Abstract
To date, the effects of deep brain stimulation (DBS) on hippocampal neurogenesis have been mainly characterized in the context of memory. Acute stimulation (i.e. for 1 h) of either the entorhinal cortex or the anterior thalamus increases both cell proliferation and survival. We investigate whether stimulation applied to targets being considered for the treatment of depression, namely the ventromedial prefrontal cortex (vmPFC) or nucleus accumbens (Acb), also increases hippocampal neurogenesis in rodents. Rats were treated with vmPFC or Acb DBS for 1 h at different settings. 5′-bromo-2′deoxyuridine (BrdU) was injected three days following stimulation onset and animals were sacrificed 24 h or 28 days later. Overall, we found that neither vmPFC nor Acb DBS increased hippocampal neurogenesis. In summary, the delivery of acute stimulation into targets homologous to those used in human depression trials does not increase hippocampal neurogenesis.
Keywords: Prefrontal cortex, Nucleus accumbens, Depression, Deep brain stimulation, Hippocampus, Neurogenesis
1. Introduction
Deep brain stimulation (DBS) is currently being investigated for the treatment of depression. Among the most commonly studied targets are the subgenual cingulate region (SCG) (Mayberg et al., 2005) and the nucleus accumbens (Acb) (Bewernick et al., 2010). Similar to humans, DBS applied to homologous regions in rodents (i.e. the ventromedial prefrontal cortex, vmPFC, and Acb) has been shown to induce antidepressant-like responses in different behavioural tests (Gersner et al., 2010; Hamani et al., 2014, 2010b; Hamani and Temel, 2012; Rea et al., 2014).
At present, there is evidence implicating dysfunction in central monoaminergic transmission, neurotrophic regulation and neurogenic processes in the depressive brain. In rodents, DBS increases levels of hippocampal serotonin (Adachi et al., 2005; Hamani et al., 2010b) and brain derived neurotrophic factor (Gersner et al., 2010; Hamani et al., 2012). In a recent series of studies, stimulation of limbic structures (Encinas et al., 2011; Hamani et al., 2011; Stone et al., 2011; Toda et al., 2008) or the Acb (Schmuckermair et al., 2013) has been shown to increase hippocampal neurogenesis. As the vmPFC has direct and indirect connections with the hippocampus (Vertes, 2004, 2006), it is conceivable to hypothesize that treatments that modulate its activity (e.g. vmPFC DBS) may favourably increase neurogenesis.
In the present study, we investigate the effects of acute vmPFC or Acb DBS on hippocampal neurogenesis.
2. Methods and materials
Procedures were approved by the Animal Care Committees of the Centre for Addiction and Mental Health and the Senate of Berlin, Germany. Male Sprague Dawley rats (200 g) had stainless steel electrodes (cathodes; 250 μm in diameter) bilaterally implanted into the vmPFC or Acb, as previously described (Hamani et al., 2014; Rea et al., 2014). Similar electrodes connected to a bone screw implanted over the somatosensory cortex were used as anodes. Controls had electrodes implanted in either the vmPFC or Acb but did not receive stimulation. DBS was conducted for 1 h at 100 μA or 300 μA, 130 Hz, and 90 μs (Hamani et al., 2014).
In Study 1, 5-bromo-2′deoxyuridine (BrdU; Sigma) was injected on post-stimulation day 3 (50 mg/kg every 6 h for 24 h) and animals were sacrificed the next day, as previously described (Encinas et al., 2011; Hamani et al., 2011; Stone et al., 2011; Toda et al., 2008). In Study 2, BrdU was given from post-stimulation days 3–5 (50 mg/kg every 6 h) and animals were sacrificed 28 days later.
On the 4th or the 28th day following DBS, animals were anesthetized and transcardially perfused with 1xPBS, followed by 4% paraformaldehyde. Free-floating 40 μm sections were processed (Toda et al., 2008) and incubated with the following primary antibodies: rat anti-BrdU (1:200; Axyl), mouse anti-doublecortin (1:1000, Millipore), guinea pig anti-GFAP (1:500; Harlan). Secondary antibodies were goat anti-rat Alexa Fluor 488 (1:200; Life Tech, city, state) and goat anti-mouse Rhodamine Red X. (1:200; Jackson Lab). Cell counting was performed in the dentate gyrus (DG) granule cell layer and the region comprising the 50 μm border along the hilar margin. Stained BrdU+ nuclei were scored in every 6th section throughout the rostrocaudal extent of the granule cell layer. For confocal microscopy an Olympus Fluoview FV1200 microscope was used. The number of BrdU+ cells co-stained for doublecortin (DCX) was determined in every 12th section throughout the rostrocaudal extent of the DG. GFAP was not counted since virtually no BrdU+ cells co-stained for this marker.
