Abstract
Thymic nurse cells (TNCs) are specialized epithelial cells that reside in the thymic cortex. The initial report of their discovery in 1980 showed TNCs to contain up to 200 thymocytes within specialized vacuoles in their cytoplasm. Much has been reported since that time to determine the function of this heterotypic internalization event that exists between TNCs and developing thymocytes. In this review, we discuss the literature reported that describes the internalization event and the role TNCs play during T cell development in the thymus as well as why these multicellular complexes may be important in inhibiting the development of autoimmune diseases.
Keywords: Thymic nurse cells, internalization, MHC restriction, lupus erythromatosus
Introduction
The idea of a viable cell containing another living cell, cell-in-cell, is not entirely a new concept [1-5]. This type of cellular association was describe by Eberth in 1864, showing lymphocytes residing within intestinal epithelium [1]. Since that time, a host of reports have been generated documenting these types of association between various cell types in a variety of species [6-9]. The internalization process can be classified as either homotypic or heterotypic [10-12]. Homotypic internalization primarily involves neoplastic cells although this type of internalization may also include non-tumorigenic cell as well. In heterotypic cell-in-cell internalization, the host cell possesses the ability to internalize a variety of viable cell types. The heterogeneous population of internalized cells was shown to move vigorously within host cytoplasm with some of the cells exiting [13-15]. This intra-cytoplasmic cellular dynamism and exit is termed emperipolesis [16, 17]. These internalization events may differ from the phagocytosis performed by macrophages to rid the organism’s body of dead or dying cells.
A heterotypic interaction best defines the mode of internalization of thymocytes by TNCs (Figs. 1, 2 panels 2 and 3). TNCs have been enigmatic since their discovery in 1980 [18]. On one hand, they are stromal epithelial cells that express cytokeratins. On the other hand, TNCs have phagocytic capabilities but are not classical cells of the immune system such as macrophages or dendritic cells which traditionally perform that function. For this reason, TNCs define a special cell type that is epithelial but functions very much like cells of the immune system. Early in the study of TNCs, there were questions about the authenticity of this multicellular structure in vivo (Figs. 3, 4). Do TNCs exist in the thymus or do they assemble as an artifact of the extensive digestion procedure required for their ex vivo isolation? Are the internalized thymocytes enclosed cytoplasmically? What is the phenotype of the internalized cells, and what is the mechanism employed to facilitate these cell-in-cell structures? What function does this internalization event have during T cell development, and for NIH purposes, are there diseases specific to their malfunction?
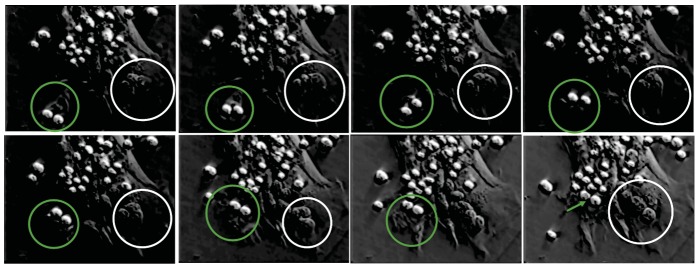
Fig. (1).
Phase contrast video microscopic analysis of TNC thymocyte interaction. Surface bound thymocytes are phase bright. Figure shows a time-lapse movement of the two thymocytes being brought into the TNC cell body via cytoplasmic membrane extensions (green circles and arrow). White circles illustrate thymocytes located within specialized cytoplasmic channels [24]. (Color figure available online).
Fig. (2).
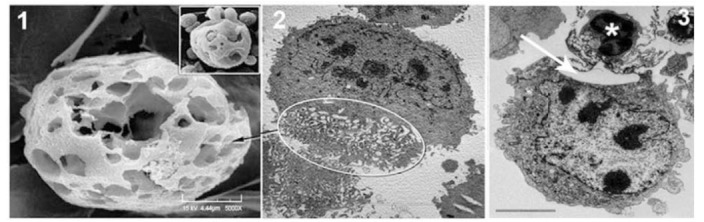
Microscopic identification of membrane extensions and fenestrated structures of TNCs during binding and internalization. Panels 1 (SEM) and 2 (TEM) show fenestrated TNC structures. Panel 3 shows TNC membrane extension interacting with a thymocyte (*). Inset shows thymocytes with cage-like structure as well as thymocytes being released for cage [24].
Fig. (3).
