Abstract
There is strong evidence supporting the role of the plasminogen activator system in head and neck squamous cell carcinoma (HNSCC), particularly of its uPA (urokinase plasminogen activator) / uPAR (urokinase plasminogen activator receptor) and SERPINE1 components. Overexpression of uPA/uPAR and SERPINE1 enhances tumor cell migration and invasion and plays a key role in metastasis development, conferring poor prognosis. The apparent paradox of uPA/uPAR and its inhibitor SERPINE1 producing similar effects is solved by the identification of SERPINE1 activated signaling pathways independent of uPA inhibition. Both uPA/uPAR and SERPINE1 are directly linked to the induction of epithelial-to-mesenchymal transition, the acquisition of stem cell properties and resistance to antitumor agents. The aim of this review is to provide insight on the deregulation of these proteins in all these processes.
We also summarize their potential value as prognostic biomarkers or potential drug targets in HNSCC patients. Concomitant overexpression of uPA/uPAR and SERPINE1 is associated with a higher risk of metastasis and could be used to identify patients that would benefit from an adjuvant treatment. In the future, the specific inhibitors of uPA/uPAR and SERPINE1, which are still under development, could be used to design new therapeutic strategies in HNSCCs.
Keywords: head and neck cancer, uPA, uPAR, SERPINE1, prognosis
INTRODUCTION
Head and neck cancer is the sixth most common cancer in incidence worldwide [1]. More than 500,000 new cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed each year (http://globocan.iarc.fr). Two thirds of patients are diagnosed at advanced stages, as lymph node metastases are often the first sign of the disease [2]. In advanced stages, surgery can significantly impact organ function, produce damage to the structures involved in swallowing and speech, and greatly reduce patient quality of life [3]. In order to avoid these effects, initial radical surgery has been progressively replaced by multimodal treatments that combine surgery, radiation and chemotherapy. Multimodal treatments have improved loco-regional disease control and organ preservation in head and neck patients, but five-year survival remains around 50% [2]. A high percentage of patients develop recurrences, metastasis or secondary tumors after treatment, which results in a poor clinical outcome [4-8].
The molecular mechanisms associated with head and neck tumor invasion, metastasis, dissemination and drug resistance remain largely unknown. The identification of new biomarkers associated with tumor spread could be very useful in classifying patients according to their risk of recurrence [9]. An appropriate classification would make it possible to optimize and rationalize treatment, management and HNSCC patient care after diagnosis [10, 11].
The plasminogen activator (PA) system plays a key role in extracellular matrix (ECM) remodeling, which in turn is crucial in the first steps of tumor progression and spreading [12]. Its main components include the plasminogen activators (uPA, urokinase plasminogen activator; tPA, tissue plasminogen activator), the cell membrane receptor for uPA (uPAR), the plasminogen activator inhibitors (PAI-1, plasminogen activator inhibitor 1; PAI-2, plasminogen activator inhibitor 2) and plasmin (Figure 1) [13]. The PA system regulates the generation of plasmin that results from the activation of plasminogen by uPA or tPA. The uPAR receptor in turn accumulates plasminogen conversion at cell surfaces [12]. In addition, the plasminogen activator inhibitors (PAI-1; PA1-2), also known as, SERPINE1 and SERPINB2 are the main inhibitors of uPA and tPA [13, 14].
Figure 1. Schematic representation of the main components of the plasminogen activator system and their role in extracellular matrix remodeling, growth factor activation, tumor growth and dissemination.
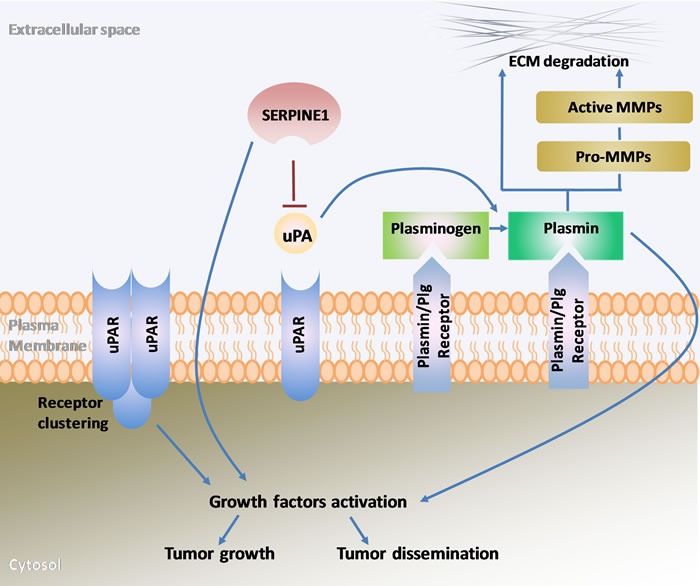
uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor; SERPINE1, serpin family E member 1 also known as plasminogen activator inhibitor-1(PAI-1); Plg, plasminogen; MMPs, metalloproteinases; ECM, extracellular matrix.
Plasmin activation is a key factor for fibrinolysis control and prevents health problems due to the formation of blood clots. Deregulation of uPA/uPAR and SERPINE1 has been associated with thrombosis, cardiovascular diseases and alterations of wound healing [14, 15]. Moreover, the PA system effect on cell adhesion and migration is particularly important in cancer progression. Active plasmin degrades the ECM directly or indirectly though the activation of several metalloproteinases [14]. ECM degradation facilitates the migration of tumor cells to other tissues and structures. SERPINE1 regulates this process and the adhesion/deadhesion balance of cells to the ECM, which is essential to control and promote tumor cell migration [16, 17]. A high expression of uPA/uPAR and SERPINE1 has been observed in numerous cancer types, being associated with poor patient prognosis [12, 18]. In this regard, several studies supporting the association between the activation of the PA system and head and neck cancer prognosis have been reported over the last years.
In this review, we first describe the effect of uPA, uPAR and SERPINE1 in head and neck cancer cell migration, metastatic dissemination and drug resistance. Secondly, we summarize their value as prognostic markers in patients with HNSCC. Finally, we discuss about the potential use of SERPINE1, uPAR, uPA and associated pathways as therapeutic targets.
ROLE OF UPA/UPAR IN TUMOR CELL PROLIFERATION, MIGRATION, INVASION AND METASTASIS
The uPA gene (Gene ID: 5328; http://www.ncbi.nlm.nih.gov/) encodes a 411 amino acid serine protease that consists of two α and two anti-parallel β strands [19]. This protein is secreted as a zymogen (pro-uPA) that is activated by cleavage of the peptide bond Lys158-Ile159 [20]. uPA has an amino-terminal EGF-like domain, a kringle domain, an interdomain linker and a catalytic domain [21]. The pro-uPA to uPA conversion is catalyzed by plasmin. Kallikrein, T cell-associated serine proteinase, cathepsin B, cathepsin L, nerve growth factor-γ, human mast cell tryptase and prostate specific antigen are other proteinases that can also catalyze “in vitro” the conversion of pro-uPA [12]. uPA converts plasminogen to plasmin by specific cleavage of an Arg-Val bond in plasminogen. The interaction of uPA with its receptor uPAR is also important for cell migration.
The uPAR gene (Gene ID: 5329; http://www.ncbi.nlm.nih.gov/gene) encodes a single polypeptide chain of 313 aminoacid residues that after the maturation generates a 55-60kDa protein [21, 22]. The uPA/uPAR interaction on the cell surface induces conformational changes that facilitate the formation of a new complex with integrins and with several ECM proteins such as vitronectin [23, 24]. uPAR overexpression can also enhance growth factor activation and cell migration through the regulation of several pathways independent from its capacity of binding uPA and promoting plasmin activation [25].
The uPA/uPAR complex expression plays a significant role on the invasive and metastatic potential of HNSCC [22, 26-28] (Figure 2). An active uPA increases the production of plasmin from plasminogen and this lead to ECM degradation, which in turn facilitates the invasion of cancer cells into the surrounding tissue, as well as their access to blood and lymph node vessels. α5β6 integrin can also activate uPA, which facilitates HNSCC cell motility [27, 29]. uPA and uPAR have been associated with an increase in the growth of head and neck cancer cells and with the activation of FAK and ERK1/2 signaling [30, 31]. Thombospondin has also been associated with the activation of uPA and cell invasion in HNSCC cells [32]. Plasmin cleaves a range of ECM proteins, such laminin and fibrin, and also activates metalloproteinases, such as the MMP2, previously associated with an increase of head and neck cancer cell invasion and metastasis [33-35]. Moreover, uPA and plasmin are involved in the activation of several growth factors, including HGF/SF and MSP, TGF-β, and FGF [12].
Figure 2. The pleiotropic effect of uPA/uPAR and SERPINE1 in head and neck squamous cell carcinoma.
