterization variables, history of coarctation requiring intervention at the time of the pre-Fontan catheterization, history of superior cavopulmonary anastomosis (SCPA, e.g., bidirectional Glenn shunt, hemi-Fontan), and type of Fontan procedure (e.g., lateral tunnel connection, direct right atrial to pulmonary artery connection). Clinical data obtained at the time of the study echocardiogram included BNP level (B-type natriuretic peptide), Child Health Questionnaire (CHQ) parental report (CHQ-PF50) (10) Physical and Psychosocial summary scores, and medications, including history of and current angiotensin converting enzyme (ACE) inhibitor usage. Subjects also underwent exercise stress testing and the following parameters were recorded: peak oxygen consumption (VO2), ventilatory anaerobic threshold (VAT), %predicted peak VO2, % predicted VAT, maximal O2 pulse/body surface area (BSA), maximal work rate, %predicted maximal O2 pulse, %predicted maximal work rate and %predicted maximal heart rate.
Statistical Methods
Descriptive statistics were reported as mean ± standard deviation and median (interquartile range). Logistic regression was used to model the presence vs. absence of a restrictive pattern as a function of age, and a generalized additive model was used to assess potential nonlinearity between age and restrictive pattern. Spearman correlation coefficients were used to examine the association between continuous diastolic function measures and age. Cumulative logistic regression was used to model the relationship between DFG and age. An interaction was fit between age and ventricular morphology to determine whether associations between DFG and age were dependent on anatomy. Similarly, tests of interaction were fit to assess whether associations of DFG and other clinical measurements depended on ventricular morphology. We used a chi-square test to assess the association of DFG and categorical patient and clinical variables. ANOVA and Kruskal-Wallis tests were used to compare parameters of systolic function (EF, ESV, EDV and mass:volume z-scores (11)), current AVV or semilunar valve regurgitation, current use of ACE inhibition, exercise performance, and functional status by DFG. Multivariable modeling was not conducted because few variables were associated with DFG. Analysis of variance was used to compare the distributions of E/E’ ratio and other continuous diastolic function parameters and their z-scores indexed to age (11) by age group and by ventricular morphology. To determine whether mass-to-volume ratio differed according to DFG and ACE inhibitor use, we used the Kruskal-Wallis test. A p-value of less than 0.05 was considered significant. All analyses were conducted in SAS version 9.2 (Statistical Analysis System, Inc., Cary, NC) and S-Plus (Insightful Corp., Seattle, WA).
RESULTS
A summary of the parameters by DFG, with z-scores where applicable, is included in Table 1, and baseline demographic characteristics for the cohort are presented in Table 2. Of the 546 subjects who underwent a study echocardiogram, 326 (60%) had echocardiograms adequate to assign a DFG, and in 343 (63%) it was possible to determine the presence or absence of a restrictive pattern. The 220 subjects for whom a DFG could not be calculated were similar to those with a calculated grade with respect to time since the Fontan procedure, gender, and ventricular morphology, but differed slightly with regard to race (84% vs. 77% white). The distribution of DFG in the overall cohort was: DFG 0, 32%; DFG 1, 9%; DFG 2, 37%; and DFG 3, 22%.
Table 1.
Summary of Parameters by Diastolic Function
| Variable | Normal (Grade 0) |
Impaired Relaxation (Grade 1) |
Pseudo- normalization (Grade 2) |
Restrictive (Grade 3) |
|---|---|---|---|---|
| N | 104 | 30 | 120 | 72 |
| E:E’ | 7.3±2.2 | 6.8±3.0 | 9.6±5.0 | 9.0±3.7 |
| Z-score | 0.6±1.1 | 0.4±1.4 | 1.7±2.4 | 1.4±1.8 |
| E:A | 1.5±0.3 | 0.9±0.1 | 1.4±0.3 | 2.5±0.4 |
| Z-score | −1.2±0.4 | −2.1 ±0.2 | −1.3±0.5 | 0.4±0.6 |
| DT (msec) | 204±56 | 125±34 | 120±39 | 193±87 |
| Z-score | 1.6±1.6 | −0.7±1.0 | −0.8±1.2 | 1.3±2.6 |
| Vfp (cm/sec)* | 63±22 | 67±17 | 64 ±20 | 64±17 |
| Z-score | −0.3±1.0 | −0.1 ±0.7 | −0.2±0.9 | −0.2±0.8 |
| EF (%)** | 59±10 | 56±12 | 61 ±11 | 60±10 |
| Z-score | −1.0±2.3 | −1.7±2.7 | −0.6±2.2 | −0.7±2.1 |
| Mass:Volume*** | 1.1±0.3 | 1.3±0.6 | 1.2±0.4 | 1.2±0.4 |
| Z-score | 2.1 ±2.1 | 3.2±5.2 | 2.5±3.4 | 2.9±3.0 |
DT = deceleration time; EF = ejection fraction; Vfp = ventricular flow propagation
Vfp sample sizes by grade are 39; 7; 57; 38
EF sample sizes by grade are 84; 25; 94; 61
Mass:volume sample sizes by grade are 80; 25; 92; 61
Table 2.