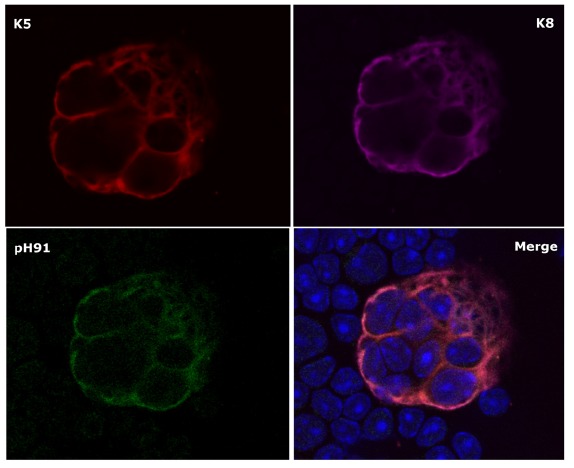
Confocal microscopic analysis of K5+ K8+ pH91+ TNCs. Figure shows freshly isolated TNC stained with anti-K5 (red); anti-K8 (magenta) and the TNC-specific monoclonal antibody (green). The lower right panel indicates a merge of all three stains. Original magnification 40X [22].
Fig. (4).
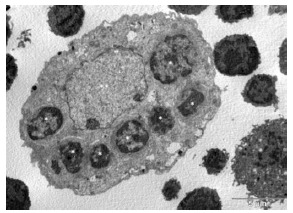
Transmission electron micrograph of engulfed thymocytes by TNC. Isolated TNCs were fixed and prepared for TEM analysis. The large TNC nucleus is indicated by (**). Engulfed thymocytes are visible throughout TNC cytoplasm (*) [24].
In short, the answer is yes. Thymic nurse cells do exist in the thymus [19-22] (Figs. 3, 4). They are not artifacts produced by the isolation procedure. Most of the thymocytes within the multicellular complex are not cytoplasmic [18]. Those thymocytes that become apoptotic eventually enter the cytoplasm and are degraded by TNC-specific lysosomes [23]. The large numbers of thymocytes that are visible within the complex reside in a unique 3D fenestrated cage-like structure believed to be important to the T cell developmental process [24] (Figs. 2, 3). It is reasonable to state that the internalization event is a function of the T cell developmental process because the thymocytes that interact with TNCs are αβTCRloCD4+CD8+ cells, which is the thymocyte phenotype that undergoes major histocompatibility complex (MHC) restriction [23, 25]. The uptake event of thymocytes by TNCs requires the active participation of both cell types. The rearrangement of both microfilaments and microtubules within TNCs, along with the formation of uropods by the thymocytes being internalized are required to facilitate the heterotypic internalization event observed between thymocytes and TNCs, resulting in the formation of this unique multicellular structure found within the thymic cortex [26] (Figs. 2, 4). If one examines the thymic cortices of autoimmune mice, the number of TNCs is significantly reduced [27-29]. It has been proposed that insufficient numbers of TNCs within the thymus may be directly correlated to self-antigen recognition in peripheral organs because the antigen presentation function of TNCs has been diminished in autoimmune animals. The details of the studies related to all of the issues presented above will be addressed in this review.
Thymic Nurse Cells Identity and Function
Thymic nurse cells, a subset of cortical epithelial cells (cTEC) of the thymus were first identified in mice by Wekerle and Ketelsen, 1980. A TNC may contain as many as 200 proliferating lymphocytes within highly specialized cytoplasmic vacuoles (Fig. 3) [19] and also express both class I and class II MHC complexes on their cell membrane [25, 30]. The expression of class II antigens by TNCs was quite interesting since only cells of the immune system possess the capacity to present antigen. TNCs have been identified in many microenvironments of the thymus ranging from the subcapsular region of the thymus to corticomedullary junction (CMJ) and they also express cytokeratins 5 and 8 (K5 and K8), which is a hallmark phenotype used to identify epithelial cells in the thymus. Their discovery in rodents has since led to identifying them in numerous vertebrate species including birds, fish, frogs, chicken and humans [31-34]. TNC expression of class II antigens on their cell surfaces suggests that they play a vital role in T cell development and MHC restriction. Recent findings suggest that TNCs also provide a microenvironment in which secondary T cell receptor alpha rearrangement occurs [35]. Hendrix et al. 2010 [24], have also shown TNCs to have progenitor potential. The nurse cells located at the CMJ are believed to express the stem-cell markers K5K8 and P63 [36-38]. Further investigations by our group have shown embryonic cells destined to differentiate into thymic primordium stain positive for the TNC-specific monoclonal antibody pH91 (Fig. 3 bottom left panel) [22]. The expression of pH91 remained robust throughout development as well as in the postnatal thymus. According to studies conducted by Geenen et al. 2009 [39] TNCs also express the neuroendocrine self-antigens, oxytocin and insulin-like growth factor-2 and the autoimmune regulator AIRE. Further, their studies confirmed the presence of the transcription factor Foxp3 and AIRE expression within the TNC microenvironment suggesting a role for TNCs in natural T regulatory cell (nTreg) development. These studies establish TNCs as not only having an important role in thymocyte development but also shed light on their importance to T-cell tolerance since nTregs are known to be involved in the inactivation of autoreactive cells.