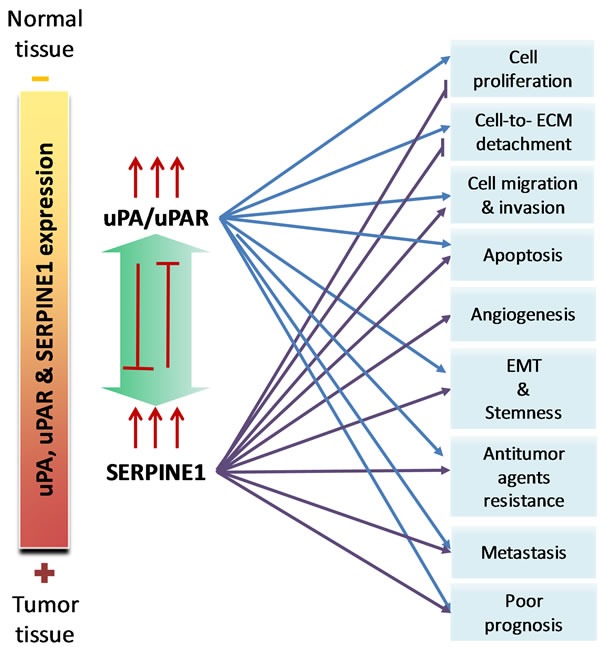
The expression of uPAR is associated with an invasive and metastatic phenotype in studies done using in vivo murine models of head and neck cancer. In oral squamous cell carcinoma xenografts, the inhibition of uPAR reduces tumor growth and downregulates the expression of genes previously associated with metastasis, such us MMP-2, MMP-9, VEGF-C, VEGF-D and VEGFR-3 [36]. A study conducted using an orthotopic murine model showed that the overexpression of uPAR in oral cancer cells generated infiltrative tumors with undefined margins and cytologic atypia [37]. These authors showed that the effect of uPAR on tumor cell invasion was associated with the activation of ERK1/2 MAP kinases and its co-localization with uPA and α3β1 integrin complex. uPAR can also promote the activation of the Ras-MAPK, Fak, Src and Rac and the PI3K-Akt pathways that have a significant effect on tumor cell migration [38]. Using an oral cancer metastatic mouse model, Zhang et al. showed that the expression of uPAR in cancer cells isolated from lymph node metastasis was higher than in cells isolated from primary tumor [39]. In nasopharyngeal carcinoma, a highly metastatic head and neck cancer [7], uPAR overexpression increases cell migration and invasion and promotes epithelial-to-mesenchymal transition and metastasis [25]. This process has been associated with the activation of the Jak-Stat pathway [40].
The inhibition of uPAR using antisense oligonucleotides reduces the invasiveness and the metastatic potential of head and neck cancer cells [41, 42].
In summary, most of the studies reported in head and neck cancer have shown that the overexpression of uPA/uPAR enhances tumor cell proliferation, migration and invasion. This effect is due to the activation of plasmin and ECM degradation, but it could also be the result of the indirect activation of several signaling pathways with a key role in tumor progression and metastasis, such as the PI3K-Akt pathway.
SERPINE1 IN CELL PROLIFERATION, MIGRATION, INVASION AND METASTASIS
The SERPINE1 gene (encodes a clade E member of the serine protease inhibitor (SERPIN) superfamily that is the main regulator of the plasminogen activator system (PAs). SERPINE1 inhibits the urokinase-type plasminogen (uPA) and tissue-type plasminogen activator (tPA), which in turn, reduce the conversion of plasminogen to the active protease plasmin [21]. The SERPINE1 gene is located at 7q21.2-q22 and codifies for a single-chain glycoprotein of about 50kDa. SERPINE1 has several polymorphisms in the promoter region that are associated with gene transcription [43]. Its expression could also be modulated by several transcription factors such as SP1, AP1, SMAD proteins, TGF-β1, and p53 [44-46]. SERPINE1 expression could be epigenetically modulated [47, 48] and it has been described as a target for the miR-145 [49-51]. SERPINE1 expression is also related to the activation of hypoxia-related factors such as HIF-1[52]. The different protein conformations displayed by SERPINE1 are one of the particular features of this protein. Its active conformation inhibits tPA and uPA forming a complex with each enzyme, whereas its latent form does not react with their target proteinases [53]. A non-inhibitory substrate form of SERPINE1 that could be cleaved by PAs has also been described [54]. After the interaction between SERPINE1 and PAs, SERPINE1 is cleaved and acquires an inactive form. This is relevant because, depending on its conformation, SERPINE1 could interact with different proteins and activate distinct molecular pathways.
SERPINE1 is the main inhibitor of the uPA/uPAR complex, which induces its internalization through a process mediated by the lipoprotein receptor protein-1 (LRP1 receptor) [55]. Based on the pro-metastatic role of plasmin that promotes cell matrix degradation and cell migration, SERPINE1 expression would be expected to develop a protective effect against tumor dissemination throughout the inhibition of uPA/uPAR complex activity. However, most of the studies conducted to date, in several cancer types, indicate that SERPINE1 expression is associated with poor outcome and increased risk of metastasis [56]. This supports a multifunctional role for SERPINE1 that promotes tumor cell migration and metastasis through several pathways independent from PAs effectors. In this regard, SERPINE1 has more ligands than PAs, including ECM components, heparin and LRP1[57].
In normal and transformed human keratinocytes, SERPINE1 is up-regulated in response to EGFR and TGF-β and is associated with an increase in cell migration and invasion [58-60]. During the process of wound healing, TGF-β induces the expression of SERPINE1 in leading edge keratinocytes, which stimulates cell migration and re-epithelization [16].
SERPINE1 expression has also been associated with tumor cell migration and invasion in head and neck cancer cells [9, 61, 62]. We showed that the ectopic overexpression of SERPINE1 enhances head and neck cancer cell migration, and this is mediated by the phosphorylation and activation of Akt [62]. These findings are in agreement with several studies conducted in endothelial cells, fibrosarcoma and breast cancer cells indicating that SERPINE1 overexpression increases tumor cell migration and invasion through the activation of the PI3K-Akt pathway [63, 64]. SERPINE1 pro-migratory effect has been associated with LRP1 interaction, which in turn stimulates the Jak/Stat pathway [65, 66]. In thyroid cancer cells, LRP1 activates ERK and inhibits JNK-dependent pathways, which maintain the invasive capacity of tumor cells [65]. It would be interesting to evaluate the interaction of SERPINE1 and LRP1 and whether it has a similar effect on cell migration in head and neck cancer cells. Similar results have been observed using “in vivo” mice models. Bajou K et al. observed a decrease in local invasion and tumor vascularization of transplanted malignant keratinocytes in mice deficient in SERPINE1 expression. [67]. Invasion was restored by transfection with a vector expressing SERPINE1. SERPINE1 may also contribute to tumor aggressiveness by promoting tumor angiogenesis [67, 68][69].
The studies addressing the connection between SERPINE1 expression and cell proliferation have generated inconsistent results. Some groups showed that SERPINE1 expression increases cell proliferation, whereas others reported a reduction in cell proliferation [57, 70]. In head and neck cancer cells, we observed that the ectopic overexpression of SERPINE1 reduces cell proliferation, whereas its inhibition with shRNA increases cell proliferation [62].
UPA/UPAR AND SERPINE1 IN APOPTOSIS REGULATION AND TUMOR RESISTANCE
Recent findings have suggested that in addition to its role in cell dissemination and metastasis, the expression of several components of the PA system could reduce the cytotoxic effect of anticancer drugs [71]. For instance, the uPAR expression has been associated with multi-drug resistance in small cell lung cancer cells [72]. uPA, uPAR, and SERPINE1 have also been associated with the efficacy of tamoxifen treatment in breast cancer [73, 74]. A high expression of these proteins increases drug resistance.
In head and neck and oesophageal cancer cells, uPA and SERPINE1 expression are upregulated after irradiation or reactive oxygen species exposure [75-79]. The activation of SERPINE1 is also associated with radiation resistance [80]. Moreover, the inhibition of uPAR has been associated with the downregulation of the multidrug resistance gene MDR1 [36]. Hypoxia is another factor that could contribute to tumor resistance. In this regard, the expression of SERPINE1 has been associated with the activation of hypoxia-related factors in head and neck cancer cells [52].
The plasminogen activator system proteins have been also associated with resistance to targeted therapies [71]. uPAR expression is associated with the development of resistance to EGFR inhibitor therapy in glioblastoma [81]. In head and neck cancer cells, the combination of uPAR down-regulation and EGFR inhibition showed a synergistic anti-tumor effect [82, 83]. Moreover, an association between the activation of EGFR signaling and SERPINE1 expression has also been described [60].
SERPINE1 (PAI-1) increases cisplatin resistance of head and neck tumor cells [62], while SERPINB2 (PAI-2) increases sensitivity [84]. Interestingly, both PA inhibitors showed an opposite effect on drug resistance. These findings and the fact that uPA/uPAR associates with resistance to therapy, suggest that the effect of SERPINE1 on tumor progression and drug resistance may be due to the activation of several signaling pathways that are independent from the inhibition of PA.
In the context of antitumor drug resistance, SERPINE1 is particularly interesting as it can enhance tumor progression through the inhibition of the apoptotic signaling. Thus, SERPINE1 over-expression has an anti-apoptotic effect [15, 59, 85, 86] that is mainly associated with the inhibition of Fas/Fas-L mediated apoptosis [68, 87]. SERPINE1 expression can also promote cell survival and block apoptosis by inhibiting caspase-3 activation [86]. Accordingly, we showed that the ectopic overexpression of SERPINE1 protects head and neck cancer cells from the apoptotic induction after cisplatin treatment [62]. This effect was mediated by PI3K-Akt pathway activation [62]. Similar findings have been reported in fibrosarcoma in which the expression of SERPINE1 protects cells from apoptosis through activation of the PI3K-Akt cell survival pathway [69, 88, 89].
On the basis of the foregoing, it may be concluded that SERPINE1 has an important role in protecting cells from apoptosis induction after the exposure to antitumor agents, and this should be considered when developing new treatment strategies, since it may be involved in cancer recurrence after therapy.
UPA/UPAR AND SERPINE1, GO OR GROWTH EFFECT, EPITHELIAL-TO-MESENCHYMAL TRANSITION AND STEMNESS
The results reported to date suggest that SERPINE1 expression could increase cell migration and simultaneously reduce cell proliferation [62, 90]. In this regard, SERPINE1 could act as a switch between tumor cell proliferation and tumor cell migration [16, 90, 91]. This phenomenon commonly known as “go or growth” supports the notion that changes in tumor cell morphology associated with an increase in cell motility and migration, such us the cytoskeletal reorganization, are incompatible with enhanced cell proliferation [92]. Thus, tumor cells could activate the migration process to spread to other locations and once these cells reach a specific tissue, they could activate cell proliferation in order to colonize the tissue and generate metastasis. Cell migration is also accompanied by a reduction in apoptotic signaling that protects cells from death during their travel towards the target tissue. We observed that SERPINE1 inhibits apoptosis while reducing tumor cell proliferation, findings that are consistent with SERPINE1 enhancement of the invasive and migratory phenotype [62].
