Abstract
Antiretroviral therapy (ART) can reduce HIV viral loads to undetectable levels and prevent disease progression. However, HIV persists in rare cellular reservoirs within ART-treated patients and rapidly reemerges if ART is stopped. Latently infected CD4+ T cells represent a major reservoir of HIV that persists during ART. Therefore, a cure for HIV must include methods that either permanently inactivate or eliminate latent virus. Experimental methods under investigation for eliminating latently infected cells include transplantation/gene therapy approaches intended to deplete the infected cells and replace them with HIV-resistant ones, and DNA editing strategies that are capable of damaging or excising non-expressing HIV proviruses. Alternatively, “activation-elimination,” also known as “shock and kill,” approaches aim to induce expression of latent virus, allowing the virus to be eliminated by viral cytopathic effects, immune effector mechanisms, or additional cells/antibodies that specifically target and kill cells expressing HIV proteins. Here, we describe these experimental approaches for eliminating latent HIV along with other recent advances in HIV cure research.
Keywords: activation, latency, reservoir
I. INTRODUCTION
Human immunodeficiency virus (HIV) infects and kills CD4+ T cells. The continuous depletion of these cells over years eventually overcomes the regenerative capacity of the immune system, resulting in the development of acquired immunodeficiency syndrome (AIDS). Potent combinations of antiretroviral drugs have been developed to combat HIV, which inhibit virus replication and often suppress plasma viral loads to undetectable levels. Yet the required continuous daily use of antiretroviral therapy (ART) is complicated by side effects of the drugs, adherence issues, the development of resistant viruses, and high financial costs. Most importantly, ART alone does not cure HIV infection. This is because replication-competent HIV is maintained in rare viral reservoirs that persist throughout ART.
The best characterized and probably the largest source of replication-competent HIV during ART is the latent HIV reservoir within resting CD4+ T cells.1–4 There is some debate over whether replication-competent HIV can also survive via other mechanisms over many years of ART. For example, virus might in some cases be maintained through very low levels of ongoing virus replication in tissues with poor antiretroviral drug penetration, such as the central nervous system or deep within lymphoid organs.5,6 Persistence of HIV in these organs may be further aided by infection of cells such as macrophages, which have longer half-lives than HIV-infected activated CD4+ T cells7 and might support rare chains of infection, where virus is transferred from one cell to another very infrequently, perhaps only once every few weeks or months. However, even if other sources of persistent HIV are present during ART, latently infected CD4+ T cells represent a key barrier to HIV cure that appears to be present in all patients, and are therefore a major focus of HIV cure research.
Latently infected cells are resting, primarily central and transitional memory CD4+ T cells that harbor intact HIV genomes integrated within their chromosomes, but express little or no viral RNA and no viral proteins.1,3,8,9 Virus within these cells can persist in a latent state for decades,9 but then revert to producing infectious virions if the host cell becomes stimulated, for example, through an encounter with its cognate antigen or exposure to a pro-inflammatory cytokine environment. The latent proviruses are not affected by ART (which only inhibits actively replicating virus) and are invisible to the immune system due to their lack of viral protein expression. Therefore, these latently infected cells can persist during ART and generate virus that rapidly spreads if ART is stopped. Resting CD4+ T cells cannot usually be productively infected with HIV, and exposure of these cells to virus typically results in abortive infection prior to integration.10 Hence, it is believed that latently infected cells are probably formed when an overtly activated or cytokine-stimulated CD4+ becomes infected by HIV and then transitions to a resting memory cell before it can be killed by the immune response or viral cytopathic effects.