The Role of TNCs in MHC Restriction
Thymocyte selection within the thymus is based upon the affinity model. The model of thymocyte fate selection relies upon the relative strength of binding that occurs between the TCR and MHC self-peptide complex [40-43]. Relatively weak interactions between the TCRs of double positive thymocytes and MHC self-peptide complexes lead to positive selection, a process that promotes the survival of such selecting cells to eventually become single positive cells. Negative selection on the other hand, relies on strong affinity of TCR for MHC self-peptide complexes and induces clonal deletion in DP cells by programmed cell death also known as apoptosis [40-43]. Neglect is a type of cell-death that occurs when DP thymocytes fail to interact with MHC self-peptide.
To address whether TNCs participate in MHC restriction, we constructed TNC cell-lines by immortalizing freshly isolated TNCs with a temperature-sensitive SV40 virus [44]. The resulting TNC-line, tsTNC-1 was shown to bind and internalize immature viable thymocytes bearing the αβTCRloCD4+CD8+ phenotype [45, 46] (Fig. 1). Further, creating such a research tool enabled us to conduct long-term co-incubation studies since freshly isolated TNCs have a limited life-span in culture and also because they do not reestablish binding and internalization of thymocytes once their thymocyte cargo has been released in culture [25]. Further, tsTNC-1 cells were shown in long-term co-incubation experiments to rescue a subpopulation of the internalized cells from apoptosis by inducing the expression of Bcl-2, an anti-apoptotic protein whereas a second subset was induced to become apoptotic. The activation of Bcl-2 in this population of thymocytes may be important to the recently published data that show secondary T cell receptor alpha rearrangement within TNCs require an extensive time period [35]. Two distinct populations of cells were released from the TNC thymocyte interactions, however, the cells that remained intra-cytoplasmically died via apoptosis [23]. Also, antibodies targeting MHC class I or class II antigens abrogated the rescue activity by TNCs previously observed. In addition, when IL1β was added to the co-culture, rescued thymocytes transitioned from an immature
phenotype αβTCRloCD69- to αβTCRhiCD69+. Taken together, these data suggest that the TNC/thymocyte interaction results in the rescue and early maturational steps of immature thymocytes.
To further demonstrate the role of TNCs in MHC restriction, we examined the HY- TCR transgenic system [9, 47]. In this transgenic system, the repertoire of immature thymocytes express an αβTCR that exclusively interact with the male-specific HY antigen within the context of MHC. We performed a comparative confocal microscopic analysis of TNCs isolated from HY-TCR females and HY-TCR males. HY-TCR transgenic females were shown to have 17 times more TNCs and their TNCs were significantly larger than that of HY males [9]. In addition, the HY-TCR females contained 5 times more thymocytes within cytoplasmic vacuoles than their male counterpart. TUNEL analyses of both female and male TCR transgenic mice showed a dramatic increase in apoptosis among male animals (42%) compare to 4% for HY females. These data strengthen the theory that TNCs create an intimate 3D microenvironment that promotes their participation in MHC restriction (Fig. 2). Further, these data clearly demonstrate that TNCs are associated with viable thymocytes and refute the notion that they are involved in the removal of nonfunctional and apoptotic thymocytes [9, 48].
Differentiation of Pre-Entotic Lymphocytes
T cell progenitors (TCPs) originate in the bone marrow and use the circulatory system as a vehicle for their transportation to the thymus [49]. The TCPs enter the thymus at the CMJ and undergo a series of interaction with thymic stromal cells that eventually result in specific cell function [49]. When they initially enter into the thymus, TCPs do not express cell-surface markers CD4 nor CD8 nor αβTCR and are termed triple negative (TNs) [50]. The TN cells undergo a series of developmental changes due to interactions with specific microenvironmental niches that result in the production of single positive CD4+ T cells or CD8+ T cells. Prior to their terminal T cell lineage commitment, immature T cells are grouped into four defined stages; DN1, DN2, DN3 and DN4 based upon cluster of differentiation (CD) 4 and 8 expression. Each T cell substage can be differentiated by the expression or absence of CD44 and or CD25 [51]. DN1 cells are predisposed to T lineage commitment, whereas DN2 cells transit the thymic cortex and initiate the rearrangement of TCR-β. After DN3 cells successfully rearrange β-chain, they transit to the DN4 stage of development. DN4 cells that successfully executed β-chain rearrangement result in the significant reduction in the expression of both CD44 as well as CD25 and concomitantly increased the expression of CD4 and CD8, which activates TCR-α chain rearrangement [52, 53]. The TCR-α rearrangement event establishes a triple positive thymocyte phenotype αβTCRloCD4+CD8+ that interacts with TNCs [45].