SERPINE1 is directly connected with the epithelial-mesenchymal transition (EMT) and the acquisition of stemness, two biological processes that are essential to control the transition from the proliferative to the invasive tumor phenotype [60, 93-97]. The EMT process is associated with the activation of the TGF-β pathway which also induces SERPINE1 activation [98]. In fact, SERPINE1 has been commonly used as a surrogate marker of EMT. EMT increases the plasticity of tumor cells and their capacity to spread to other tissues and it is closely linked to the acquisition of stem cell properties. In this sense, SERPINE1 overexpression has been observed in circulating tumor cells from breast cancer patients showing EMT-like features [97]. In head and neck tumors, Lee and colleagues showed that, SERPINE1 inhibition suppresses the self-renewal capacity of cancer stem cells through the inhibition of SOX2 [80]. The overexpression of SOX2, one of the main regulators of self-renewal in adult tissues, has also been associated with the development of different types of squamous cell carcinomas such as lung, esophagus and nasopharyngeal carcinomas [8, 99].
Recently, Yu et al. have shown a link between SERPINE1 expression and Notch signaling in differentiated thyroid cancer [100]. These authors showed that NOTCH1 induction downregulates SERPINE1 expression and it is associated with a reduction in lung metastasis development in mice. It would be interesting to explore if this effect is also present in HNSCC, as a high percentage of inactivating mutations in Notch has been observed in this tumor type [101, 102]. Notch signaling is a crucial regulator of stem cell renewal and promotes terminal differentiation of keratinocytes through the expression of p21 and caspase-3 [103-105].
In HNSCCs, the induction of EMT and the acquisition of cancer stem cell properties may partly account for the acquisition of resistance to antitumor agents after the PA system activation [80]. Changes in cell adhesion and cytoskeletal remodeling, experienced during the EMT-process, increase tumor cell plasticity and drug resistance, effects that correlate with an increase in the expression of uPAR and SERPINE1 [106, 107]. A mesenchymal-like phenotype showing stemness features has been observed in the most aggressive subtype of head and neck tumors that often overexpress SERPINE1 [108-111]. uPAR and uPA signaling could also contribute to cancer stemness, as it has been demonstrated in breast and pancreatic cancer cells [112, 113]. The high level of recurrence that occurs in nasopharyngeal carcinoma after chemoradiotherapy treatment [6] appears to be associated with the expression of EMT and cancer stem cell markers [8].
In summary, there is evidence in the literature supporting a role for SERPINE1 expression in the induction of EMT and the acquisition of stem cell properties, two key mechanisms for the generation of cancer stem cells, which are the transition from a proliferative to an invasive tumor phenotype and the development of antitumor resistance associated with late tumor recurrences.
SERPINE1, UPA AND UPAR EXPRESSION AS PROGNOSTIC MARKERS IN HEAD AND NECK CANCER
SERPINE1 expression is higher in head and neck cancer tissue than in normal mucosa [27, 48, 62, 114-119]. Although a recent publication shows that SERPINE1 is up-regulated in cancer-associated fibroblasts and promotes the invasion of oral squamous cell carcinomas [120], immunohistochemical studies showed that the expression pattern of SERPINE1 in HNSCCs often differs from that observed in other cancer types. In head and neck tumors, SERPINE1 is expressed mainly in cancer cells, whereas in breast and colon cancers SERPINE1 is predominantly expressed in stromal cells rather than in cancer cells [116]. We observed, in this regard, that head and neck cancer cells showed membrane and cytoplasmatic positivity for SERPINE1 while tumor-adjacent normal tissue and stromal tissue areas were negative or showed negligible staining [62]. This could be particularly important as SERPINE1 could activate different biological pathways to promote tumor spread based on whether it is expressed in tumor cells or stromal cells.
In microarray gene expression studies, SERPINE1 expression has been identified as a HNSCC marker [109, 111, 121-123]. We used RNA seq level 3 data of 520 head and neck tumor samples and 44 normal tissue samples included in The Cancer Genome Atlas Database (TCGA) (http://cancergenome.nih.gov/) to study changes in uPA, uPAR and SERPINE1 expression after malignant transformation and its capacity to predict patient survival. We found overexpression of SERPINE1 in tumor tissue as compared to normal tissue (Figure 3A). Strojan et al showed that SERPINE1 expression was also higher in serum from patients with head and neck cancer than in healthy controls [124]. Lindberg et al showed that SERPINE1 expression was absent in normal, hyperplastic and dysplastic epithelia whereas a high SERPINE1 expression was present in incipient carcinoma and invasive carcinoma [116]. Additional large independent studies are needed to establish if the overexpression of SERPINE1 is also present in human papillomavirus (HPV) positive HNSCCs, a tumor subtype that differs in origin, biological features and clinical behavior from HPV negative tumors [125, 126].
Figure 3. Expression profile of the uPA, uPAR and SERPINE1 genes in head and neck samples from HNSCC patients included in TCGA database.
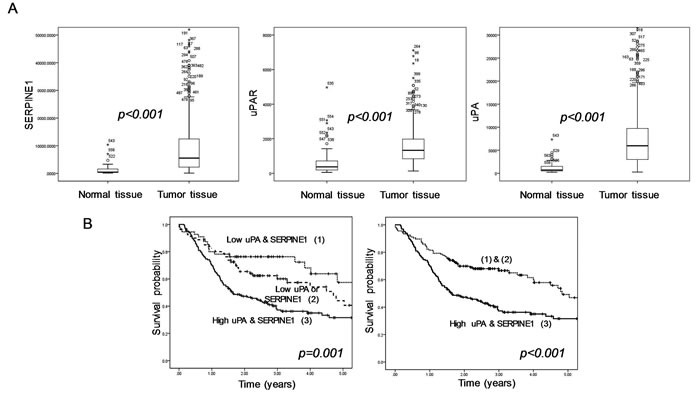
A. Differences in gene expression between normal tissue (n = 44) and tumor tissue (n = 520) (Mann Whitney U test). B. Differences in survival between patients with tumors expressing high levels of uPA or SERPINE1 and patients with low tumor expression (log-rank test and Kapplan Meier curves. In order to perform the survival analysis, we selected patients with a minimal follow-up of 18 months (n = 297). All (unpublished) results shown are based upon RNA seq level 3 data generated by TCGA Research Network;http://cancergenome.nih.gov/.
A high expression of SERPINE1 in head and neck tumor biopsies has been associated with a poor clinical outcome [27, 61, 62, 116-118, 124, 127-131] (Table 1). Most of the studies conducted to date in HNSCC have concluded that patients with tumors showing a high SERPINE1 expression had a poorer disease-free or overall survival than patients with tumors expressing low levels. Besides its prognostic value, we also showed, by analyzing three independent cohorts of patients with HNSCC, that a high SERPINE1 expression increases the risk of metastasis after treatment [62].
Table 1. Expression of SERPINE1, uPA and uPAR as prognostic factors in head and neck cancer studies.
| Study | uPA/uPAR/SERPINE1 | Protein/RNA | Tumor vs Normal | Prognostic significance | Type | N |
|---|---|---|---|---|---|---|
| Pavon et al 2015 [62] | SERPINE1 | Protein/RNA | Over-expressed | Poor prognosis | Re-/prospective | 80 & 190 |
| Pasini et al 2001 [117] | uPA/ SERPINE1 | RNA | Over-expressed | ------ | Prospective | 91 |
| Speleman et al 2007 [118] | uPA/SERPINE1 | Protein | Over-expressed | Poor prog. (SERPINE1) | Prospective | 46 |
| Yasuda et al 1997 [127] | uPA/SERPINE1 | Protein | ----- | ----- | NA | 28 |
| Lindberg et al 2006 [116] | uPAR/SERPINE1 | Protein | Over-expressed | ----- | Retrospective | 20 |
| Nozaki et al 1998 [27] | uPA/uPAR/SERPINE1 | Protein | ----- | ------ | NA | 34 |
| Strojan et al 1998 [124] | uPA/SERPINE1 | Protein | Over-expressed | ----- | Prospective | 58 |
| Chin et al 2005 [128] | uPA/SERPINE1 | Protein | Over-expressed | Poor prognosis | NA | 62 |
| Huang et al 2014 [84] | SERPINE1 | Protein | Over-expressed | NS | Retrospective | 43 |
| Magnussen et al 2014[130] | uPAR/SERPINE1 | Protein | Over-expressed | Poor prognosis | Retrospective | 115 |
| Dhanda et al 2014 [61] | SERPINE1 | Protein | Over-expressed | Poor prognosis | Prospective | 112 |
| Hundsdorfer et al 2005 [131] | uPA/SERPINE1 | Protein | Over-expressed | Poor prognosis | Prospective | 79 |
| Strojan et al 2000 [141] | uPA/SERPINE1 | Protein | --- | Poor prognosis (uPA) | NA | 47 |
| Yoshizawa et al 2011 [140] | uPA/uPAR | Protein | ---- | Poor prognosis | Retrospective | 54 |
N:number of patients included in each study ; NS:non-significant; NA.: not available
However, the association between SERPINE1 expression and the clinicopathological characteristics has generated conflicting results. Some studies have shown that the SERPINE1 expression is higher in advanced stages [124, 127]. SERPINE1 expression has been also associated with the presence of lymph node metastasis and the perineural invasion [62, 118, 132]. Dhanda et al showed that SERPINE1 expression was higher in the invasive front and could predict the extracapsular spread in patients with oral cancer [61]. Once again, these findings support the association of SERPINE1 overexpression with an invasive and migratory tumor phenotype. Other authors did not find any association between the clinicopatological characteristics and SERPINE1 expression, probably due to the small sample size included in these studies [27, 129].