Historically, the latent reservoir has been quantified by observing viral outgrowth during a single round of stimulation in a limiting dilution coculture assay. Based on this assay, it is estimated that each infected individual harbors approximately a million latently infected cells, although this number can vary substantially from person to person.2,11 This translates into around 1 per million resting CD4+ T cells containing an activation-inducible latent provirus in each infected individual. A more recent study based on the quantification of apparently intact HIV proviral sequences within resting CD4+ cells suggests that the reservoir of replication-competent proviruses might be in some cases 60-fold higher than calculated using assays involving a single round of stimulation.12 Regardless of the precise size of the latent reservoir, it is known to decay very slowly during ART, with a half-life of over 40 months.1 This slow rate of decay is likely sufficient to ensure lifelong infection despite continuous ART. The latent reservoir is also seeded very soon after primary infection, and is detectable even in patents who initiated ART within weeks of infection.13 In addition to serving as a persistent source of virus, the latent reservoir can also serve as an archive of viral variants. This can complicate treatment options since antiretroviral drug resistant variants can be maintained in the reservoir. It also poses challenges for natural immune control of virus and the use of cytotoxic T lymphocyte (CTL)-based immunotherapies to combat HIV, because CTL-resistant viruses are similarly deposited in the reservoir early in the course of infection.14,15
Efforts to eliminate the latent reservoir of HIV have historically been focused on one of several individual strategies, as we have reviewed more completely elsewhere.16,17 One potential approach involves ablation/transplantation, whereby both latently infected and uninfected cells are eliminated by powerful ablative chemotherapy/radiotherapy. Hematopoietic stem cells from an individual who is resistant to HIV (e.g., due to a lack of expression of the CCR5 coreceptor) are then introduced into the patient, which repopulate the immune system with mature HIV-resistant cells. This approach appears to have been successful in the case of the “Berlin patient,” Timothy Ray Brown, who remains free of HIV after receiving aggressive therapy to treat leukemia and two bone marrow transplants using cells from a CCR5 Δ32 homozygous individual.18,19 While the health risks, financial costs, and logistical problems associated with this treatment would preclude its use in the overwhelming majority of HIV-infected individuals, safer alternatives are being investigated. These include the genetic modification of a patient’s own stem cells to reduce or eliminate CCR5 expression, which should circumvent the significant problem of finding an appropriate CCR5-deficient donor match for transplantation.20 Safer, more gentle conditioning procedures that make space for the donor cells but fall short of complete myeloablation are also being investigated.21 Without the fully ablative therapy and graft versus host disease that occurred in the Berlin patient, it is perhaps more likely that these milder approaches would lead to extended periods of drug-free virus control, or a “functional cure,” rather than a complete sterilizing cure whereby no replication-competent HIV is detectable.
Alternative approaches directed toward eliminating latently infected cells are also being developed. Perhaps the most conceptually straightforward method to reduce the latent reservoir would be to excise or otherwise permanently inactivate the non-expressing latent HIV proviruses. In recent years, a range of approaches involving DNA-editing enzymes have been developed to achieve precisely this goal (Fig. 1). Homing endonuclease,22 zinc finger nuclease,23 evolved recombinase,24 and CRISPR/Cas9 (Ref. 25) systems have each been developed that can disrupt HIV-derived proviruses in cell lines or activated primary cells. Challenges associated with applying these methods to latently infected resting CD4+ T cells in vivo include the difficulty of expressing exogenous DNA or RNA encoding the editing enzymes in resting CD4+ T cells. Delivering native therapeutic proteins into resting cells in sufficient quantities to be active would also be problematic. It is possible that nanoparticle-mediated delivery systems could aid in this process by protecting the therapeutic DNA/RNA/proteins during in vivo delivery and help to deliver them across the plasma membrane into cells.