The TNC/Thymocyte Interaction Typifies Heterotypic Cell-in-Cell Interaction
The use of immortalized TNC cell-lines provided the first set of evidence showing the binding and internalization of immature thymocytes [44, 45]. When freshly isolated thymocytes were added to the TNC cell-line SVT-II2 and videotaped for a duration of 12 hours, membrane channels developed and became visible [44, 45]. αβTCRloCD4+CD8+ thymocytes were brought into the TNC cell body by cytoplasmically rearranged finger-like projections (Figs. 1, 22-3). Both cell types mediate the interactions. Thymocytes rearrange their shapes to produce a unique structure called a uropod (tail-like structure) that facilitated their movement into the TNCs [26]. The internalized thymocytes moved vigorously about each other, and in some cases moved unilaterally within specialized channels (Fig. 1). Philp et al. 1993 [54] demonstrated that the internalized thymocytes were completely enclosed within the TNC structure. These results however, instigated questions concerning the nature of this interaction with respect to the cytoplasmic location of captured thymocytes.
The binding of thymocytes to TNCs is mediated by an antigenic determinant on the TNC membrane. We have developed a TNC-specific monoclonal antibody (pH91) in our laboratory that recognizes a 43-kDa protein on the cell surface of TNCs [21]. In tissue culture experiments, ts-TNC-1 treatment with pH91 resulted in a significant binding reduction of thymocytes to TNCs. Further, when we co-cultured fetal thymus in the presence of pH91, we observed a marked reduction in thymocyte viability. These data suggest the interactions between immature thymocytes and the receptor defined by the pH91 antibody on TNCs provide critical signals that are necessary for thymocyte binding and the subsequent internalization event. After several attempts, using a significant number of techniques in our laboratory and in commercial laboratories, the antigen targeted by pH91 remains undefined.
The thymocyte uptake by TNCs is mediated by cytoskeletal proteins actin and tubulin [26]. Immunofluorescence analyses reveal that both cytoskeletal proteins rearrange to make contact with thymocytes at bound surfaces. The treatment of co-cultures with the cytoskeletal inhibitors colchicine or jasplakinoloid caused the binding and internalization of thymocytes by the TNCs to be significantly reduced. Similarly, Overholtzer et al. 2007 [12], examined whether cell engulfment requires actin, myosin Rho, and ROCK activity. Their results showed that when cells were treated with latrunculin B, an inhibitor of actin polymerization or blebbistatin, an inhibitor of myosin II, the treated cells formed suspension aggregates that minimally engulfed other cells. Further, inhibition analyses of Rho GTPase and their target effectors showed significant reduction in engulfment. These findings associate Rho-ROCK-actin/myosin as important players during the process of engulfment. These studies reveal the importance of cytoplasmic proteins during the internalization event, however, much remains to be done to define the role of these and other cytoplasmic proteins during heterotypic cellular uptake by different cell types.
The internalization of αβTCRloCD4+CD8+ thymocytes by TNCs is dependent on TNC-cytoplasmic filamentous extensions, pH91 binding and actin/tubulin retraction. Video evidence show the lassoing action of cytoplasmic extension around the thymocytes (Fig. 1). This actin/tubulin retraction leads to thymocyte transportation into the cage-like structure, where we believe TNC-specific MHC restriction is housed. Thymocytes are allowed to move freely within caverns and exit the fenestrae. The internalization process used by TNCs, although they have different molecular requirements, is reminiscent of the action deployed during the process of entosis. An important consideration is what happens to the thymocytes that are negatively selected within the TNC fenestrae? We believe that these dead cells are trafficked into the cytoplasm of the TNCs and are degraded by lysosomes. The mechanism used in the latter process may be similar to those of entosis or ‘eat me’ recognition signals such as phosphatidylserine used by phagocytic cells [55]. The emerging concept of the TNC structure is bipartite where cell selection occurs within the apical fenestrae (cage-like) region of the cell and negatively selected cells enter the cytoplasm through vacuoles located between the cage and the cytoplasm.
Using TEM, SEM and confocal techniques we were able to ascertain a more complete representation of the thymic nurse cell structure (Figs. 1-4). Co-cultures were grown in Terisaki plates and examined by confocal microscopy. Images show a TNC containing many internalized thymocytes compartmentalized into the body of the TNC structure. At the apical region there appears to be extensive membrane folds creating a highly fenestrated cage (Fig. 2). The fenestrated region of the TNCs may house the apparatus for MHC selection of developing thymocytes. In that study we proposed that cells undergoing negative selection may be entosed into the cytoplasm. Simply stated, most of the thymocytes within the TNC microenvironment are not cytoplasmic, although when visualized microscopically the entire population appears to be within the cytoplasmic membrane. We believe the fenestrated cage-like structure that houses the majority of the thymocytes within the multicellular structure provides an extended surface area of plasma membrane presenting both class I and II MHC proteins. It is in this area of the TNC structure that MHC restriction occurs. Those cells that are positively selected can exit the structure because this unique membranous cage-like arrangement is external to the cytoplasm of the TNC (Fig. 21). Otherwise, the release of maturing thymocytes would involve a series of undocumented membrane fusions to facilitate exit from the cytoplasm. The thymocyte subset that is negatively selected is emptied into classical cytoplasmic vacuoles. We know that negatively selected thymocytes are cytoplasmic because they fuse with lysosomes where they are destroyed [23].