HNSCC studies showed that the expression of uPA and uPAR is also commonly higher in tumor tissue than in normal tissue [114, 115, 117, 119, 127, 133-137]. We also found similar results by analyzing the expression data (RNA seq level 3 data) of HNSCC samples included in TCGA project (Figure 3A) Tobacco smoke could induce the expression of uPA which is commonly overexpressed in premalignant and malignant lesions in the oral cavity [138]. Several studies showed that uPA or uPAR expression is associated with higher T stage, low grade of differentiation and the presence of lymph node metastasis [27, 117, 134, 139]. Most analyses conducted in head and neck cancer have concluded that the expression of uPA and uPAR is also associated with a poor clinical outcome [128, 140, 141] (Table 1).
SERPINB2, another component of the plasminogen activator system, has been also analyzed in patients with head and neck cancer, being associated with clinical outcome [84, 142]. In contrast to SERPINE1, SERPINB2 was identified as a favorable prognostic marker. Moreover, down-regulation was associated with a reduced overall survival in patients with HNSCC. The opposite effect on patient prognosis of both plasminogen inhibitors (SERPINE1 and SERPINB2) supports again the notion that SERPINE1 activates signaling pathways that are independent from its role as PA system inhibitors.
Taken together, the reported findings suggest that a high expression of the PA system components, especially of uPA and SERPINE1, is associated with a poor clinical outcome in patients with head and neck cancer. In this regard, our analysis of the gene expression profile of 297 tumors included in TCGA database with a minimum follow-up of 18 months, identified a subgroup of patients with a poor survival characterized by a high expression of uPA and SERPINE1 (Figure 3B). We observed that the expression of these proteins in pre-treatment tumor biopsies could be used to identify patients with a high probability of death and to distinguish them from patients with low risk. Many studies in breast cancer patients, have establish SERPINE1 and uPA as suitable markers for therapy decision-making in patients with early lymph-node negative breast cancer [143-146]. We expect that these markers will be increasingly studied in the near future to establish if they could also be used in HNSCC patients for helping treatment decision making, especially to identify those patients with a high risk of metastasis who could be treated with adjuvant chemotherapy or chemo-radiation.
INHIBITION OF THE PLASMINOGEN ACTIVATOR COMPONENTS AS A THERAPEUTIC STRATEGY IN HNSCC
The reported results point out that the components of the PA system uPA, uPAR and SERPINE1 could be good targets for HNSCC therapy, so that their inhibition could represent a relevant strategy to increase the efficacy of current antitumor agents. In this sense, the antitumor activity of several small molecules inhibitors of SERPINE1, initially developed as antithrombotic agents, is currently being evaluated [87, 147-150]. These specific inhibitors usually block the interaction between SERPINE1 and uPA and generate conformational changes that result in the irreversible conversion of SERPINE1 into its latent or cleaved forms [15, 147, 151-153].
In vitro and in vivo xenografts models have shown an effect for these inhibitors on angiogenesis, apoptosis induction and tumor growth [87, 154-158]. In pre-clinical models, Tiplaxtinin, one of the most studied SERPINE1 inhibitors, is able to block the growth and induce apoptosis in bladder carcinoma, fibrosarcoma and head and neck cancer cells [80, 87, 156]. However, additional preclinical studies and subsequent clinical trials are necessary to show if these specific inhibitors could be used as a targeted therapy in HNSCCs patients whose tumors overexpress SERPINE1. Inhibitors of uPAR or uPA are also being developed and tested as antitumor agents in patients with breast, pancreatic and head and neck cancer in phase I-II trials [46, 159-162].
Moreover, as the activation of the PI3K-Akt pathway was commonly associated with SERPINE1 overexpression, the use of new inhibitors of PI3K-Akt pathway, currently under clinical investigation, could also be considered as an option to treat tumors overexpressing SERPINE1.
CONCLUSIONS AND PERSPECTIVES
In summary, the overexpression of uPA/uPAR enhances tumor cell proliferation, migration and invasion and plays a key role in metastasis development, conferring poor prognosis. This system appears to act mainly by activation of plasmin, involved in ECM degradation, and through the activation of several signaling pathways such as the PI3K-Akt pathway. SERPINE1 overexpression also enhances tumor cell migration and metastasis dissemination, promotes angiogenesis, protects cells from Fas/Fas-L mediated apoptosis and is associated with poor prognosis. The fact that the overexpression of uPA/uPAR and its main inhibitor SERPINE1, produce similar effects on cell migration, tumor spread and prognosis may seem contradictory, but several reports suggest that SERPINE1 activates signaling pathways independent of the inhibition of the uPA/uPAR complex. Both, uPA/uPAR and SERPINE1 are closely associated with the induction of EMT and the acquisition of cancer stem cell properties, which could contribute to resistance to therapy.
uPA/uPAR and SERPINE1 may be useful as prognostic biomarkers, since they are commonly overexpressed in HNSCCs and are associated with a poor clinical outcome. The determination of these markers in pre-treatment tumor biopsies could be used to stratify patients according to their risk of metastasis development. In the future, these markers, especially uPA and SERPINE1, could be used for treatment decision making to identify patients with a high risk of metastasis development, who could benefit from adjuvant chemotherapy or chemo-radiotherapy. However, they should be validated in independent clinical trials in order to clarify their clinical value and to identify suitable parameters for their detection and patient stratification. We also expect in the future that the specific inhibitors of uPA/uPAR and SERPINE1, already in clinical trials, could be tested in HNSCCs in combination with other drugs or radiation in an attempt to improve current antitumor therapy. The inhibition of the PA system could be particularly relevant to reduce lymph node recurrences and metastatic dissemination, one of the major challenges to prevent deaths for head and neck cancer.
Acknowledgments
This work was supported by AGAUR (Grants SGR1437 and 2014 PROD 00055) ; Fundació Marató de TV3 (Grant 416/C/2013-2030); CIBER-BBN (Grant 06/01/1031) and Plan Estatal de I+D+I of the Instituto de Salud Carlos III (co-funding from FEDER)(Grants PI14/01918, PI15/00378, PI15/00500 and PIE15/00028)
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 3.Licitra L, Mesia R, Keilholz U. Individualised quality of life as a measure to guide treatment choices in squamous cell carcinoma of the head and neck. Oral Oncol. 2015 doi: 10.1016/j.oraloncology.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Leon X, Martinez V, Lopez M, Garcia J, Venegas Mdel P, Esteller E, Quer M. Second, third, and fourth head and neck tumors. A progressive decrease in survival. Head Neck. 2012;34:1716–1719. doi: 10.1002/hed.21977. [DOI] [PubMed] [Google Scholar]
- 5.Licitra L, Vermorken JB. Is there still a role for neoadjuvant chemotherapy in head and neck cancer? Ann Oncol. 2004;15:7–11. doi: 10.1093/annonc/mdh001. [DOI] [PubMed] [Google Scholar]
- 6.Wang HY, Chang YL, To KF, Hwang JS, Mai HQ, Feng YF, Chang ET, Wang CP, Kam MK, Cheah SL, Lee M, Gao L, Zhang HZ, He JH, Jiang H, Ma PQ, et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chinese journal of cancer. 2016;35:41. doi: 10.1186/s40880-016-0103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen LJ, Wang SY, Xie GF, Zeng Q, Chen C, Dong AN, Huang ZM, Pan CC, Xia YF, Wu PH. Subdivision of M category for nasopharyngeal carcinoma with synchronous metastasis: time to expand the M categorization system. Chinese journal of cancer. 2015;34:450–458. doi: 10.1186/s40880-015-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lun SW, Cheung ST, Lo KW. Cancer stem-like cells in Epstein-Barr virus-associated nasopharyngeal carcinoma. Chinese journal of cancer. 2014;33:529–538. doi: 10.5732/cjc.014.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takes RP, Rinaldo A, Rodrigo JP, Devaney KO, Fagan JJ, Ferlito A. Can biomarkers play a role in the decision about treatment of the clinically negative neck in patients with head and neck cancer? Head Neck. 2008;30:525–538. doi: 10.1002/hed.20759. [DOI] [PubMed] [Google Scholar]
- 10.Polanska H, Raudenska M, Gumulec J, Sztalmachova M, Adam V, Kizek R, Masarik M. Clinical significance of head and neck squamous cell cancer biomarkers. Oral Oncol. 2014;50:168–177. doi: 10.1016/j.oraloncology.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 11.da Silva SD, Ferlito A, Takes RP, Brakenhoff RH, Valentin MD, Woolgar JA, Bradford CR, Rodrigo JP, Rinaldo A, Hier MP, Kowalski LP. Advances and applications of oral cancer basic research. Oral Oncol. 2011;47:783–791. doi: 10.1016/j.oraloncology.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Duffy MJ. The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des. 2004;10:39–49. doi: 10.2174/1381612043453559. [DOI] [PubMed] [Google Scholar]