FIG. 1.
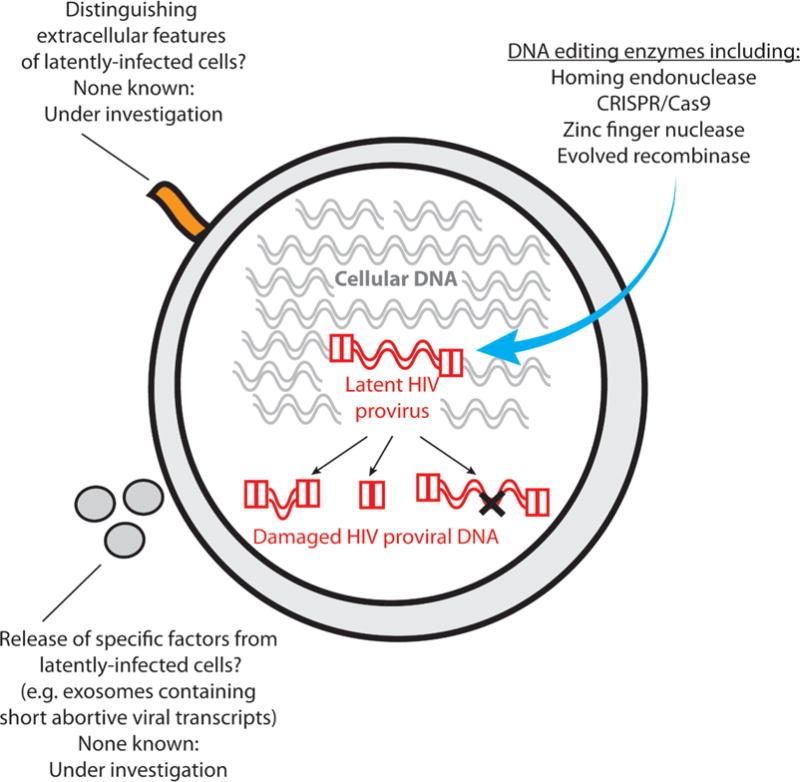
Methods under investigation for eliminating latent HIV proviruses without first inducing HIV expression
Additional challenges associated with directly editing latent HIV genomes include the fact that latent proviruses can be integrated in relatively inaccessible regions of the chromosomal DNA, which could afford them some protection from exogenous DNA editing enzymes faced with the already difficult task of targeting a single 10 kilobase pair HIV genome within approximately 3 billion base pairs of human DNA present in each cell. Indeed, one of the factors that might contribute to establishment of HIV latency is the integration of virus into non-expressing regions of cellular DNA such as heterochromatin.26 Finally, any therapy directly targeting the HIV DNA genome will have to contend with the extremely large genetic diversity of HIV, which is a well-known challenge in the field of vaccine development, and is critically important for the ability of HIV to evade the immune response and evolve resistance to antiretroviral drugs. This genetic diversity will also be a challenge when designing DNA editing approaches for eliminating latent HIV from primary cells, although targeting conserved regions of the virus and the simultaneous editing of multiple regions of the viral genome are feasible approaches to circumvent these problems. Hence, the approach of directly editing the non-expressing HIV genome is not without challenges. Nevertheless, editing of latent proviral HIV DNA represents an attractive and direct potential pathway to eliminating the latent reservoir.
If the HIV DNA cannot be targeted directly, then an alternative approach for eliminating latently infected cells would be to selectively kill only those cells harboring HIV genomes (Fig. 1). This could potentially be achieved if latently infected cells bear any external features that distinguished them from their uninfected counterparts. However, beyond the broad phenotype of predominantly memory CD4+ T cells, no definitive markers of latently infected cells have yet been found. Research in this area is continuing, with membrane proteomics being coupled with HIV latency models to try to identify markers that are enriched in latently infected cells. Along these lines, high levels of CD2 expression have recently been associated with latently infected cells in both an in vitro model system and ART-treated patient samples.27 Alternative surrogate markers for latently infected cells are also being searched for. For example, while latently infected cells do not express viral proteins, they might under some circumstances express short transcripts such as the TAR stem loop that is present in all HIV RNA, including abortive short transcripts. TAR RNA has been shown to be present in exosomes that are released from infected cells.28 Therefore, efforts are underway to identify whether latently infected cells produce exosomes containing HIV RNA that could aid in their identification, quantification, and elimination.
A third potential method for eliminating latent virus has variously been termed an “activation-elimination,” “shock and kill,” or “kick and kill” approach (Fig. 2). This involves first flushing the virus out of latency leading to the expression of viral proteins. Once this has been achieved, the host cell would potentially become susceptible to viral cytopathic effects, immune effector mechanisms, or therapeutics that can target cells producing viral proteins. If this were performed under the cover of antiretroviral therapy, then any newly produced virions should be inhibited by the antiretroviral drugs and therefore should not be able to initiate new rounds of replication.
FIG. 2.