Patient Characteristics by Diastolic Function Grade
| Variable | No assignable DFG |
All with assigned DFG |
P1 | Normal (Grade 0) |
Impaired Relaxation (Grade 1) |
Pseudo- normalization (Grade 2) |
Restrictive (Grade 3) |
P2 |
|---|---|---|---|---|---|---|---|---|
| N | 220 | 326 | 104 | 30 | 120 | 72 | ||
| Time since Fontan, yr |
8.6±3.6 | 8.5±3.3 | .83 | 8.9±3.3 | 7.8±3.8 | 8.2±3.2 | 8.9±3.2 | 0.18 |
| Age at echo, yr |
12.1±3.5 | 11.7±3.3 | .31 | 12.3±3.5 | 11.4±3.4 | 11.2±3.1 | 12.0±3.4 | 0.09 |
| Heart rate, bpm* |
79±15 | 71 ±13 | <.001 | 68±11 | 80±9 | 77±13 | 64±12 | <.001 |
| Male | 57% | 62% | .24 | 61% | 50% | 66% | 64% | 0.43 |
|
Ventricular Type |
.75 | 0.30 | ||||||
| LV | 48% | 49% | 53% | 47% | 41% | 58% | ||
| RV | 35% | 33% | 28% | 37% | 39% | 26% | ||
| Mixed | 17% | 18% | 19% | 17% | 20% | 15% |
For heart rate N=160 without DFG, N=277 with DFG; N=88 DFG0, N=25 DFG1, N=100 DFG2; N=64 DFG3. DFG=Diastolic Function Grade
P1=p-value comparing subjects with vs. without an assignable DFG
P2=p-value comparing the 4 DFG groups
Age-Related Patterns of Diastolic Function
DFG was not associated with time since Fontan or age at echo (Table 2). There were few associations with age among the individual variables used to determine diastolic function grade. Mitral valve DT was positively correlated with age (Spearman R = 0.15, p=0.007) but this was not significant after adjustment for heart rate (p=0.14). None of the other variables had statistically significant associations with age after adjustment for heart rate.
Diastolic Function Grade Associations with Pre-Fontan Variables
Ventricular morphology
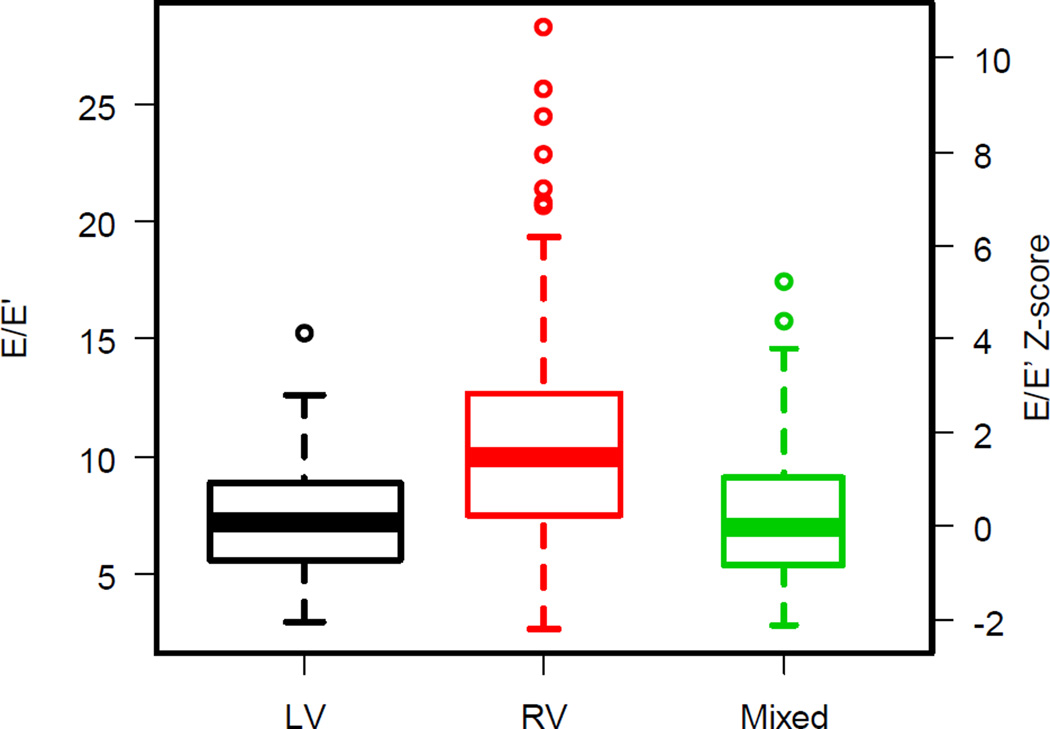
E’ was lowest in RV morphology subjects (mean±SD: RV, 8.0±2.8; LV, 10.0±3.2; Mixed 10.4±3.9, p <.001) (Table 3) and this finding remained statistically significant when using z-scores– for-age (p <.001). The E/E’ ratio was higher in the RV group than in the others, due to the lower E’. (Figure 2).