- 14.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 15.Van De Craen B, Declerck PJ, Gils A. The Biochemistry, Physiology and Pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2012;130:576–585. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Simone TM, Longmate WM, Law BK, Higgins PJ. Targeted Inhibition of PAI-1 Activity Impairs Epithelial Migration and Wound Closure Following Cutaneous Injury. Adv Wound Care (New Rochelle) 2015;4:321–328. doi: 10.1089/wound.2014.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simone TM, Higgins PJ. Inhibition of SERPINE1 Function Attenuates Wound Closure in Response to Tissue Injury: A Role for PAI-1 in Re-Epithelialization and Granulation Tissue Formation Journal of Developmental Biology. 2015;3:11–24. [Google Scholar]
- 18.McMahon BJ, Kwaan HC. Components of the Plasminogen-Plasmin System as Biologic Markers for Cancer. Adv Exp Med Biol. 2015;867:145–156. doi: 10.1007/978-94-017-7215-0_10. [DOI] [PubMed] [Google Scholar]
- 19.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Behrens MA, Botkjaer KA, Goswami S, Oliveira CL, Jensen JK, Schar CR, Declerck PJ, Peterson CB, Andreasen PA, Pedersen JS. Activation of the zymogen to urokinase-type plasminogen activator is associated with increased interdomain flexibility. J Mol Biol. 2011;411:417–429. doi: 10.1016/j.jmb.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Stack MS. Urinary-type plasminogen activator (uPA) and its receptor (uPAR) in squamous cell carcinoma of the oral cavity. Biochem J. 2007;407:153–159. doi: 10.1042/BJ20071037. [DOI] [PubMed] [Google Scholar]
- 23.Kwaan HC, Mazar AP, McMahon BJ. The apparent uPA/PAI-1 paradox in cancer: more than meets the eye. Semin Thromb Hemost. 2013;39:382–391. doi: 10.1055/s-0033-1338127. [DOI] [PubMed] [Google Scholar]
- 24.McMahon B, Kwaan HC. The plasminogen activator system and cancer. Pathophysiol Haemost Thromb. 2008;36:184–194. doi: 10.1159/000175156. [DOI] [PubMed] [Google Scholar]
- 25.Jo M, Takimoto S, Montel V, Gonias SL. The urokinase receptor promotes cancer metastasis independently of urokinase-type plasminogen activator in mice. The American journal of pathology. 2009;175:190–200. doi: 10.2353/ajpath.2009.081053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petruzzelli GJ, Snyderman CH, Johnson JT. In vitro urokinase type plasminogen activator levels and total plasminogen activator activity in squamous cell carcinomas of the head and neck. Arch Otolaryngol Head Neck Surg. 1994;120:989–992. doi: 10.1001/archotol.1994.01880330067012. [DOI] [PubMed] [Google Scholar]
- 27.Nozaki S, Endo Y, Kawashiri S, Nakagawa K, Yamamoto E, Yonemura Y, Sasaki T. Immunohistochemical localization of a urokinase-type plasminogen activator system in squamous cell carcinoma of the oral cavity: association with mode of invasion and lymph node metastasis. Oral Oncol. 1998;34:58–62. doi: 10.1016/s1368-8375(97)00028-6. [DOI] [PubMed] [Google Scholar]
- 28.Li HF, Liu YQ, Shen ZJ, Gan XF, Han JJ, Liu YY, Li HG, Huang ZQ. Downregulation of MACC1 inhibits invasion, migration and proliferation, attenuates cisplatin resistance and induces apoptosis in tongue squamous cell carcinoma. Oncol Rep. 2015;33:651–660. doi: 10.3892/or.2014.3612. [DOI] [PubMed] [Google Scholar]
- 29.Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, Batakis P, Wiechec E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis. 2014;35:747–759. doi: 10.1093/carcin/bgu045. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguirre Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–2524. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- 32.Albo D, Tuszynski GP. Thrombospondin-1 up-regulates tumor cell invasion through the urokinase plasminogen activator receptor in head and neck cancer cells. J Surg Res. 2004;120:21–26. doi: 10.1016/j.jss.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal EL, Hotary K, Bradford C, Weiss SJ. Role of membrane type 1-matrix metalloproteinase and gelatinase A in head and neck squamous cell carcinoma invasion in vitro. Otolaryngol Head Neck Surg. 1999;121:337–343. doi: 10.1016/S0194-5998(99)70217-2. [DOI] [PubMed] [Google Scholar]
- 34.Shimada T, Nakamura H, Yamashita K, Kawata R, Murakami Y, Fujimoto N, Sato H, Seiki M, Okada Y. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human oral squamous cell carcinomas: implications for lymph node metastasis. Clin Exp Metastasis. 2000;18:179–188. doi: 10.1023/a:1006749501682. [DOI] [PubMed] [Google Scholar]
- 35.Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, Maune S. Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck. 2006;28:31–39. doi: 10.1002/hed.20298. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Tang Y, Liang X, Yang X, Yang J, Zhu G, Zheng M, Zhang C. RNAi targeting urokinase-type plasminogen activator receptor inhibits metastasis and progression of oral squamous cell carcinoma in vivo. Int J Cancer. 2009;125:453–462. doi: 10.1002/ijc.24360. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Koblinski J, Johnson J, Liu Y, Ericsson A, Davis JW, Shi Z, Ravosa MJ, Crawford S, Frazier S, Stack MS. Urinary-type plasminogen activator receptor/alpha 3 beta 1 integrin signaling, altered gene expression, and oral tumor progression. Mol Cancer Res. 2010;8:145–158. doi: 10.1158/1541-7786.MCR-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Liu Y, Gilcrease MZ, Yuan XH, Clayman GL, Adler-Storthz K, Chen Z. A lymph node metastatic mouse model reveals alterations of metastasis-related gene expression in metastatic human oral carcinoma sublines selected from a poorly metastatic parental cell line. Cancer. 2002;95:1663–1672. doi: 10.1002/cncr.10837. [DOI] [PubMed] [Google Scholar]
- 40.Bao YN, Cao X, Luo DH, Sun R, Peng LX, Wang L, Yan YP, Zheng LS, Xie P, Cao Y, Liang YY, Zheng FJ, Huang BJ, Xiang YQ, Lv X, Chen QY, et al. Urokinase-type plasminogen activator receptor signaling is critical in nasopharyngeal carcinoma cell growth and metastasis. Cell Cycle. 2014;13:1958–1969. doi: 10.4161/cc.28921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozaki S, Endo Y, Nakahara H, Yoshizawa K, Hashiba Y, Kawashiri S, Tanaka A, Nakagawa K, Matsuoka Y, Kogo M, Yamamoto E. Inhibition of invasion and metastasis in oral cancer by targeting urokinase-type plasminogen activator receptor. Oral Oncol. 2005;41:971–977. doi: 10.1016/j.oraloncology.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Ignar DM, Andrews JL, Witherspoon SM, Leray JD, Clay WC, Kilpatrick K, Onori J, Kost T, Emerson DL. Inhibition of establishment of primary and micrometastatic tumors by a urokinase plasminogen activator receptor antagonist. Clin Exp Metastasis. 1998;16:9–20. doi: 10.1023/a:1006503816792. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Cao Q, Wang X, Li B, Tang M, Yuan W, Fang J, Qian J, Qin C, Zhang W. PAI-1 4G/5G polymorphism contributes to cancer susceptibility: evidence from meta-analysis. PLoS One. 2013;8:e56797. doi: 10.1371/journal.pone.0056797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fink T, Kazlauskas A, Poellinger L, Ebbesen P, Zachar V. Identification of a tightly regulated hypoxia-response element in the promoter of human plasminogen activator inhibitor-1. Blood. 2002;99:2077–2083. doi: 10.1182/blood.v99.6.2077. [DOI] [PubMed] [Google Scholar]
- 46.Ulisse S, Baldini E, Sorrenti S, D’Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. doi: 10.2174/156800909787314002. [DOI] [PubMed] [Google Scholar]
- 47.Gao S, Skeldal S, Krogdahl A, Sorensen JA, Andreasen PA. CpG methylation of the PAI-1 gene 5′-flanking region is inversely correlated with PAI-1 mRNA levels in human cell lines. Thromb Haemost. 2005;94:651–660. [PubMed] [Google Scholar]
- 48.Gao S, Nielsen BS, Krogdahl A, Sorensen JA, Tagesen J, Dabelsteen S, Dabelsteen E, Andreasen PA. Epigenetic alterations of the SERPINE1 gene in oral squamous cell carcinomas and normal oral mucosa. Genes Chromosomes Cancer. 2010;49:526–538. doi: 10.1002/gcc.20762. [DOI] [PubMed] [Google Scholar]
- 49.Cao P, Zhou L, Zhang J, Zheng F, Wang H, Ma D, Tian J. Comprehensive expression profiling of microRNAs in laryngeal squamous cell carcinoma. Head Neck. 2013;35:720–728. doi: 10.1002/hed.23011. [DOI] [PubMed] [Google Scholar]
- 50.Villadsen SB, Bramsen JB, Ostenfeld MS, Wiklund ED, Fristrup N, Gao S, Hansen TB, Jensen TI, Borre M, Orntoft TF, Dyrskjot L, Kjems J. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br J Cancer. 2012;106:366–374. doi: 10.1038/bjc.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotte M, Mohr C, Koo CY, Stock C, Vaske AK, Viola M, Ibrahim SA, Peddibhotla S, Teng YH, Low JY, Ebnet K, Kiesel L, Yip GW. miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene. 2010;29:6569–6580. doi: 10.1038/onc.2010.386. [DOI] [PubMed] [Google Scholar]
- 52.Sun ZJ, Yu GT, Huang CF, Bu LL, Liu JF, Ma SR, Zhang WF, Liu B, Zhang L. Hypoxia induce TFE3 expression in head and neck squamous cell carcinoma. Oncotarget. 2016;7:11651–63. doi: 10.18632/oncotarget.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gils A, Declerck PJ. The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors. Thromb Haemost. 2004;91:425–437. doi: 10.1160/TH03-12-0764. [DOI] [PubMed] [Google Scholar]