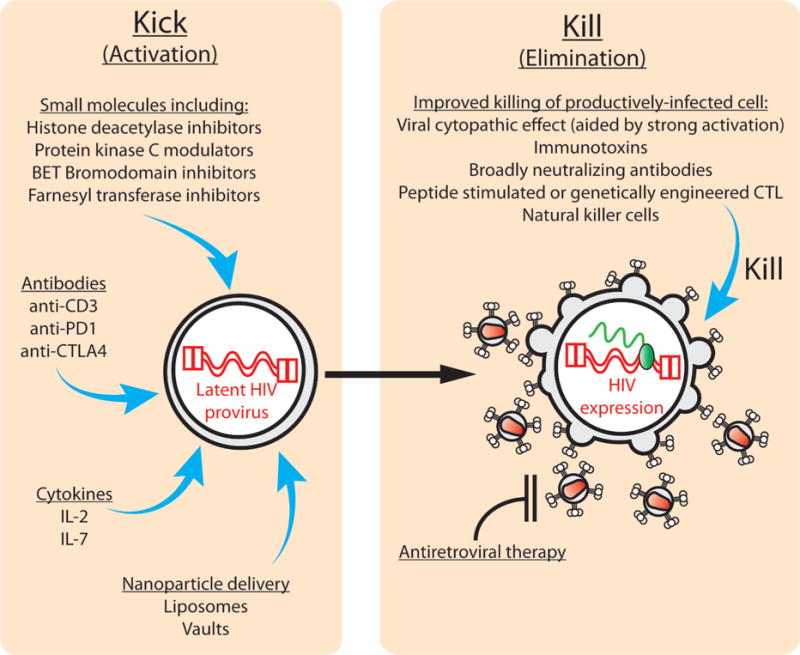
“Kick and kill” approaches for inducing expression of latent HIV then killing of the virus expressing cell. In the left-hand panel several experimental approaches for inducing expression of latent HIV are shown. The right-hand panel lists possible approaches for accelerating the killing of cells induced to express viral proteins.
Methods for inducing expression of latent HIV in a safe and effective manner are currently under intense investigation (Fig. 2). Approaches that have been tested in ART-treated patients include the use of antibodies (anti-CD3), cytokines (IL-2), and histone deacetylase inhibitors (e.g., valproic acid and vorinostat).29–32 These efforts produced important information regarding the feasibility of latency activating approaches. However, they had varying effects on in vivo HIV expression and latent reservoir size, and none of the tested approaches have successfully eliminated persistent HIV reservoirs entirely or prevented viral rebound if therapy is stopped. Additional agents that are under investigation for inducing latent HIV expression include small molecules such as protein kinase C (PKC) modulators. These include prostratin, bryostatin, and synthetic analogs based on these compounds, which have greatly improved characteristics compared with the parental compounds.33–37 BET bromodomain inhibitors and farnesyl transferase inhibitors have also been shown to be capable of activating latent HIV in some circumstances, and are therefore being further pursued.38,39 Immunomodulatory antibodies including anti-PD1 and anti-CTLA4 might overcome inhibitory signals in latently infected cells and thereby help in efforts to activate the host cell and induce latent virus expression.8,40–42
There is also some concern that HIV latency activating agents could have unwanted side effects on cells that are not targets for HIV infection. Therefore, we have investigated nanoparticle delivery systems for latency reversing agents.43,44 These are still under development but offer potential advantages including the possibility of targeting latency reversing agents to CD4+ T cells by attaching antibodies or other targeting domains to the nanoparticles, which may reduce the possibility of toxic effects mediated through non-HIV target cells. Nanoparticles also allow for the co-packaging of multiple latency reversing agents into single particles, and simultaneous delivery of latency reversing agents and antiretroviral drugs such as protease inhibitors to latently infected cells, which could serve as a safety feature that prevents the newly activated cell from producing infectious virions.44
If a high level of virus expression is produced by latency reversing agents, then the host cell could be killed by viral cytopathic effects. However, cell damage induced by virus production might not be sufficient if latency reversing agents only induce low levels of viral protein production. The natural antiviral immune response might also be insufficient in these circumstances, particularly since the latent reservoir is rapidly populated by virus that is resistant to immunodominant CTL epitopes.14,15,45 Therefore, augmentation of cell killing by infusion of peptide stimulated CTL, which are specific for subdominant HIV epitopes,14 or CTL that have been genetically engineered with T cell receptors that are specific for conserved HIV epitopes,46 might help in rapidly eliminating latently infected cells (Fig. 2). Natural killer cells could also serve a similar function but have not yet been thoroughly evaluated in relevant in vivo latency models.
HIV envelope proteins are expressed on the surface of productively infected cells. Therefore, another approach for rapidly killing latently infected cells that have been induced to express viral proteins by a “kick and kill” strategy would be to use broadly neutralizing antibodies that target HIV gp120 and can facilitate antibody-dependent cell mediated cytotoxicity, or immunotoxins that can bind to Env-expressing cells and directly kill them with the toxic moieties they carry.41,47 These targeted, augmented killing approaches are also attractive because if any ongoing virus replication does occur in ART-treated individuals, then chronically/productively infected cells could be eliminated at the same time as the latently infected cells that have been induced to express virus.