The Role of TNCs in Autoimmunity
The idea that autoimmune diseases develop as a consequence of the activity of aberrant lymphocytes that cannot tolerate self-antigens was first presented by Burnet in 1972 [56]. Conceivably, these auto-reactive lymphocytes may result from disruptions in both central (recessive) and peripheral (dominant) tolerance mechanisms. Genetic evidence was provided to support a role for dominant tolerance in the prevention of autoimmunity with the discovery that a genetic mutation in the Foxp3 gene resulted in the reduction of immunosuppressive activity among regulatory T cells in the periphery [56, 57]. Additionally, mutations in the gene encoding AIRE were found to result in the development of diseases like Type I diabetes and autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) [58]. Although this particular finding seems to suggest that genetic mutations can impair recessive tolerance mechanisms and consequently result in autoimmune disorders, genetic mutations are insufficient in explaining the contribution of disrupted central tolerance to autoimmune regulation. This observation is reinforced by the recent finding that negative selection is intact in NOD mice [59].
An abnormal thymic architecture is a common feature associated with several models of autoimmune disease. For example, large acellular spaces referred to as “cortical holes” have been described in the thymic cortex of several murine models of systemic lupus erythematosus (SLE) including MRL/MP-lpr/lpr, BXSB/MpJ Yaa, and C3H/HeJ-gld/gld [28]. Epithelial cell free zones and disruptions in the cortical epithelial network were also observed in the thymi of other autoimmune mouse models such as NOD, NOD-scid and NZB [60]. Since, both positive and negative selection of thymocytes relies on the number of profitable contacts occurring between developing T cells and the thymic epithelium, stromal abnormalities in the autoimmune thymus are of great interest. It was suggested that thymic architectural abnormalities in autoimmune animals resulted from accelerated degeneration of thymic stromal cells [29]. Since thymic structural abnormalities were observed in animals prior to manifestations of disease and were never observed in normal mice, they were considered to be a cause and not a consequence of the autoimmune disease etiology. Of particular interest was the finding that TNC numbers decreased exponentially in the thymi of diseased SLE-prone mice [29]. This reduction in TNC number was always coupled with a reduction in apoptotic frequencies among thymocytes.
However, such significant decreases in TNC number were not unique to the autoimmune disease SLE. It was also reported in the thymi of obese chickens that develop spontaneous autoimmune thyroiditis [32].
In our own studies, using the lupus prone NZB/W model we have observed marked reduction in TNC numbers with a strong correlation to serum conversion from IgM to IgG (unpublished). In addition, the thymic architecture appears to be irregularly arranged with no defined cortices or medulla and the presence of cortical holes appear throughout the thymic tissue.
In a recent study (2008) [39], examined TNCs participation in the development of effector and natural regulatory T cells. Regulatory T cells are important to the process of immune tolerance although the mechanism of how they are generated in the thymus is not fully understood. Their findings show that the thymocytes internalized by TNCs express both AIRE and FoxP3 within their nuclei. FoxP3 is a marker used to identify Tregs. Their study is important to the understanding of SLE because if TNCs participate in Treg development then TNC deficiencies as a result of cortical-hole formation would cause a dramatic reduction in the number of Tregs being generated in the affected thymus. Therefore, affected animals with significantly less Tregs maybe more prone to develop autoimmune diseases.
TNCs are among the major cell types engaged in the selection and differentiation of T cells in the thymus, and their functional loss seems to result in the immune dysregulation seen in many autoimmune diseases. The development of cellular therapies for autoimmune diseases which focus on restoring immune regulation should include methods to reestablish the intrathymic TNC profile. Such therapies have the potential to reestablish negative selection and differentiation of nTregs in the thymus. There are several precedents which suggest that cellular therapies may be successfully used as treatment regimens for autoimmune disease. For example, Peterson et al. (2011) [61] demonstrated that disease onset was inhibited in a mouse model of autoimmune arthritis by increasing the number of Tregs in the thymi and spleen of these animals. Another experiment demonstrated that the transfer of human amniotic epithelial cells (hAEC) into mice prone to autoimmune encephalomyelitis resulted in an increase in the number of CD4+ CD25+ FoxP3+ Tregs in peripheral lymph nodes [62]. Additionally, hematopoietic stem cell transplants have been used successfully to treat patients with severe forms of autoimmune diseases such as multiple sclerosis [63], SLE [64] and rheumatoid arthritis for over thirteen years [65]. Thus the development of TNC-based cellular therapies may provide alternatives to conventional treatments for autoimmune diseases.
Acknowledgements
The work was supported by Grant Number 8G12MD007603-29 from the National Institute on Minority Health and Health Disparities.
We thank Mr. Zachariah Olushoga for his patience, dedication, image processing skills and manuscript preparation.
I would like to dedicate this manuscript to my son Omari Claude Samms. His inspiration was one of the major driving forces behind the completion of this document.