Table 3.
Comparison of E, E’ and E/E’ by ventricular type
| LV | RV | Mixed | |||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | P | |
| E z-score* |
169 | 0.7 ± 0.2 −1.4 ±1.1 |
108 | 0.8 ± 0.2 −0.9 ± 1.1 |
66 | 0.7 ± 0.1 −1.3 ± 0.9 |
<.001 <.001 |
| E’ z-score* |
222 | 10.0 ± 3.2 −1.3 ± 0.7 |
155 | 8.0 ± 2.8 −1.8 ± 0.6 |
75 | 10.4 ± 3.9 −1.2 ± 0.9 |
<.001 <.001 |
| E/E’ z-score* |
147 | 7.2 ± 2.3 0.6 ± 1.1 |
93 | 10.9 ± 5.1 2.4 ± 2.4 |
56 | 7.4 ± 3.0 0.6 ± 1.4 |
<.001 <.001 |
z-score for age
Figure 2.
E/E’ ratio and E/E’ ratio z-score by ventricular morphology; p-value <0.001, LV = left ventricle, RV = right ventricle. The width of each box is proportional to the square root of the group size.
Age at Fontan Surgery
We stratified the cohort by age at Fontan surgery as <2 years, 2-<3 years, 3-<4 years and ≥ 4 years. DFG did not differ significantly among the groups (p=.50); nor did E/E’ z-score (p=0.45; Table 4).
Table 4.
Mean±SD of E/E’ Ratio Z-score by Age at Fontan, and Ventricular Type
| Age at Fontan, yr | |||||
|---|---|---|---|---|---|
| Ventricular Type (n) |
< 2 (n=66) |
2 to < 3 (n=109) |
3 to < 4 (n=52) |
≥ 4 (n=69) |
P-value* |
| LV (147) | 0.67±1.07 (27) | 0.42±1.18 (57) | 0.79±1.17 (29) | 0.71±1.00 (34) | 0.27 |
| RV (93) | 3.58±3.19 (25) | 2.03±1.90 (36) | 1.91±1.61 (12) | 1.82±2.01 (20) | 0.15 |
| Mixed (56) | 0.48±1.57 (14) | 0.81±1.56 (156) | 0.73±1.28 (11) | 0.54±1.45 (15) | 0.79 |
| All (296) | 1.73±2.61 (66) | 1.01±1.66 (109) | 0.97±1.41 (52) | 0.99±1.53 (69) | 0.45 |
P-values were calculated by ANOVA. Similar inferences were drawn using non-parametric testing (Kruskal-Wallis test).
Other Pre-Fontan Medical History Factors
Neither the individual diastolic function variables nor the DFG differed between subjects who underwent a prior SCPA surgery compared to those who did not, even after adjusting for age (Table 5). Among those who underwent volume unloading surgery, the age at surgery was very mildly negatively correlated with E (R= − 0.13, p=.02) and E/E’(R = −0.15, p = .01) and positively correlated with E’ (R=0.15, p=.002). The DFG also did not differ by type of Fontan procedure performed, presence or absence of a fenestration, presence of coarctation intervention at the time of the pre-Fontan catheterization or pre-Fontan cardiac catheterization pressures (Table 5).
Table 5.