Location of electrode tracks was confirmed with cresyl violet staining and was similar our previous descriptions (Hamani et al., 2014; Rea et al., 2014). Comparison between each DBS group and its correspondent controls was ascertained using a Student t-test.
3. Results
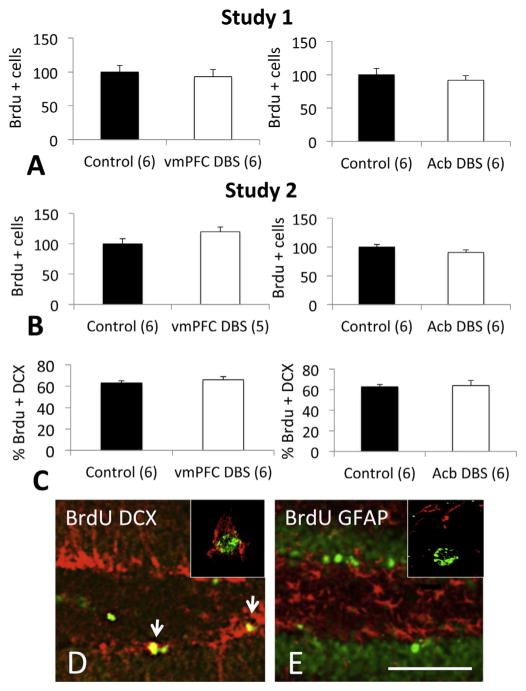
In Study 1, no differences were found between the number of BrdU+ cells recorded in controls and animals receiving vmPFC (p = 0.6) or Acb stimulation (p = 0.5) (Fig. 1A). To characterize the phenotype of Brdu+ cells, we have studied whether they co-expressed DCX, a marker of immature neurons, or GFAP, a marker as astrocytes. Approximately 60% of newly borne cells in either controls or DBS treated animals were co-labelled with DCX, (Fig. 1C–D), whereas virtually no cells co-expressed GFAP (Fig. 1E).
Fig. 1.
DBS applied to the ventromedial prefrontal cortex (vmPFC) or nucleus accumbens (Acb) did not increase hippocampal proliferation and neurogenesis. A) In Study 1, animals received 1 h of vmPFC or Acb DBS (100 μA, 90 μs, 130 Hz). BrdU was injected on post-stimulation day 3 and animals were sacrificed 1 day later. B) In Study 2, animals received vmPFC or Acb DBS (300 μA, 90 μs, 130 Hz). BrdU was injected from post-stimulation days 3–5 and animals were sacrificed on post-stimulation day 28 day. In neither circumstance the administration of DBS was associated with changes in hippocampal proliferation/neurogenesis. C) Phenotype of BrdU+ cells. Sections from animals in Study 1 were co-stained for doublecortin (DCX), a marker o immature neurons, or GFAP, a marker of astrocytes. In all studied groups, approximately 60% of BrdU+ cells co-expressed DCX. D) Photomicrograph of the hippocampal dentate gyrus of a vmPFC DBS-treated rat showing cells stained for BrdU (green) and DCX (red). Note that various cells co-stained for both (yellow; arrows). In the right upper corner, example of a single cell co-expressing BrdU (green; nucleus) and doublecortin (red; cytoplasm). E) Photomicrograph of the hippocampal dentate gyrus of an Acb DBS-treated rat showing cells stained for BrdU (green) and GFAP (red). Virtually no cells co-stained for both markers. In the right upper corner, example of a single BrdU stained cell (green; nucleus) that does not co-label with GFAP (red). Horizontal bar in E = 100 μm. Bar graphs represent mean and SEM. Numbers in parenthesis represent animals per group.
In Study 2, we have delivered DBS at a higher current (300 μA), injected BrdU for a longer period of time (3 days), and sacrificed the animals 28 days after stimulation (i.e. to assess cell survival). Once again, no differences were found between controls and animals given vmPFC (p = 0.1) or Acb DBS (p = 0.2; Fig. 1B). As most cells in our initial study assumed a neuronal phenotype, double labelling experiments were not conducted in Study 2.
4. Discussion
In contrast to previous studies showing that DBS applied to limbic targets (e.g. entorhinal cortex and anterior thalamic nucleus) increased hippocampal neurogenesis, we found no differences in the number of BrdU+ cells recorded in non-stimulated controls and animals receiving vmPFC or Acb DBS.