I would like to thank my mentor, Dr. Jerry Charles Guyden for his devotion to academic excellence and his humanitarianism. His belief in people has changed the trajectory of so many ordinary lives.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Eberth J. Über die feineren bau der darmschleithaut. Wurzb Naturwiss Zeitschr. 1864;5:11. [Google Scholar]
- 2.Eimer A.D. Zur beckerfrage. Virchows Arch. 1867;40:282. [Google Scholar]
- 3.Von Arnstein C. Über becherzellen und ihre beziehung zur fettresorption und sekretion. Arch Mikrosk Anat. 1867;39:527–547. [Google Scholar]
- 4.Paneth J. Über die sezernierenden zellen der dünndarmepithels. Arch F Mikrosk Anat. 1888;31:113–191. [Google Scholar]
- 5.Bauchwitz M.A. The bird's eye cell: cannibalism or abnormal division of tumor cells. Acta Cytol Abstr. 1981;25:92. [Google Scholar]
- 6.Fischer A., Dolschansky L. Uber das wachstum von milzstromazellen in vitro. Arch Entwmech Org. 1929;116:123. doi: 10.1007/BF02145224. [DOI] [PubMed] [Google Scholar]
- 7.Shelton E., Rice M. Studies on mouse lymphomas. III. Behavior of tumor- and non-tumor-cell populations during growth of three ascites lymphomas. J. Natl. Cancer Inst. 1958;21:163–191. [PubMed] [Google Scholar]
- 8.Wheatley D.N. Cellular engulfment of erythrocytes. Br. J. Exp. Pathol. 1968;49:541–543. [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez M., Samms M., Hendrix T.M., Adeosun O., Pezzano M., Guyden J.C. Thymic nurse cell multicellular complexes in HY-TCR transgenic mice demonstrate their association with MHC restriction. Exp. Biol. Med. (Maywood) 2007;232:780–788. [PubMed] [Google Scholar]
- 10.Hong J.S. The exfoliative cytology of endometrial stromal sarcoma in peritoneal fluid. Acta Cytol. 1981;25:277–281. [PubMed] [Google Scholar]
- 11.Kumar P.V., Hosseinzadeh M., Bedayat G.R. Cytologic findings of medulloblastoma in crush smears. Acta Cytol. 2001;45:542–546. doi: 10.1159/000327862. [DOI] [PubMed] [Google Scholar]
- 12.Overholtzer M., Mailleux A., Mouneimne G., et al. A Nonapoptotic Cell Death Process, Entosis, that Occurs by Cell-in-Cell Invasion. Cell. 2007;131(5):966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Hughes D., Raine C.S., Field E.J. Invasion of neurones in vitro by non immune lymphocytes. An electron microscopic study. Br. J. Exp. Pathol. 1968;49:356–359. [PMC free article] [PubMed] [Google Scholar]
- 14.de Waal Malefijt R., Leene W., Roholl P.J., Wormmeester J., Hoeben K.A. T cell differentiation within thymic nurse cells. Lab. Invest. 1986;55:25–34. [PubMed] [Google Scholar]
- 15.Tsunoda R., Heinen E., Sugai N. Follicular dendritic cells in vitro modulate the expression of Fas and Bcl-2 on germinal center B cells. Cell Tissue Res. 2000;299:395–402. doi: 10.1007/s004419900172. [DOI] [PubMed] [Google Scholar]
- 16.Burns E.R., Zucker-Franklin D., Valentine F. Characterization of the cell population mediating cytotoxicity and emperipolesis in human malignant melanomas. Trans. Assoc. Am. Physicians. 1981;94:366–371. [PubMed] [Google Scholar]
- 17.Humble J.G., Jayne W.H., Pulvertaft R.J. Biological interaction between lymphocytes and other cells. Br. J. Haematol. 1956;2:283–294. doi: 10.1111/j.1365-2141.1956.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 18.Pezzano M., Samms M., Martinez M., Guyden J. Questionable Thymic Nurse Cell. Micro Mol Bio Rev. 2001;65(3):390–403. doi: 10.1128/MMBR.65.3.390-403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wekerle H. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. J. Exp. Med. 1980;151(4):925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyewski B. Thymic nurse cells: possible sites of T-cell selection. Immunol. Today. 1986;7(12):374–379. doi: 10.1016/0167-5699(86)90030-7. [DOI] [PubMed] [Google Scholar]
- 21.Pezzano M., King K., Philp D., et al. A Thymic Nurse Cell-Specific Monoclonal Antibody. Cell. Immunol. 1998;185(2):123–133. doi: 10.1006/cimm.1998.1279. [DOI] [PubMed] [Google Scholar]
- 22.Chilukuri Rajendra V.E., Patel Viral K., Martinez M., Guyden J.C., Samms M.D. The Antigenic Determinant That Defines Thymic Nurse Cells Is Expressed by Thymic Epithelial Progenitor Cells. Front. Cell Dev. Biol. 2014;2:1–12. doi: 10.3389/fcell.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samms M., Emanus F., Osuji O., Pezzano M., Guyden J.C. Lysosomal-mediated degradation of apoptotic thymocytes within thymic nurse cells. Cell. Immunol. 1999;197:108–115. doi: 10.1006/cimm.1999.1559. [DOI] [PubMed] [Google Scholar]