Other Pre-Fontan Medical History Factors by Diastolic Function Grade
| Variable | Normal | Impaired Relaxation |
Pseudo- normalization |
Restrictive | chisq p |
|---|---|---|---|---|---|
| N | 104 | 30 | 120 | 72 | |
| SCPA performed | 68% | 73% | 78% | 69% | 0.43 |
| In LVs | 56% | 64% | 67% | 71% | 0.45 |
| In RVs | 86% | 91% | 91% | 84% | 0.81 |
| In Mixed | 75% | 60% | 71% | 36% | 0.15 |
| Type of Fontan | 0.60 | ||||
| Atriopulmonary connection | 17% | 10% | 13% | 13% | |
| Intracardiac lateral tunnel | 50% | 70% | 65% | 63% | |
| Extracardiac conduit | 29% | 21% | 20% | 22% | |
| Extracardiac lateral tunnel | 1% | 0% | 2% | 0% | |
| Other | 3% | 0% | 1% | 3% | |
| Fenestrated Fontan | 65% | 67% | 70% | 65% | 0.80 |
| Pre-Fontan coarctation catheterization intervention |
3% | 3% | 8% | 7% | 0.42 |
| N with catheterization | 104 | 30 | 120 | 72 | |
| End-diastolic pressure, mmHg (median) |
7.1±4.4 (8.0) | 7.4±3.6 (8.0) | 7.0±4.2 (7.0) | 7.4±6.0 (7.0) | 0.95/0.67* |
| PA pressure, mmHg (median) | 9.6±6.8 (11.0) |
11.0±4.5 (11.0) | 8.9±7.6 (11.0) | 8.7±7.6 (10.0) |
0.65/0.13* |
| O2 saturation, % (median) |
81±21 (85) | 83±5 (84) | 80±19 (85) | 78±22 (83) | 0.57/0.75* |
ANOVA p-value/Kruskal-Wallis test p-value
SCPA = superior cavopulmonary anastomosis; PA=pulmonary artery
Diastolic Function Grade Associations with Measures of Current Status
No association was seen between DFG and parameters of systolic function (EDV z-score, ESV z-score, EF, EF z-score, end-systolic global average fiber stress, or stroke volume z-score), nor between DFG and functional health status (CHQ Physical summary score or Psychosocial summary score). There was no significant association between DFG and atrioventricular valve regurgitation (p=0.20) or semilunar valve regurgitation (p=0.52). There was no difference in DFG between patients currently taking ACE inhibitor therapy versus those who were not (p=0.70)
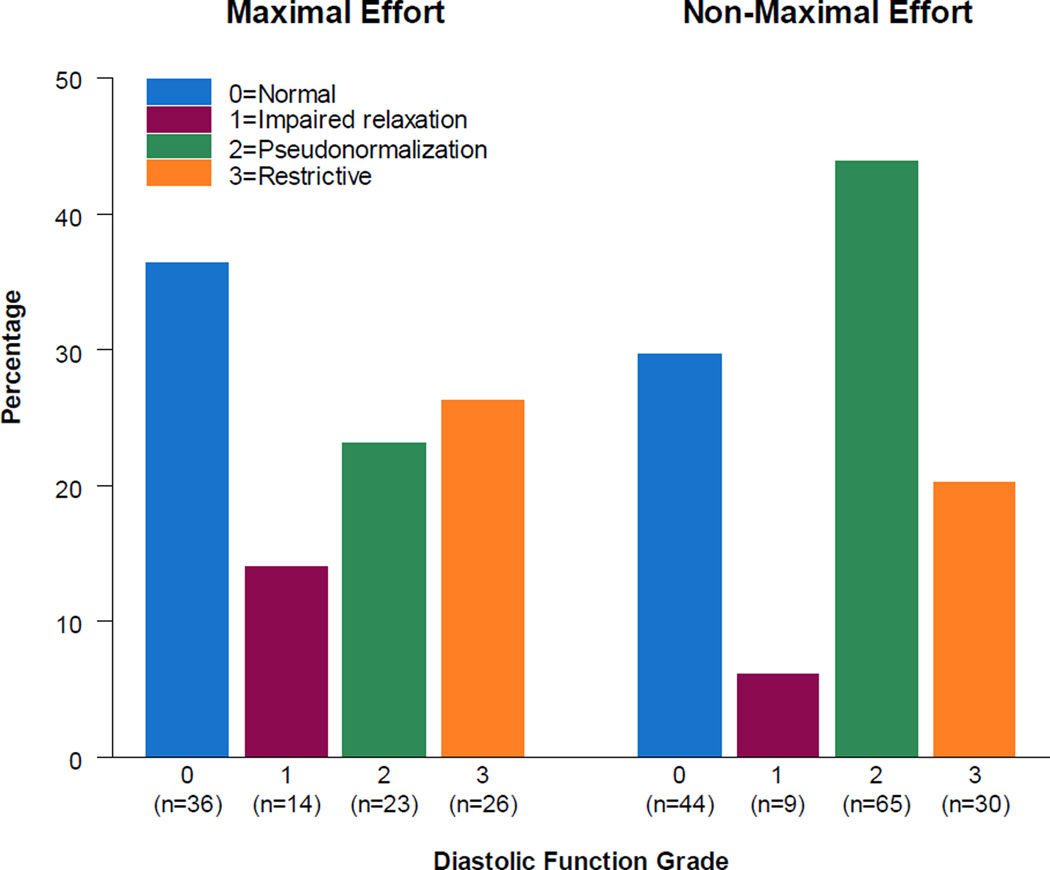
Of the 326 subjects for whom DFG was calculable, 254 underwent an exercise test, with 99 subjects achieving maximum effort defined as respiratory exchange ratio ≥1.1. The percentage achieving maximal effort differed by DFG (p=0.004; Figure 3). Of the nine individual exercise parameters, only maximal O2 pulse/BSA (p=.03) and maximal work rate differed by DFG (p=.04). The DFG 0 and 3 groups had the highest mean maximal O2 pulse/BSA (5.8±1.4 and 58±1.8 ml O2/BSA), while the other two groups had means of 5.03 and 5.35 ml O2/BSA. For maximal work rate, the DFG 0 group had the highest mean (2.0±0.5 W), while the other groups had means between 1.8 and 1.9 W. When the analysis was restricted to the maximal effort group no significant association was seen in maximal O2 pulse/BSA (p=.28) or maximal work rate (p=.874). For maximal O2 pulse/BSA, the loss of significance was in part due to the restricted sample size; the DFG group mean was 6.1 ml O2/BSA while the other group means were 5.4 to 5.5 ml O2/BSA.