In the Acb DBS group, the number of BrdU+ cells was approximately 90% of that in controls. While a similar percentage was recorded after vmPFC DBS at 100 μA, this was increased to 120% when current was raised to 300 μA. It is possible that the administration of even higher currents could have yielded a significant increase in hippocampal neurogenesis. Yet, we decided not to pursue these experiments for various reasons. First, stimulation settings in our study were similar to those associated with antidepressant-like responses, serotonin release and increased BDNF expression in previous reports (Hamani et al., 2010b; Hamani and Temel, 2012). In our experience, currents above 300 μA are detrimental and may worsen behavioural responses in the forced swim test (Hamani et al., 2010a). Finally, amplitudes above the ones used in this study have been reported to induce side effects when delivered to the Acb (e.g. stereotypic movements) (Hamani et al., 2014). In a recent study, Schmuckermair and colleagues reported an increase in neurogenesis after Acb DBS in mice with high trait anxiety (Schmuckermair et al., 2013). Interestingly, this was only noticed when BrdU was administered before DBS and not while the animals were receiving stimulation. Such results are intriguing and the mechanisms for this effect remain to be elucidated. Those findings, however, are somewhat in line with ours inasmuch we did not record an increase in neurogenesis when DBS was delivered prior to BrdU injections. Also relevant to our study, hippocampal neurogenesis following the administration of selective serotonin reuptake inhibitors is only detected after chronic treatment (i.e. several weeks) (David et al., 2009; Santarelli et al., 2003). It is possible that vmPFC or Acb DBS may induce neurogenesis when delivered chronically (e.g. for weeks to months).
Acknowledgments
Role of funding
Experimental work was supported by funds from the Era Net Neuron consortium (DBS_F20rat), the Canadian Institutes for Health Research (CIHR) and the Federal Ministry of Education and Research, Germany (BMBF 01EW1103). MV is financed by the German Research Foundation (DFG KFO 247) (WI 2140/1-1+2).
Footnotes
- Christine Winter – Helped designing experiments and writing the manuscript.
- Tatiana Bregman – Conducted part of the experiments.
- Mareike Voget – Conducted part of the experiments.
- Roger Raymond – Conducted part of the experiments.
- Ravit Hadar – Conducted part of the experiments.
- José N. Nobrega – Helped writing the manuscript.
- Clement Hamani – Helped designing and conducted part of the experiments. Helped writing the manuscript.
Conflict of interest
C.H. is a consultant for St Jude Medical. The other authors do not have a conflict of interest.
Contributor Information
Christine Winter, Department of Psychiatry and Psychotherapy, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany.
Tatiana Bregman, Behavioural Neurobiology Laboratory, Centre for Addiction and Mental Health, 250 College Street, Toronto, ON, M5T 1R8, Canada.
Mareike Voget, Department of Psychiatry and Psychotherapy, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany.
Roger Raymond, Behavioural Neurobiology Laboratory, Centre for Addiction and Mental Health, 250 College Street, Toronto, ON, M5T 1R8, Canada.
Ravit Hadar, Department of Psychiatry and Psychotherapy, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany.
José N. Nobrega, Behavioural Neurobiology Laboratory, Centre for Addiction and Mental Health, 250 College Street, Toronto, ON, M5T 1R8, Canada
Clement Hamani, Behavioural Neurobiology Laboratory, Centre for Addiction and Mental Health, 250 College Street, Toronto, ON, M5T 1R8, Canada; Campbell Family Mental Health Research Institute, CAMH, Canada; Division of Neurosurgery, Toronto Western Hospital, 399 Bathurst Street, Toronto, ON, M5T 2S8, Canada.
References
- Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T. Isoflurane anesthesia induces biphasic effect on dopamine release in the rat striatum. Brain Res Bull. 2005;67:176–81. doi: 10.1016/j.brainresbull.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Hamani C, Lozano AM, Enikolopov G. Neurogenic hippocampal targets of deep brain stimulation. J Comp Neurol. 2011;519:6–20. doi: 10.1002/cne.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol Psychiatry. 2010;67:125–32. doi: 10.1016/j.biopsych.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Hamani C, Amorim BO, Wheeler AL, Diwan M, Driesslein K, Covolan L, et al. Deep brain stimulation in rats: different targets induce similar antidepressant-like effects but influence different circuits. Neurobiol Dis. 2014;71:205–14. doi: 10.1016/j.nbd.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010a;44:683–7. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010b;67:117–24. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, et al. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–5. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011;232:100–4. doi: 10.1016/j.expneurol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv8. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Rea E, Rummel J, Schmidt TT, Hadar R, Heinz A, Mathe AA, et al. Anti-anhedonic effect of deep brain stimulation of the prefrontal cortex and the dopaminergic reward system in a genetic rat model of depression: an intracranial self-stimulation paradigm study. Brain Stimul. 2014;7:21–8. doi: 10.1016/j.brs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmuckermair C, Gaburro S, Sah A, Landgraf R, Sartori SB, Singewald N. Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology. 2013;38:1234–44. doi: 10.1038/npp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108:132–8. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]