II. CONCLUSION
The challenges to developing an HIV cure are formidable. However, over the last several years there has been a growing sentiment in the field that a cure for HIV is possible. This has been fueled by the (so far) unique case of the Berlin patient,18 but also additional cases where a shrinking of the latent reservoir by bone marrow transplants in two adults or through very early and intensive ART initiation in an infant each resulted in delayed viral rebound on stopping therapy.48,49 It is hoped that advances in therapeutic approaches for eliminating latent HIV described herein, coupled with the availability of relevant in vivo models for evaluating their effects latent HIV,50–52 will provide a pathway for the development of scalable and safe methods for eliminating persistent reservoirs of HIV in the future.
Acknowledgments
Research in the authors’ laboratory is supported by National Institutes of Health Grants No. AI70010 and No. U19AI096113 (Project No. 3.4 to JZ), and the UCLA center for AIDS research (Grant No. 5P30 AI028697).
BRIEF COMMENTS REGARDING JOHN FAHEY
One of the authors, Jerome Zack, would like to briefly acknowledge the contributions of Dr. John L. Fahey to AIDS research, to UCLA, and to his personal research career. First, it is without question that John contributed greatly to AIDS research. Aside from his well-known contributions to immunology, John was the first to bring the new technology of flow cytometry to UCLA. His suggestion to use this new technology to examine the levels of CD4+ T lymphocytes in the blood of patients suffering from what we now recognize as HIV infection led to the first description of the syndrome by Drs. Gottlieb and Saxon, and led to a major way of monitoring disease progression.53,54 John contributed greatly to multiple studies in the Multicenter AIDS Cohort Study (MACS), thereby defining the course and confounders of HIV disease. In later years, John worked tirelessly to bring improved technologies and knowledge to other countries, most notably India. In his role as Director of the International Activities Core of the UCLA CFAR/AIDS Institute, he brought attention to international issues, and galvanized many of our investigators to work in these areas. Regarding the author’s own research career, John Fahey was instrumental on many levels. First, while not being the actual postdoctoral mentor, there was his constant encouragement and considerable discussion regarding immunology. More specifically, John provided office space to a young postdoctoral fellow new to UCLA, in a laboratory filled with more senior scientists. This provided an environment to have scientific discussions with many outstanding young investigators with diverse interests, and facilitated the establishment of many friendships, which still exist today. Finally, John helped mentor one of Jerry Zack’s trainees who had chosen to work on topics exploring the interaction of the nervous system and the immune system, a topic with which he was familiar, but Jerry was not. This trainee has gone on to enjoy a distinguished research career, which may not have been as successful without John’s help. John will be greatly missed for his valued advice, his innovative science, and his enduring friendship.
ABBREVIATIONS
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- CTL
cytotoxic T lymphocytes
- HIV
human immunodeficiency virus
References
- 1.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94(24):13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111(6):2307–12. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28(15):2251–8. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, Skillman D, Meltzer MS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167(4):1428–41. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sékaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 10.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61(2):213–22. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O’Doherty U, Palmer S, Deeks SG, Siliciano JD. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathogens. 2013;9(2):e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–51. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95(15):8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L, Siliciano RF. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–5. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsden MD, Zack JA. Double trouble: HIV latency and CTL escape. Cell Host Microbe. 2015;17(2):141–2. doi: 10.1016/j.chom.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsden MD, Zack JA. HIV/AIDS eradication. Bioorg Med Chem Lett. 2013;23(14):4003–10. doi: 10.1016/j.bmcl.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsden MD, Zack JA. Establishment and maintenance of HIV latency: model systems and opportunities for intervention. Future Virol. 2010;5(1):97–109. doi: 10.2217/fvl.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kücherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 19.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117(10):2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 20.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003;100(1):183–8. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiem HP, Jerome KR, Deeks SG, McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell. 2012;10(2):137–47. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubert M, Ryu BY, Banks L, Rawlings DJ, Scharenberg AM, Jerome KR. Successful targeting and disruption of an integrated reporter lentivirus using the engineered homing endonuclease Y2 I-AniI. PLoS One. 2011;6(2):e16825. doi: 10.1371/journal.pone.0016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu X, Wang P, Ding D, Li L, Wang H, Ma L, Zhou X, Liu S, Lin S, Wang X, Zhang G, Liu S, Liu L, Wang J, Zhang F, Lu D, Zhu H. Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells. Nucleic Acids Res. 2013;41(16):7771–82. doi: 10.1093/nar/gkt571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkar I, Hauber I, Hauber J, Buchholz F. HIV-1 proviral DNA excision using an evolved recombinase. Science. 2007;316(5833):1912–5. doi: 10.1126/science.1141453. [DOI] [PubMed] [Google Scholar]