- 24.Hendrix T., Chilukuri R., Martinez M., et al. Thymic nurse cells exhibit epithelial progenitor phenotype and create unique extra-cytoplasmic membrane space for thymocyte selection. Cell. Immunol. 2010;261(2):81–92. doi: 10.1016/j.cellimm.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezzano M., Philp D., Stephenson S., et al. Positive Selection by Thymic Nurse Cells Requires IL-1β and Is Associated with an Increased Bcl-2 Expression. Cell. Immunol. 1996;169(2):174–184. doi: 10.1006/cimm.1996.0108. [DOI] [PubMed] [Google Scholar]
- 26.Webb O., Kelly F., Benitez J., et al. The identification of thymic nurse cells in vivo and the role of cytoskeletal proteins in thymocyte internalization. Cell. Immunol. 2004;228(2):119–129. doi: 10.1016/j.cellimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Takeoka Y., Chen S., Boyd R., et al. A Comparative Analysis of the Murine Thymic Microenvironment in Normal, Autoimmune, and Immunodeficiency States. Dev. Immunol. 1997;5(2):79–89. doi: 10.1155/1997/69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeoka Y., Yoshida S., Van de Water J., et al. Thymic Microenvironmental Abnormalities in MRL/MP-lpr/lpr, BXSB/MpJYaaand C3H HeJ-gld/gldMice. J Auto. 1995;8(2):145–161. doi: 10.1006/jaut.1995.0012. [DOI] [PubMed] [Google Scholar]
- 29.Takeoka Y., Taguchi N., Shultz L., et al. Apoptosis and the Thymic Microenvironment in Murine Lupus. J Auto. 1999;13(3):325–334. doi: 10.1006/jaut.1999.0325. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz R., Allen P. Thymic cortical epithelial cells lack full capacity for antigen presentation. Nature. 1989;340(6234):557–559. doi: 10.1038/340557a0. [DOI] [PubMed] [Google Scholar]
- 31.Boyd R., Oberhuber G., Hala K., Wick G. Obese strain (OS) chickens with spontaneous autoimmune thyroiditis have a deficiency in thymic nurse cells. J. Immunol. 1984;132:718–724. [PubMed] [Google Scholar]
- 32.Hiramine C., Hojo K., Koseto M., Nakagawa T., Mukasa A. Establishment of a murine thymic epithelial cell line capable of inducing both thymic nurse cell formation and thymocyte apoptosis. Lab. Invest. 1990;62:41–54. [PubMed] [Google Scholar]
- 33.Ritter A., Suavage C.A., Cotmore S.F. The human thymus microenvironment: in vivo identification of thymic nurse cells and other antigenically-distinct subpopulations of epithelial cells. Immunology. 1981;44:439–446. [PMC free article] [PubMed] [Google Scholar]
- 34.Wekerle H., Ketelson U.P. Thymic nurse cells- Ia bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980;283:402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa Y., Ohigashi I., Nitta T., et al. Thymic nurse cells provide microenvironment for secondary T cell receptor rearrangement in cortical thymocytes. Proc. Natl. Acad. Sci. USA. 2012;109(50):20572–20577. doi: 10.1073/pnas.1213069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popa I., Zubkova I., Medvedovic M., et al. Regeneration of the adult thymus is preceded by the expansion of K5+K8+ epithelial cell progenitors and by increased expression of Trp63, cMyc and Tcf3 transcription factors in the thymic stroma. Int. Immunol. 2007;19(11):1249–1260. doi: 10.1093/intimm/dxm092. [DOI] [PubMed] [Google Scholar]
- 37.Senoo M., Pinto F., Crum C., McKeon F. p63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell. 2007;129(3):523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 38.Bennett A., Farley A., Blair N., Gordon J., Sharp L., Blackburn C. Identification and Characterization of Thymic Epithelial Progenitor Cells. Immunity. 2002;16(6):803–814. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 39.Hansenne I., Louis C., Martens H., et al. Aire and Foxp3 Expression in a Particular Microenvironment for T Cell Differentiation. Neuroimmunomodulation. 2009;16(1):35–44. doi: 10.1159/000179665. [DOI] [PubMed] [Google Scholar]
- 40.Kappler J., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 41.Kisielow P., Blüthmann H., Staerz U., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 42.Von Boehmer H., Kishi H., Borgulya P., et al. Control of T-cell Development by the TCR for Antigen. Cold Spring Harb. Symp. Quant. Biol. 1989;54(0):111–118. [PubMed] [Google Scholar]