Figure 3.
Percentage of subjects in each diastolic function grade, by those who achieved maximal effort (defined as achieving respiratory exchange ratio ≥ 1.1) and those achieving non-maximal effort.
We next examined whether E’ alone was associated with clinical outcome. A total of 452 subjects had a measurable annular E’ velocity, and 24% had an abnormal value (z-score < −2). No association was seen between E’ and CHQ summary score, BNP or exercise study parameters.
Associations with Presence of a Restrictive Pattern
We sought to determine whether a simplified approach of assessment of diastolic function, i.e., presence or absence of a restrictive pattern, would reveal associations not seen with the 0–3 grading scale of DFG. Over half (52%) had a restrictive pattern. This pattern was associated with not achieving maximal effort on exercise testing (32% in the restrictive pattern group achieved maximal effort vs. 48% in those without a restrictive pattern, p=.012). Catheterization intervention for coarctation was marginally associated (7.3% in the restrictive pattern group vs. 3.0% in those without a restrictive pattern, p=.08). Maximal work rate was also associated, but not within the subset who achieved maximal effort on testing. No significant associations with age, pre-Fontan variables or current outcomes were identified with this approach.
Associations with Mass-to-Volume Ratio
Of the 326 subjects with a calculable DFG, 258 had a determination of mass-to-volume ratio by echocardiography. There was no significant difference in mass-to-volume ratio or mass-to-volume ratio z-score by DFG (Kruskal-Wallis p>0.3). Mass-to-volume ratio z-score was positively associated with the number of years since the Fontan procedure was performed (R=0.20, p<.001). Mass-to-volume ratio was not associated with current or historical use of ACE inhibitors. Mass-to-volume ratio did not correlate with exercise performance or CHQ summary scores.
DISCUSSION
The surgical strategy of early ventricular volume unloading in patients with functional single ventricles has been developed to mitigate the myocardial changes which occur with chronic volume overload. However, this strategy results in an abnormal physiological state with low resting cardiac output, elevated systemic vascular resistance, abnormal ventricular-vascular coupling, and increased total ventricular work due to the absence of a second ventricle and other power losses throughout the Fontan circulation (12).
Our finding that the majority of subjects have preserved systolic function with abnormal diastolic function is consistent with prior studies (2, 12, 13). In the current era, systolic dysfunction is seldom a major problem until late after the Fontan procedure, especially when it is assessed at the ages in our cohort. Noninvasive evidence of diastolic dysfunction, however, is likely to be present early, primarily due to the hemodynamic abnormalities that result from the Fontan surgical pathway (12, 14). Although the mechanisms are not completely clear, our data from this large cohort confirm the high prevalence of diastolic dysfunction after the Fontan procedure, as determined by standard algorithms. Noninvasive diastolic function indices do not seem to have the same implications for the single ventricle as they do for a two-ventricle circulation. Compared to the single ventricle cohort presented here, correlation between E/E’ and EDP is stronger in children with aortic stenosis, where there are two ventricles that interact in series and in parallel and the disease affects only the left ventricle (15).