- 54.Declerck PJ, De Mol M, Vaughan DE, Collen D. Identification of a conformationally distinct form of plasminogen activator inhibitor-1, acting as a noninhibitory substrate for tissue-type plasminogen activator. J Biol Chem. 1992;267:11693–11696. [PubMed] [Google Scholar]
- 55.Harbeck N, Kates RE, Gauger K, Willems A, Kiechle M, Magdolen V, Schmitt M. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I: novel tumor-derived factors with a high prognostic and predictive impact in breast cancer. Thromb Haemost. 2004;91:450–456. doi: 10.1160/TH03-12-0798. [DOI] [PubMed] [Google Scholar]
- 56.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 57.Lee C, Huang T. Plasminogen Activator Inhibitor-1: The Expression, Biological Functions, and Effects on Tumorigenesis and Tumor Cell Adhesion and Migration. Journal of Cancer Molecules. 2005;1:25–36. [Google Scholar]
- 58.Wilkins-Port CE, Ye Q, Mazurkiewicz JE, Higgins PJ. TGF-beta1 + EGF-initiated invasive potential in transformed human keratinocytes is coupled to a plasmin/MMP-10/MMP-1-dependent collagen remodeling axis: role for PAI-1. Cancer Res. 2009;69:4081–4091. doi: 10.1158/0008-5472.CAN-09-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins SP, Samarakoon R, Higgins CE, Freytag J, Wilkins-Port CE, Higgins PJ. TGF-beta1-Induced Expression of the Anti-Apoptotic PAI-1 Protein Requires EGFR Signaling. Cell Commun Insights. 2009;2:1–11. doi: 10.4137/cci.s2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samarakoon R, Higgins CE, Higgins SP, Higgins PJ. TGF-beta1-Induced Expression of the Poor Prognosis SERPINE1/PAI-1 Gene Requires EGFR Signaling: A New Target for Anti-EGFR Therapy. J Oncol. 2009;2009:342391. doi: 10.1155/2009/342391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhanda J, Triantafyllou A, Liloglou T, Kalirai H, Lloyd B, Hanlon R, Shaw RJ, Sibson DR, Risk JM. SERPINE1 and SMA expression at the invasive front predict extracapsular spread and survival in oral squamous cell carcinoma. Br J Cancer. 2014;111:2114–2121. doi: 10.1038/bjc.2014.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavon MA, Arroyo-Solera I, Tellez-Gabriel M, Leon X, Viros D, Lopez M, Gallardo A, Cespedes MV, Casanova I, Lopez-Pousa A, Mangues MA, Quer M, Barnadas A, Mangues R. Enhanced cell migration and apoptosis resistance may underlie the association between high SERPINE1 expression and poor outcome in head and neck carcinoma patients. Oncotarget. 2015;6:29016–29033. doi: 10.18632/oncotarget.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balsara RD, Castellino FJ, Ploplis VA. A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. J Biol Chem. 2006;281:22527–22536. doi: 10.1074/jbc.M512819200. [DOI] [PubMed] [Google Scholar]
- 64.Webb DJ, Thomas KS, Gonias SL. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes MCF-7 cell growth. J Cell Biol. 2001;152:741–752. doi: 10.1083/jcb.152.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langlois B, Perrot G, Schneider C, Henriet P, Emonard H, Martiny L, Dedieu S. LRP-1 promotes cancer cell invasion by supporting ERK and inhibiting JNK signaling pathways. PLoS One. 2010;5:e11584. doi: 10.1371/journal.pone.0011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 67.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 68.Bajou K, Peng H, Laug WE, Maillard C, Noel A, Foidart JM, Martial JA, DeClerck YA. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell. 2008;14:324–334. doi: 10.1016/j.ccr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutierrez LS, Schulman A, Brito-Robinson T, Noria F, Ploplis VA, Castellino FJ. Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res. 2000;60:5839–5847. [PubMed] [Google Scholar]
- 70.Jing Y, Kovacs K, Kurisetty V, Jiang Z, Tsinoremas N, Merchan JR. Role of plasminogen activator inhibitor-1 in urokinase's paradoxical in vivo tumor suppressing or promoting effects. Mol Cancer Res. 2012;10:1271–1281. doi: 10.1158/1541-7786.MCR-12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonias SL, Hu J. Urokinase receptor and resistance to targeted anticancer agents. Front Pharmacol. 2015;6:154. doi: 10.3389/fphar.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutova M, Najbauer J, Gevorgyan A, Metz MZ, Weng Y, Shih CC, Aboody KS. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS One. 2007;2:e243. doi: 10.1371/journal.pone.0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meijer-van Gelder ME, Look MP, Peters HA, Schmitt M, Brunner N, Harbeck N, Klijn JG, Foekens JA. Urokinase-type plasminogen activator system in breast cancer: association with tamoxifen therapy in recurrent disease. Cancer Res. 2004;64:4563–4568. doi: 10.1158/0008-5472.CAN-03-3848. [DOI] [PubMed] [Google Scholar]
- 74.Eastman BM, Jo M, Webb DL, Takimoto S, Gonias SL. A transformation in the mechanism by which the urokinase receptor signals provides a selection advantage for estrogen receptor-expressing breast cancer cells in the absence of estrogen. Cell Signal. 2012;24:1847–1855. doi: 10.1016/j.cellsig.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schilling D, Bayer C, Geurts-Moespot A, Sweep FC, Pruschy M, Mengele K, Sprague LD, Molls M. Induction of plasminogen activator inhibitor type-1 (PAI-1) by hypoxia and irradiation in human head and neck carcinoma cell lines. BMC Cancer. 2007;7:143. doi: 10.1186/1471-2407-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Artman T, Schilling D, Gnann J, Molls M, Multhoff G, Bayer C. Irradiation-induced regulation of plasminogen activator inhibitor type-1 and vascular endothelial growth factor in six human squamous cell carcinoma lines of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76:574–582. doi: 10.1016/j.ijrobp.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 77.Bayer C, Kielow A, Schilling D, Maftei CA, Zips D, Yaromina A, Baumann M, Molls M, Multhoff G. Monitoring PAI-1 and VEGF levels in 6 human squamous cell carcinoma xenografts during fractionated irradiation. Int J Radiat Oncol Biol Phys. 2012;84:e409–417. doi: 10.1016/j.ijrobp.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 78.Jerhammar F, Ceder R, Garvin S, Grenman R, Grafstrom RC, Roberg K. Fibronectin 1 is a potential biomarker for radioresistance in head and neck squamous cell carcinoma. Cancer Biol Ther. 2010;10:1244–1251. doi: 10.4161/cbt.10.12.13432. [DOI] [PubMed] [Google Scholar]
- 79.Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, Yamagishi H. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer. 2004;91:1543–1550. doi: 10.1038/sj.bjc.6602187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee YC, Yu CC, Lan C, Lee CH, Lee HT, Kuo YL, Wang PH, Chang WW. Plasminogen activator inhibitor-1 as regulator of tumor-initiating cell properties in head and neck cancers. Head Neck. 2015 doi: 10.1002/hed.24124. [DOI] [PubMed] [Google Scholar]
- 81.Wykosky J, Hu J, Gomez GG, Taylor T, Villa GR, Pizzo D, VandenBerg SR, Thorne AH, Chen CC, Mischel PS, Gonias SL, Cavenee WK, Furnari FB. A urokinase receptor-Bim signaling axis emerges during EGFR inhibitor resistance in mutant EGFR glioblastoma. Cancer Res. 2015;75:394–404. doi: 10.1158/0008-5472.CAN-14-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abu-Ali S, Fotovati A, Shirasuna K. Tyrosine-kinase inhibition results in EGFR clustering at focal adhesions and consequent exocytosis in uPAR down-regulated cells of head and neck cancers. Mol Cancer. 2008;7:47. doi: 10.1186/1476-4598-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waldron NN, Oh S, Vallera DA. Bispecific targeting of EGFR and uPAR in a mouse model of head and neck squamous cell carcinoma. Oral Oncol. 2012;48:1202–1207. doi: 10.1016/j.oraloncology.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Z, Li H, Huang Q, Chen D, Han J, Wang L, Pan C, Chen W, House MG, Nephew KP, Guo Z. SERPINB2 down-regulation contributes to chemoresistance in head and neck cancer. Mol Carcinog. 2014;53:777–786. doi: 10.1002/mc.22033. [DOI] [PubMed] [Google Scholar]
- 85.Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, Hayashida S, Shimeno H. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- 86.Schneider DJ, Chen Y, Sobel BE. The effect of plasminogen activator inhibitor type 1 on apoptosis. Thromb Haemost. 2008;100:1037–1040. [PubMed] [Google Scholar]
- 87.Fang H, Placencio VR, DeClerck YA. Protumorigenic activity of plasminogen activator inhibitor-1 through an antiapoptotic function. J Natl Cancer Inst. 2012;104:1470–1484. doi: 10.1093/jnci/djs377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romer MU, Larsen L, Offenberg H, Brunner N, Lademann UA. Plasminogen activator inhibitor 1 protects fibrosarcoma cells from etoposide-induced apoptosis through activation of the PI3K/Akt cell survival pathway. Neoplasia. 2008;10:1083–1091. doi: 10.1593/neo.08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lademann UA, Romer MU. Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1) Thromb Haemost. 2008;100:1041–1046. [PubMed] [Google Scholar]