- 43.Teh H., Kisielow P., Scott B., et al. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 44.Pezzano M., Li Y., Yang Y., Guyden J. The immortalization of thymic nurse cells by SV40 virus. Cell. Immunol. 1991;133(2):434–445. doi: 10.1016/0008-8749(91)90116-s. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Pezzano M., Philp D., Reid V., Guyden J. Thymic nurse cells exclusively bind and internalize CD4+CD8+ thymocytes. Cell. Immunol. 1992;140(2):495–506. doi: 10.1016/0008-8749(92)90214-a. [DOI] [PubMed] [Google Scholar]
- 46.Pezzano M., Li Y., Philp D., et al. Thymic nurse cell rescue of early CD4+CD8+ thymocytes from apoptosis. Cell Mol Biol (Noisy-le-grand) 1995;41(8):1099–1111. [PubMed] [Google Scholar]
- 47.Strasser A., Harris A., von Boehmer H., Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc. Natl. Acad. Sci. USA. 1994;91(4):1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguilar L.K., Agilar-Cordova E., Cartwright J., Jr, Belmont J.W. Thymic nurse cells are sites of thymocyte apoptosis. J. Immunol. 1994;152:2645–2651. [PubMed] [Google Scholar]
- 49.Lind E. Mapping Precursor Movement through the Postnatal Thymus Reveals Specific Microenvironments Supporting Defined Stages of Early Lymphoid Development. J. Exp. Med. 2001;194(2):127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuniga-Pflucker J. When Three Negatives Made a Positive Influence in Defining Four Early Steps in T Cell Development. J. Immunol. 2012;189(9):4201–4202. doi: 10.4049/jimmunol.1202553. [DOI] [PubMed] [Google Scholar]
- 51.Shah D., Zuniga-Pflucker J. An Overview of the Intrathymic Intricacies of T Cell Development. J. Immunol. 2014;192(9):4017–4023. doi: 10.4049/jimmunol.1302259. [DOI] [PubMed] [Google Scholar]
- 52.Ciofani M., Zuniga-Pflucker J. Determining γδ versus αβ T cell development. Nat. Rev. Immunol. 2010;10:657–663. doi: 10.1038/nri2820. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald H., Budd R., Howe R. A cd3− subset of cd4−8+ thymocytes: a rapidly cycling intermediate in the generation of cd4+8+ cells. Eur. J. Immunol. 1988;18(4):519–524. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 54.Philp D., Pezzano M., Li Y., Omene C., Boto W., Guyden J. The Binding, Internalization, and Release of Thymocytes by Thymic Nurse Cells. Cell. Immunol. 1993;148(2):301–315. doi: 10.1006/cimm.1993.1114. [DOI] [PubMed] [Google Scholar]
- 55.Brown S., Heinisch I., Ross E., Shaw K., Buckley C., Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418(6894):200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 56.Burnet F. A reassessment of the forbidden clone hypothesis of autoimmune disease. Aust. J. Exp. Biol. Med. Sci. 1972;50(1):1–9. doi: 10.1038/icb.1972.1. [DOI] [PubMed] [Google Scholar]
- 57.Brunkow M.E., Jeffery E.W., Hjerrild K.A., et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 58.Chatila T., Blaeser F., Ho N., et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 2000;106(12):R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heino M., Peterson P., Kudoh J., et al. APECED mutations in the autoimmune regulator (AIRE) gene. Hum. Mutat. 2001;18(3):205–211. doi: 10.1002/humu.1176. [DOI] [PubMed] [Google Scholar]
- 60.Mingueneau M., Jiang W., Feuerer M., Mathis D., Benoist C. Thymic negative selection is functional in NOD mice. J. Exp. Med. 2012;209(3):623–637. doi: 10.1084/jem.20112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peterson L., Shaw L., Joetham A., Sakaguchi S., Gelfand E., Dragone L. SLAP Deficiency Enhances Number and Function of Regulatory T Cells Preventing Chronic Autoimmune Arthritis in SKG Mice. J. Immunol. 2011;186(4):2273–2281. doi: 10.4049/jimmunol.1003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y., Chan J., Vaghjiani V., Murthi P., Manuelpillai U., Toh B. Human amniotic epithelial cells suppress relapse of corticosteroid-remitted experimental autoimmune disease. Cytotherapy. 2014;16(4):535–544. doi: 10.1016/j.jcyt.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Farge D., Henegar C., Carmagnat M., et al. Analysis of immune reconstitution after autologous bone marrow transplantation in systemic sclerosis. Arth Rheum. 2005;52(5):1555–1563. doi: 10.1002/art.21036. [DOI] [PubMed] [Google Scholar]
- 64.Alexander T., Thiel A., Rosen O., et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2008;113(1):214–223. doi: 10.1182/blood-2008-07-168286. [DOI] [PubMed] [Google Scholar]
- 65.Roord S.T., de Jager W., Boon L., et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111(10):5233–5241. doi: 10.1182/blood-2007-12-128488. [DOI] [PubMed] [Google Scholar]