Overall, there are numerous factors in this population that would diminish the coupling between E/E’ and EDP, as we observed. Mechanistically, E/E’ reflects the combined effects of the pressure gradient between the left atrium and the left ventricle at the time of mitral valve opening (this gradient is the determinant of E) and the speed of myocardial relaxation (E’ correlates with Tau, the time constant of myocardial relaxation). The peak early transmitral gradient (and hence E) depends directly on left atrial end-systolic pressure which is in turn dependent on the combination of end-diastolic pressure and left atrial compliance. E/E’ therefore increases in conjunction with higher EDP or lower left atrial compliance and also increases with a lower value of Tau. Differential trends in any of these variables will disrupt the correlation between EDP and E/E’. Impaired relaxation that does not result in elevated EDP or is associated with a compliant atrium may fail to manifest a significant elevation in E/E’. Similarly, normal relaxation rate may mask the effect of elevated EDP on E/E’. It is important to note that the finding of elevated E/E’ was more prevalent in the RV group, where the “normal” value for E/E’ may be quite different than the normal value in a LV. There is, of course, no “normal” value for E’ in a single RV but in the study population the RV E’ of 8.0±2.8 is lower than the LV E’ of 10.0±3.2. Due to its relatively under-developed conduction system the RV may have more diastolic dyssynchrony than the LV and in general, the single ventricles may have more diastolic dyssynchrony than normal ventricles, which would result in an elevated Tau and hence lower E’. Ventricular volume overload is always present in these patients prior to the Fontan and is associated with variably increased ventricular compliance. Higher ventricular compliance results in a higher peak E value, which would mask the impact of a lower E’ on the E/E’.
Our study is the first to attempt to establish predictors or determine the clinical correlations of abnormal diastolic function in a large cohort of Fontan patients. We found that subjects with RV morphology were more likely to have diastolic dysfunction as measured by standard guidelines (8, 9), even when adjusted for heart rate. We found few significant associations with anatomic variables or current status, and no associations were found between mass-to-volume ratio and DFG, ACE inhibitor use or exercise performance.
The group of subjects with systemic RVs demonstrated more abnormalities in DFG than other groups. Similar findings have been described in other smaller series (16, 17), which reported lower S’ and E’ velocities as well as higher E/E’ in subjects with RV morphology compared to those with LV morphology. Systemic RVs in biventricular circulations have also been shown to have decreased longitudinal velocities (18) compared to systemic LVs. It should be noted that E’ measures peak velocity of longitudinal lengthening. Because the fiber structure of the RV has fewer longitudinally oriented muscle fibers (19), it is not surprising that longitudinal velocity is lower. In the present cohort, AVV regurgitation was worse in the RV subgroup (reported previously (1)). Along with other factors intrinsic to the RV, this may have contributed to the overall higher DFG in the RV group, since DGF groups 2 and 3 were more likely to have higher degrees of AVVR.
The use of standard algorithms to assess diastolic function takes advantage of multiple parameters to define abnormalities. However, most other studies in this population have relied on single variables to describe diastolic function. In a small series of 32 patients (mean age 30 months ± 22 months) with single ventricle palliation, which included pre- and post-Glenn as well as post-Fontan palliation, Menon et al found modest correlations between mean ventricular end-diastolic pressure with pulmonary vein A wave reversal duration and E’ (20). Along similar lines, Vitarelli and colleagues described lower E’ velocities in patients following Fontan compared to healthy controls regardless of whether or not the patient had systolic dysfunction (21). One morphologic reason that individual variables may not be adequate to assess associations in patients with single ventricles is that the size of the AVV in single ventricles is quite variable, influencing AVV inflow patterns. For the same atrium-to-ventricle pressure differential, flow velocity through a larger valve will be lower.
The overall exercise capacity of this cohort has been previously reported (22), demonstrating a reduced maximal aerobic capacity. No echocardiographic measure of systolic or diastolic function correlated with %predicted VO2, supporting the notion that exercise capacity in this population is restricted by factors other than the properties of the single ventricle. The principal factor influencing the ability to exercise after the Fontan procedure appears to be the known inability of these patients to increase transpulmonary blood flow appropriately with exercise (23). A fenestration, when present, can also increase cardiac output, but limitation of transpulmonary blood flow remains an issue. Following this line of reasoning, Goldberg and colleagues have recently reported a proof-of-concept placebo-controlled trial of an oral pulmonary vasodilator, sildenafil, to test this question (24). They were able to demonstrate improved ventilatory efficiency and exercise performance at the anaerobic threshold. Even though VO2 max was unchanged, these early results merit further investigation and provide promise for a therapeutic option.
The potential impact of diastolic dysfunction on exercise capacity in Fontan patients remains uncertain. During exercise, there is normally a rise in stroke volume related to a rise in end-diastolic volume (preload reserve) and a fall in end-systolic volume (contractile reserve) (25). It has been reported that utilization of preload reserve is subnormal in adult patients with diastolic dysfunction with normal systolic function (26) and with systolic dysfunction (27). In a recent study by Schmitt et al (14) evaluating the response of the Fontan circulation to dobutamine stress testing, pulmonary vascular resistance was found to drop appropriately but the expected preload reserve response was not observed secondary to a dobutamine-induced rise in the end-diastolic pressure-volume curve (decreased compliance). In normal subjects, the exercise-associated rise in cardiac output is mediated primarily by heart rate response, with a much smaller effect due to a rise in stroke volume (28). In contrast, analysis of the factors influencing exercise response in the cohort of Fontan patients presented here (previously published by Paridon et al (22)) found that oxygen pulse (a surrogate measure of stroke volume) had the closest association with the rise in maximum oxygen consumption during treadmill exercise. Nonetheless, a relationship between the usual echocardiographic indices of diastolic function and maximum oxygen consumption was not observed. There are a number of potential explanations for the apparent differences in the Schmitt and Paridon observations, including differences in the response to supine dobutamine stress compared to upright treadmill exercise, but an important possibility is that the currently available techniques for noninvasive detection of abnormal compliance are unreliable in the single ventricle heart. Insofar as most of these indices are highly correlated with end-diastolic pressure, diminished sensitivity of these indices may well relate to the limited ability of the Fontan circulation to substantially elevate end-diastolic pressure.