- 90.Czekay RP, Wilkins-Port CE, Higgins SP, Freytag J, Overstreet JM, Klein RM, Higgins CE, Samarakoon R, Higgins PJ. PAI-1: An Integrator of Cell Signaling and Migration. Int J Cell Biol. 2011;2011:562481. doi: 10.1155/2011/562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simone TM, Higgins CE, Czekay RP, Law BK, Higgins SP, Archambeault J, Kutz SM, Higgins PJ. SERPINE1: A Molecular Switch in the Proliferation-Migration Dichotomy in Wound-”Activated” Keratinocytes. Adv Wound Care (New Rochelle) 2014;3:281–290. doi: 10.1089/wound.2013.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fedotov S, Iomin A. Migration and proliferation dichotomy in tumor-cell invasion. Phys Rev Lett. 2007;98:118101. doi: 10.1103/PhysRevLett.98.118101. [DOI] [PubMed] [Google Scholar]
- 93.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gemenetzidis E, Gammon L, Biddle A, Emich H, Mackenzie IC. Invasive oral cancer stem cells display resistance to ionising radiation. Oncotarget. 2015;6:43964–43977. doi: 10.18632/oncotarget.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aneta G, Tomas V, Daniel R, Jan B. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget. 2016;7:25022–49. doi: 10.18632/oncotarget.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta -induced physical and functional interactions between smads and Sp1. J Biol Chem. 2000;275:40014–40019. doi: 10.1074/jbc.C000508200. [DOI] [PubMed] [Google Scholar]
- 99.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu XM, Jaskula-Sztul R, Georgen MR, Aburjania Z, Somnay YR, Leverson G, Sippel RS, Lloyd RV, Johnson BP, Chen H. Notch1 Signaling Regulates the Aggressiveness of Differentiated Thyroid Cancer and Inhibits SERPINE1 Expression. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sakamoto K, Fujii T, Kawachi H, Miki Y, Omura K, Morita K, Kayamori K, Katsube K, Yamaguchi A. Reduction of NOTCH1 expression pertains to maturation abnormalities of keratinocytes in squamous neoplasms. Lab Invest. 2012;92:688–702. doi: 10.1038/labinvest.2012.9. [DOI] [PubMed] [Google Scholar]
- 102.Brakenhoff RH. Cancer. Another NOTCH for cancer. Science. 2011;333:1102–1103. doi: 10.1126/science.1210986. [DOI] [PubMed] [Google Scholar]
- 103.Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, Millar SE, Pear WS, Parmacek MS. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006;66:7438–7444. doi: 10.1158/0008-5472.CAN-06-0793. [DOI] [PubMed] [Google Scholar]
- 104.Okuyama R, Nguyen BC, Talora C, Ogawa E, Tommasi di Vignano A, Lioumi M, Chiorino G, Tagami H, Woo M, Dotto GP. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev Cell. 2004;6:551–562. doi: 10.1016/s1534-5807(04)00098-x. [DOI] [PubMed] [Google Scholar]
- 105.Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL. Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem. 2009;284:22825–22833. doi: 10.1074/jbc.M109.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Cecco L, Nicolau M, Giannoccaro M, Daidone MG, Bossi P, Locati L, Licitra L, Canevari S. Head and neck cancer subtypes with biological and clinical relevance: Meta-analysis of gene-expression data. Oncotarget. 2015;6:9627–9642. doi: 10.18632/oncotarget.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pavon MA, Parreno M, Tellez-Gabriel M, Sancho FJ, Lopez M, Cespedes MV, Casanova I, Lopez-Pousa A, Mangues MA, Quer M, Barnadas A, Leon X, Mangues R. Gene expression signatures and molecular markers associated with clinical outcome in locally advanced head and neck carcinoma. Carcinogenesis. 2012;33:1707–1716. doi: 10.1093/carcin/bgs207. [DOI] [PubMed] [Google Scholar]
- 110.Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X, Shockley WW, Weissler MC, Dressler LG, Shores CG, Yarbrough WG, Perou CM. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 111.Roepman P, Wessels LF, Kettelarij N, Kemmeren P, Miles AJ, Lijnzaad P, Tilanus MG, Koole R, Hordijk GJ, van der Vliet PC, Reinders MJ, Slootweg PJ, Holstege FC. An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nat Genet. 2005;37:182–186. doi: 10.1038/ng1502. [DOI] [PubMed] [Google Scholar]
- 112.Jo M, Eastman BM, Webb DL, Stoletov K, Klemke R, Gonias SL. Cell signaling by urokinase-type plasminogen activator receptor induces stem cell-like properties in breast cancer cells. Cancer Res. 2010;70:8948–8958. doi: 10.1158/0008-5472.CAN-10-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Asuthkar S, Stepanova V, Lebedeva T, Holterman AL, Estes N, Cines DB, Rao JS, Gondi CS. Multifunctional roles of urokinase plasminogen activator (uPA) in cancer stemness and chemoresistance of pancreatic cancer. Mol Biol Cell. 2013;24:2620–2632. doi: 10.1091/mbc.E12-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clayman G, Wang SW, Nicolson GL, el-Naggar A, Mazar A, Henkin J, Blasi F, Goepfert H, Boyd DD. Regulation of urokinase-type plasminogen activator expression in squamous-cell carcinoma of the oral cavity. Int J Cancer. 1993;54:73–80. doi: 10.1002/ijc.2910540113. [DOI] [PubMed] [Google Scholar]
- 115.Curino A, Patel V, Nielsen BS, Iskander AJ, Ensley JF, Yoo GH, Holsinger FC, Myers JN, El-Nagaar A, Kellman RM, Shillitoe EJ, Molinolo AA, Gutkind JS, Bugge TH. Detection of plasminogen activators in oral cancer by laser capture microdissection combined with zymography. Oral Oncol. 2004;40:1026–1032. doi: 10.1016/j.oraloncology.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Lindberg P, Larsson A, Nielsen BS. Expression of plasminogen activator inhibitor-1, urokinase receptor and laminin gamma-2 chain is an early coordinated event in incipient oral squamous cell carcinoma. Int J Cancer. 2006;118:2948–2956. doi: 10.1002/ijc.21568. [DOI] [PubMed] [Google Scholar]
- 117.Pasini FS, Brentani MM, Kowalski LP, Federico MH. Transforming growth factor beta1, urokinase-type plasminogen activator and plasminogen activator inhibitor-1 mRNA expression in head and neck squamous carcinoma and normal adjacent mucosa. Head Neck. 2001;23:725–732. doi: 10.1002/hed.1103. [DOI] [PubMed] [Google Scholar]
- 118.Speleman L, Kerrebijn JD, Look MP, Meeuwis CA, Foekens JA, Berns EM. Prognostic value of plasminogen activator inhibitor-1 in head and neck squamous cell carcinoma. Head Neck. 2007;29:341–350. doi: 10.1002/hed.20527. [DOI] [PubMed] [Google Scholar]
- 119.He Y, Shao F, Pi W, Shi C, Chen Y, Gong D, Wang B, Cao Z, Tang K. Largescale Transcriptomics Analysis Suggests Over-Expression of BGH3, MMP9 and PDIA3 in Oral Squamous Cell Carcinoma. PLoS One. 2016;11:e0146530. doi: 10.1371/journal.pone.0146530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bagordakis E, Sawazaki-Calone I, Macedo CC, Carnielli CM, de Oliveira CE, Rodrigues PC, Rangel AL, Dos Santos JN, Risteli J, Graner E, Salo T, Leme AF, Coletta RD. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumour Biol. 2016 doi: 10.1007/s13277-015-4629-y. [DOI] [PubMed] [Google Scholar]
- 121.Mendez E, Houck JR, Doody DR, Fan W, Lohavanichbutr P, Rue TC, Yueh B, Futran ND, Upton MP, Farwell DG, Heagerty PJ, Zhao LP, Schwartz SM, Chen C. A genetic expression profile associated with oral cancer identifies a group of patients at high risk of poor survival. Clin Cancer Res. 2009;15:1353–1361. doi: 10.1158/1078-0432.CCR-08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu CJ, Liu TY, Kuo LT, Cheng HW, Chu TH, Chang KW, Lin SC. Differential gene expression signature between primary and metastatic head and neck squamous cell carcinoma. J Pathol. 2008;214:489–497. doi: 10.1002/path.2306. [DOI] [PubMed] [Google Scholar]
- 123.Schmalbach CE, Chepeha DB, Giordano TJ, Rubin MA, Teknos TN, Bradford CR, Wolf GT, Kuick R, Misek DE, Trask DK, Hanash S. Molecular profiling and the identification of genes associated with metastatic oral cavity/pharynx squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:295–302. doi: 10.1001/archotol.130.3.295. [DOI] [PubMed] [Google Scholar]
- 124.Strojan P, Budihna M, Smid L, Vrhovec I, Skrk J. Urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (PAI-1) in tissue and serum of head and neck squamous cell carcinoma patients. Eur J Cancer. 1998;34:1193–1197. doi: 10.1016/s0959-8049(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 125.Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget. 2014;5:3956–3969. doi: 10.18632/oncotarget.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fakhry C, Psyrri A, Chaturvedhi A. HPV and head and neck cancers: state-of-the-science. Oral Oncol. 2014;50:353–355. doi: 10.1016/j.oraloncology.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 127.Yasuda T, Sakata Y, Kitamura K, Morita M, Ishida T. Localization of plasminogen activators and their inhibitor in squamous cell carcinomas of the head and neck. Head Neck. 1997;19:611–616. doi: 10.1002/(sici)1097-0347(199710)19:7<611::aid-hed8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 128.Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB. Novel markers for poor prognosis in head and neck cancer. Int J Cancer. 2005;113:789–797. doi: 10.1002/ijc.20608. [DOI] [PubMed] [Google Scholar]
- 129.Huang CF, Yu GT, Wang WM, Liu B, Sun ZJ. Prognostic and predictive values of SPP1, PAI and caveolin-1 in patients with oral squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:6032–6039. [PMC free article] [PubMed] [Google Scholar]