- 25.Ebina H, Misawa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. Embo J. 2003;22(8):1868–77. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iglesias-Ussel M, Vandergeeten C, Marchionni L, Chomont N, Romerio F. High levels of CD2 expression identify HIV-1 latently infected resting memory CD4+ T cells in virally suppressed subjects. J Virol. 2013;87(16):9148–58. doi: 10.1128/JVI.01297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanan A, Iordanskiy S, Das R, Van Duyne R, Santos S, Jaworski E, Guendel I, Sampey G, Dalby E, Iglesias-Ussel M, Popratiloff A, Hakami R, Kehn-Hall K, Young M, Subra C, Gilbert C, Bailey C, Romerio F, Kashanchi F. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013;288(27):20014–33. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkosky J, Nunnari G, Otero M, Calarota S, Dornadula G, Zhang H, Malin A, Sullivan J, Xu Y, DeSimone J, Babinchak T, Stern J, Cavert W, Haase A, Pomerantz RJ. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002;186(10):1403–11. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 30.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, Davey RT, Jr, Dybul M, Kovacs JA, Metcalf JA, Mican JM, Berrey MM, Corey L, Lane HC, Fauci AS. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5(6):651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 31.Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, Martinson JA, Wiegand A, Bandarenko N, Schmitz JL, Bosch RJ, Landay AL, Coffin JM, Margolis DM. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22(10):1131–5. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, Pomerantz RJ. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98(10):3006–15. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 34.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–23. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qatsha KA, Rudolph C, Marme D, Schachtele C, May WS. Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc Natl Acad Sci U S A. 1993;90(10):4674–8. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem. 2012;4(9):705–10. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, Murray D, Chun TW, Zack JA, Wender PA. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A. 2013;110(29):11698–703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, Sebastiani P, Margolis DM, Montano M. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J Leukoc Biol. 2012;92(6):1147–54. doi: 10.1189/jlb.0312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnard R, Powell D, Karn J, Das B, Dobrowolski C, Margolis D, Archin N, Holloway K, Wang I-M, Erica Cook, Li J, Adam G, Newhard W, Miller MD, Hazuda D. Farnesyl-Transferase inhibitors: Identification and validation of a class which reactivates HIV latent expression and is synergistic with other mechanisms in vitro. 6th International Workshop on HIV Persistence during Therapy. Miami. 2013 [Google Scholar]
- 40.Porichis F, Kaufmann DE. Role of PD-1 in HIV pathogenesis and as target for therapy. Curr HIV/AIDS Rep. 2012;9(1):81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich T1, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989–99. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsden MD, Zack JA. Neutralizing the HIV reservoir. Cell. 2014;158(5):971–2. doi: 10.1016/j.cell.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buehler DC, Marsden M, Shen S, Toso DB, Wu X, Loo JA, Zhou ZH, Kickhoefer VA, Wender PA, Zack JA, Rome LH. Bioengineered vaults: Self-assembling protein shell-lipophilic core nanoparticles for drug delivery. ACS Nano. 2014;8(8):7723–32. doi: 10.1021/nn5002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovochich M, Marsden MD, Zack JA. Activation of latent HIV using drug-loaded nanoparticles. PLoS One. 2011;6(4):e18270. doi: 10.1371/journal.pone.0018270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph A, Zheng JH, Follenzi A, Dilorenzo T, Sango K, Hyman J, Chen K, Piechocka-Trocha A, Brander C, Hooijberg E, Vignali DA, Walker BD, Goldstein H. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82(6):3078–89. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, Kitchen CM, Berger EA, Zack JA. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19(3):413–23. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 48.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319–27. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW, Strain M, Richman D, Luzuriaga K. Absence of detectable HIV-1 viremia after treatment cessation in an infant. The N Engl J Med. 2013;369(19):1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, Bristol G, An DS, Zack JA. HIV latency in the humanized BLT mouse. J Virol. 2012;86(1):339–47. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, Margolis DM, Garcia JV. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86(1):630–4. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84(6):2913–22. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305(24):1425–31. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 54.Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30(21):250–2. [PubMed] [Google Scholar]