Most of the diastolic function indices have been developed through study of adult patients with very different cardio-pulmonary systems than those of our patients. In congenital heart disease patients who have increased pressure loads (e.g., aortic stenosis) similar patterns of diastolic indices are seen in children (29) as have been described in adults. However, increased pressure load is less common in the single ventricle population. Altered diastolic indices likely have a fundamentally different etiology, and for multifactorial reasons.
Diastolic behavior of the ventricle relates to both chamber factors (e.g.shape and mass to volume ratio) and myocardial factors (e.g. relaxation and muscle compliance). When diastolic dysfunction occurs in the presence of hypertrophy, the impact of this chamber property must be accounted for before it is possible to ascertain the impact of myocardial properties. We hypothesized that the chamber factor mass to volume ratio may make an important contribution to diastolic function in this population: the force required to expand the ventricle (diastolic wall stress) is proportional to pressure x (radius/thickness). This means a lower radius/thickness (which is equivalent to a higher mass/volume) requires a higher pressure to achieve the same wall stress. Therefore, geometric relationships dictate that a higher pressure is required to fill thicker ventricles even if the compliance of the myocardium remains the same. The failure of this relationship to be important in these patients implies that factors other than the geometric influence of hypertrophy dominate the variation in diastolic function.
From a technical standpoint, of the 546 echocardiograms submitted to the core lab, we were able to calculate a DFG on 60%. The presence of a summation wave, common at pediatric heart rates, precluded assessment on 84 studies (16% of the total and 38% of those without an assignable DFG). Determination of whether a restrictive pattern was present was able to be made in 344 patients or 63% of the total group, indicating that although a majority of single ventricles can be assessed in such a manner, the technical difficulties in obtaining on-axis Doppler signals in a significant fraction of the patients additionally restricts the data.
Limitations
Our data have important limitations. Age association analysis was restricted by the relatively young age of the cohort studied (maximum age 18 years). As this group reaches the third and fourth decades of life, more significant differences may appear. Additionally, this is a descriptive study of the outcome of a constantly evolving treatment strategy. Patients with single ventricle physiology born today will not be managed in the same manner as our cohort, thereby limiting the generalizability of the results. Assessment of the impact of individual medications on diastolic function was limited due to small sample sizes. For example only 19 subjects cohort-wide were taking beta blockers at the time of evaluation.
Conclusions
Echocardiographic assessment of diastolic function in patients with single ventricle physiology using current algorithms is technically possible and a high percentage of patients have abnormal results. However, we found few clinically or statistically significant associations with diastolic dysfunction within our cohort. While this may imply a lack of impact of abnormal diastolic function upon clinical outcome in this cohort, our results could also indicate that the methodology developed for echocardiographic assessment of diastolic function in adults with biventricular hearts may not be applicable to pediatric single ventricle patients. Further efforts are needed to develop non-invasive methods to evaluate diastolic function in this unique circulation.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288). This work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration #: NCT00132782
References
- 1.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52(2):85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung YF, Penny DJ, Redington AN. Serial assessment of left ventricular diastolic function after Fontan procedure. Heart. 2000;83(4):420–424. doi: 10.1136/heart.83.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rychik J, Jacobs ML, Norwood WI., Jr Acute changes in left ventricular geometry after volume reduction operation. Ann Thorac Surg. 1995;60(5):1267–1273. doi: 10.1016/0003-4975(95)00704-O. discussion 74. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Blackstone EH, Kirklin JW, Pacifico AD, Bargeron LM., Jr The Fontan operation. Ventricular hypertrophy, age, and date of operation as risk factors. J Thorac Cardiovasc Surg. 1986;92(6):1049–1064. [PubMed] [Google Scholar]