- 130.Magnussen S, Rikardsen OG, Hadler-Olsen E, Uhlin-Hansen L, Steigen SE, Svineng G. Urokinase plasminogen activator receptor (uPAR) and plasminogen activator inhibitor-1 (PAI-1) are potential predictive biomarkers in early stage oral squamous cell carcinomas (OSCC) PLoS One. 2014;9:e101895. doi: 10.1371/journal.pone.0101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hundsdorfer B, Zeilhofer HF, Bock KP, Dettmar P, Schmitt M, Kolk A, Pautke C, Horch HH. Tumour-associated urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1 in normal and neoplastic tissues of patients with squamous cell cancer of the oral cavity - clinical relevance and prognostic value. J Craniomaxillofac Surg. 2005;33:191–196. doi: 10.1016/j.jcms.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 132.Inoue Y, Sugiura T, Matsuki R, Ishii K, Seki K, shirasuna K. Plaminogen Activator Inhibitor-1 in Oral Squamous Cell Carcinoma. Oral Science International. 2007:38–44. [Google Scholar]
- 133.Schmidt M, Schler G, Gruensfelder P, Muller J, Hoppe F. Urokinase receptor up-regulation in head and neck squamous cell carcinoma. Head Neck. 2000;22:498–504. doi: 10.1002/1097-0347(200008)22:5<498::aid-hed9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 134.Baker EA, Leaper DJ, Hayter JP, Dickenson AJ. Plasminogen activator system in oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2007;45:623–627. doi: 10.1016/j.bjoms.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 135.Pacheco MM, Kowalski LP, Nishimoto IN, Brentani MM. Differential expression of c-jun and c-fos mRNAs in squamous cell carcinoma of the head and neck: associations with uPA, gelatinase B, and matrilysin mRNAs. Head Neck. 2002;24:24–32. doi: 10.1002/hed.10009. [DOI] [PubMed] [Google Scholar]
- 136.Leto G, Tumminello FM, Gebbia N, Bazan V, Tomasino RM, Dardanoni G, Russo A. Differential expression levels of urokinase-type plasminogen activator and cathepsin D in locally advanced laryngeal squamous cell carcinoma: clinical implications. Int J Biol Markers. 2001;16:245–249. doi: 10.1177/172460080101600404. [DOI] [PubMed] [Google Scholar]
- 137.Nisa L, Aebersold DM, Giger R, Caversaccio MD, Borner U, Medova M, Zimmer Y. Profiling invasiveness in head and neck cancer: recent contributions of genomic and transcriptomic approaches. Cancers (Basel) 2015;7:585–597. doi: 10.3390/cancers7020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du B, Leung H, Khan KM, Miller CG, Subbaramaiah K, Falcone DJ, Dannenberg AJ. Tobacco smoke induces urokinase-type plasminogen activator and cell invasiveness: evidence for an epidermal growth factor receptor dependent mechanism. Cancer Res. 2007;67:8966–8972. doi: 10.1158/0008-5472.CAN-07-1388. [DOI] [PubMed] [Google Scholar]
- 139.Nagata M, Fujita H, Ida H, Hoshina H, Inoue T, Seki Y, Ohnishi M, Ohyama T, Shingaki S, Kaji M, Saku T, Takagi R. Identification of potential biomarkers of lymph node metastasis in oral squamous cell carcinoma by cDNA microarray analysis. Int J Cancer. 2003;106:683–689. doi: 10.1002/ijc.11283. [DOI] [PubMed] [Google Scholar]
- 140.Yoshizawa K, Nozaki S, Kitahara H, Kato K, Noguchi N, Kawashiri S, Yamamoto E. Expression of urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor and maspin in oral squamous cell carcinoma: Association with mode of invasion and clinicopathological factors. Oncol Rep. 2011;26:1555–1560. doi: 10.3892/or.2011.1419. [DOI] [PubMed] [Google Scholar]
- 141.Strojan P, Budihna M, Smid L, Vrhovec I, Skrk J. Urokinase-type plasminogen activator, plasminogen activator inhibitor type 1 and cathepsin D: analysis of their prognostic significance in squamous cell carcinoma of the head and neck. Anticancer Res. 2000;20:3975–3981. [PubMed] [Google Scholar]
- 142.Hasina R, Hulett K, Bicciato S, Di Bello C, Petruzzelli GJ, Lingen MW. Plasminogen activator inhibitor-2: a molecular biomarker for head and neck cancer progression. Cancer Res. 2003;63:555–559. [PubMed] [Google Scholar]
- 143.Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014;16:428. doi: 10.1186/s13058-014-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harbeck N, Schmitt M, Meisner C, Friedel C, Untch M, Schmidt M, Sweep CG, Lisboa BW, Lux MP, Beck T, Hasmuller S, Kiechle M, Janicke F, Thomssen C. Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur J Cancer. 2013;49:1825–1835. doi: 10.1016/j.ejca.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 145.Kantelhardt EJ, Vetter M, Schmidt M, Veyret C, Augustin D, Hanf V, Meisner C, Paepke D, Schmitt M, Sweep F, von Minckwitz G, Martin PM, Jaenicke F, Thomssen C, Harbeck N. Prospective evaluation of prognostic factors uPA/PAI-1 in node-negative breast cancer: phase III NNBC3-Europe trial (AGO, GBG, EORTC-PBG) comparing 6xFEC versus 3xFEC/3xDocetaxel. BMC Cancer. 2011;11:140. doi: 10.1186/1471-2407-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kolben T, Augustin D, Armbrust R, Kolben TM, Degenhardt T, Burgmann M, Goess C, Ditsch N, Kates R, Harbeck N, Wuerstlein R. Impact of guideline-based use of uPA/PAI-1 on patient outcome in intermediate-risk early breast cancer. Breast Cancer Res Treat. 2016;155:109–115. doi: 10.1007/s10549-015-3653-3. [DOI] [PubMed] [Google Scholar]
- 147.Placencio VR, DeClerck YA. Plasminogen Activator Inhibitor-1 in Cancer: Rationale and Insight for Future Therapeutic Testing. Cancer Res. 2015;75:2969–2974. doi: 10.1158/0008-5472.CAN-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fortenberry YM. Plasminogen activator inhibitor-1 inhibitors: a patent review (2006-present) Expert Opin Ther Pat. 2013;23:801–815. doi: 10.1517/13543776.2013.782393. [DOI] [PubMed] [Google Scholar]
- 149.Rockway TW, Nienaber V, Giranda VL. Inhibitors of the protease domain of urokinase-type plasminogen activator. Curr Pharm Des. 2002;8:2541–2558. doi: 10.2174/1381612023392676. [DOI] [PubMed] [Google Scholar]
- 150.Rupin A, Gaertner R, Mennecier P, Richard I, Benoist A, De Nanteuil G, Verbeuren TJ. S35225 is a direct inhibitor of Plasminogen Activator Inhibitor type-1 activity in the blood. Thromb Res. 2008;122:265–270. doi: 10.1016/j.thromres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 151.Fjellstrom O, Deinum J, Sjogren T, Johansson C, Geschwindner S, Nerme V, Legnehed A, McPheat J, Olsson K, Bodin C, Paunovic A, Gustafsson D. Characterization of a small molecule inhibitor of plasminogen activator inhibitor type 1 that accelerates the transition into the latent conformation. J Biol Chem. 2013;288:873–885. doi: 10.1074/jbc.M112.371732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simone TM, Higgins SP, Higgins CE, Lennartz MR, Higgins PJ. Chemical Antagonists of Plasminogen Activator Inhibitor-1: Mechanisms of Action and Therapeutic Potential in Vascular Disease. J Mol Genet Med. 2014:8. doi: 10.4172/1747-0862.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin Z, Jensen JK, Hong Z, Shi X, Hu L, Andreasen PA, Huang M. Structural insight into inactivation of plasminogen activator inhibitor-1 by a small-molecule antagonist. Chem Biol. 2013;20:253–261. doi: 10.1016/j.chembiol.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Placencio VR, Ichimura A, Miyata T, DeClerck YA. Small Molecule Inhibitors of Plasminogen Activator Inhibitor-1 Elicit Anti-Tumorigenic and Anti-Angiogenic Activity. PLoS One. 2015;10:e0133786. doi: 10.1371/journal.pone.0133786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Masuda T, Hattori N, Senoo T, Akita S, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Kohno N. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol Cancer Ther. 2013;12:2378–2388. doi: 10.1158/1535-7163.MCT-13-0041. [DOI] [PubMed] [Google Scholar]
- 156.Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol Cancer Ther. 2013;12:2697–2708. doi: 10.1158/1535-7163.MCT-13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mashiko S, Kitatani K, Toyoshima M, Ichimura A, Dan T, Usui T, Ishibashi M, Shigeta S, Nagase S, Miyata T, Yaegashi N. Inhibition of plasminogen activator inhibitor-1 is a potential therapeutic strategy in ovarian cancer. Cancer Biol Ther. 2015;16:253–260. doi: 10.1080/15384047.2014.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leik CE, Su EJ, Nambi P, Crandall DL, Lawrence DA. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J Thromb Haemost. 2006;4:2710–2715. doi: 10.1111/j.1538-7836.2006.02244.x. [DOI] [PubMed] [Google Scholar]
- 159.Frederickson M, Callaghan O, Chessari G, Congreve M, Cowan SR, Matthews JE, McMenamin R, Smith DM, Vinkovic M, Wallis NG. Fragment-based discovery of mexiletine derivatives as orally bioavailable inhibitors of urokinase-type plasminogen activator. J Med Chem. 2008;51:183–186. doi: 10.1021/jm701359z. [DOI] [PubMed] [Google Scholar]
- 160.Meyer JE, Brocks C, Graefe H, Mala C, Thans N, Burgle M, Rempel A, Rotter N, Wollenberg B, Lang S. The Oral Serine Protease Inhibitor WX-671 - First Experience in Patients with Advanced Head and Neck Carcinoma. Breast Care (Basel) 2008;3:20–24. doi: 10.1159/000151736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goldstein LJ, Stemmer SM, Schmalfeldt B, Gottschalk N, Cardoso F, Dushkin H, Mala C, Uebler N, Bevan P, Harbeck N. Phase II, two-arm, double-blind, multicenter, randomized study of the combination of oral WX-671 plus capecitabine versus capecitabine in first-line HER2-negative metastatic breast cancer (MBC) Journal of Clinical Oncology. 2010:28. [Google Scholar]
- 162.Heinemann V, Ebert MP, Pinter T, Bevan P, Neville G, Mala C. Randomized phase II trial with an uPA inhibitor (WX-671) in patients with locally advanced nonmetastatic pancreatic cancer. Journal of Clinical Oncology. 2010;28:2010. [Google Scholar]