- 5.McCrindle BW, Zak V, Sleeper LA, Paridon SM, Colan SD, Geva T, et al. Laboratory measures of exercise capacity and ventricular characteristics and function are weakly associated with functional health status after Fontan procedure. Circulation. 121(1):34–42. doi: 10.1161/CIRCULATIONAHA.109.869396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleeper LA, Anderson P, Hsu DT, Mahony L, McCrindle BW, Roth SJ, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152(3):427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study) Am J Cardiol. 2009;104(3):419–428. doi: 10.1016/j.amjcard.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51(7):679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30(1):8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 10.Landgraf JMAL, Ware JE. The Child Health Questionnaire (CHQ) User’s Manual. Boston, MA: HealthAct; 1999. [Google Scholar]
- 11.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99(2):445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 12.Senzaki H, Masutani S, Kobayashi J, Kobayashi T, Sasaki N, Asano H, et al. Ventricular afterload and ventricular work in Fontan circulation: comparison with normal two-ventricle circulation and single-ventricle circulation with Blalock-Taussig shunts. Circulation. 2002;105(24):2885–2892. doi: 10.1161/01.cir.0000018621.96210.72. [DOI] [PubMed] [Google Scholar]
- 13.Olivier M, O’Leary PW, Pankratz VS, Lohse CM, Walsh BE, Tajik AJ, et al. Serial Doppler assessment of diastolic function before and after the Fontan operation. J Am Soc Echocardiogr. 2003;16(11):1136–1143. doi: 10.1067/S0894-7317(03)00635-7. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt B, Steendijk P, Ovroutski S, Lunze K, Rahmanzadeh P, Maarouf N, et al. Pulmonary vascular resistance, collateral flow, and ventricular function in patients with a Fontan circulation at rest and during dobutamine stress. Circ Cardiovasc Imaging. 2010;3(5):623–631. doi: 10.1161/CIRCIMAGING.109.931592. [DOI] [PubMed] [Google Scholar]
- 15.Friedman KG, McElhinney DB, Colan SD, Porras D, Powell AJ, Lock JE, et al. Left ventricular remodeling and improvement in diastolic function after balloon aortic valvuloplasty for congenital aortic stenosis. Circ Cardiovasc Interv. 2012;5(4):549–554. doi: 10.1161/CIRCINTERVENTIONS.112.968958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershenson JA, Zaidi AN, Texter KM, Moiduddin N, Stefaniak CA, Hayes J, et al. Differences in tissue Doppler imaging between single ventricles after the Fontan operation and normal controls. Am J Cardiol. 2010;106(1):99–103. doi: 10.1016/j.amjcard.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Menon SC, Dearani JA, Cetta F. Long-term outcome after atrioventricular valve surgery following modified Fontan operation. Cardiol Young. 2011;21(1):83–88. doi: 10.1017/S1047951110001538. [DOI] [PubMed] [Google Scholar]
- 18.Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol. 2007;49(25):2450–2456. doi: 10.1016/j.jacc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 19.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation. 2014;129(9):1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 20.Menon SC, Gray R, Tani LY. Evaluation of ventricular filling pressures and ventricular function by Doppler echocardiography in patients with functional single ventricle: correlation with simultaneous cardiac catheterization. J Am Soc Echocardiogr. 2011;24(11):1220–1225. doi: 10.1016/j.echo.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Vitarelli A, Conde Y, Cimino E, D’Angeli I, D’Orazio S, Ventriglia F, et al. Quantitative assessment of systolic and diastolic ventricular function with tissue Doppler imaging after Fontan type of operation. Int J Cardiol. 2005;102(1):61–69. doi: 10.1016/j.ijcard.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52(2):99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 23.Gewillig M, Brown SC, Eyskens B, Heying R, Ganame J, Budts W, et al. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg. 2010;10(3):428–433. doi: 10.1510/icvts.2009.218594. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, et al. Impact of oral sildenafil on exercise performance in children and young adults after the Fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123(11):1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84(3):1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17(5):1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 27.Dahan M, Aubry N, Baleynaud S, Ferreira B, Yu J, Gourgon R. Influence of preload reserve on stroke volume response to exercise in patients with left ventricular systolic dysfunction: a Doppler echocardiographic study. J Am Coll Cardiol. 1995;25(3):680–686. doi: 10.1016/0735-1097(94)00449-Z. [DOI] [PubMed] [Google Scholar]
- 28.Hosenpud JD, Morton MJ, Wilson RA, Pantely GA, Norman DJ, Cobanoglu MA, et al. Abnormal exercise hemodynamics in cardiac allograft recipients 1 year after cardiac transplantation. Relation to preload reserve. Circulation. 1989;80(3):525–532. doi: 10.1161/01.cir.80.3.525. [DOI] [PubMed] [Google Scholar]
- 29.Friedman KG, McElhinney DB, Rhodes J, Powell AJ, Colan SD, Lock JE, et al. Left ventricular diastolic function in children and young adults with congenital aortic valve disease. Am J Cardiol. 2013;111(2):243–249. doi: 10.1016/j.amjcard.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]