Abstract
Introduction
Despite its desirable half-life and low energy Auger electrons that travel further than for other radionuclides, 67Ga has been neglected as a therapeutic radionuclide. Here, 67Ga is compared with Auger electron emitter 111In as a potential therapeutic radionuclide.
Methods
Plasmid pBR322 studies allowed direct comparison between 67Ga and 111In (1 MBq) in causing DNA damage, including the effect of chelators (EDTA and DTPA) and the effects of a free radical scavenger (DMSO). The cytotoxicity of internalized (by means of delivery in the form of oxine complexes) and non-internalized 67Ga and 111In was measured in DU145 prostate cancer cells after a one-hour incubation using cell viability (trypan blue) and clonogenic studies. MDA-MB-231 and HCC1954 cells were also used.
Results
Plasmid DNA damage was caused by 67Ga and was comparable to that caused by 111In; it was reduced in the presence of EDTA, DTPA and DMSO. The A50 values (internalized activity of oxine complexes per cell required to kill 50% of cells) as determined by trypan blue staining was 1.0 Bq/cell for both 67Ga and 111In; the A50 values determined by clonogenic assay were 0.7 Bq/cell and 0.3 Bq/cell for 111In and 67Ga respectively. At the concentrations required to achieve these uptake levels, non-internalized 67Ga and 111In caused no cellular toxicity. Qualitatively similar results were found for MDA-MB-231 and HCC1954 cells.
Conclusion
67Ga causes as much damage as 111In to plasmid DNA in solution and shows similar toxicity as 111In at equivalent internalized activity per cell. 67Ga therefore deserves further evaluation for radionuclide therapy.
Advances in knowledge and implications for patient care
The data presented here is at the basic level of science. If future in vivo and clinical studies are successful, 67Ga could become a useful radionuclide with little healthy tissue toxicity in the arsenal of weapons for treating cancer.
Keywords: Gallium-67, Radionuclide therapy, Auger electrons, Clonogenic assay
1. Introduction
Radiopharmaceutical therapies, such as 131I–MIBG, anti-CD20 antibodies (labeled with 90Y or 131I), and 177Lu-Octreotate, have become standard in the clinic. These beta particle-emitting treatments, however, are generally not curative and can cause toxicity to healthy tissue due to the long range (up to 1 cm for 90Y) by high beta energies. Radioisotopes emitting Auger electrons with a much shorter range (<1 μm) are now being considered for targeted radionuclide therapy and could become useful tools in targeting micrometastases that play a detrimental role in tumor recurrence.
Gamma camera imaging with 67Ga has been used regularly in the clinic since the 1980s to image lymphoma where it was useful in disease staging, monitoring disease progression and relapse, and predicting therapy response [1]. In Hodgkin's disease, the detection sensitivity is 70 to 83% [2]. In non-Hodgkin's lymphoma the detection sensitivity depends on cell differentiation status; less differentiated cells show higher avidity for gallium [1]. Other gallium-avid cancers include lung cancer, melanoma and multiple myeloma [1], [2]. In these applications 67Ga is administered as 67Ga-citrate and 67Ga uptake by cells is believed to be transferrin-mediated [3], although there is also evidence for transferrin-independent mechanisms [4], [5], [6].
A feature of 67Ga is that besides its gamma emissions for scintigraphy and SPECT imaging, it also emits Auger electrons [7] and thus has potential as a therapeutic radionuclide. As such it could form part of a “theranostic pair” with the generator-produced positron emitter 68Ga. Despite producing fewer Auger electrons per decay (average of 4.7) than fellow Auger electron emitter 111In (14.7), the average total Auger electron energy released per decay of 67Ga (6.3 keV) is similar to that of 111In (6.8 keV) [8]. In fact, amongst Auger electron emitters, 67Ga produces amongst the most energetic (7 to 9 KeV) and longest ranging (up to 2.4 μm in water) Auger electrons [7]. This may reduce the need for the radionuclide to be localized in specific subcellular compartments in order to be effective.
67Ga has been explored previously, to a limited extent, as a radionuclide for therapeutic applications [9], [10], [11], [12], [13]. In vitro results were promising and showed that treatment with 67Ga diminished clonogenic capacity in human U937 lymphoma cells [9] and in myeloid leukemic blasts from acute myeloid leukemia patients [10]. A feasibility study in eight patients with relapsed acute leukemia was less successful due to the low cell uptake of 67Ga-citrate [11], which might have arisen in part from using higher citrate concentrations in the clinical preparations than used in the in vitro work. Others have explored the therapeutic potential of 67Ga in lymphoma when coupled to anti-CD74 antibodies [12], [14] or anti-LL1 antibodies [13]. Michel et al. showed that 67Ga was two to three times more potent than 111In when coupled to anti-CD74 antibodies on the basis of equivalent total disintegrations in the medium per viable cell [12]. Low specific activities and lack of purpose-designed gallium chelators and conjugates at that time, however, led to a lack of further enquiry in this field.
In recent years the development of peptides and proteins labeled with 68Ga, arising from the growing popularity of the 68Ga generator for positron emission tomography applications, has led to a new generation of effective bifunctional chelators for gallium [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]. For example, the trishydroxypyridinone chelator allows radiolabelling of molecular targeting agents with gallium using fast and simple one-step procedures [15], [20]. The resulting enhanced versatility and range of potentially useful targeting agents now presents an opportunity to reconsider 67Ga as a targeted therapeutic radionuclide.
Here a comparison is made between 67Ga and the well-characterized and clinically evaluated radionuclide 111In, which has been successfully tested preclinically as a therapeutic radionuclide attached to cell surface or intracellular targets [26], [27], [28]. 67Ga and 111In have similar half-lives (78 and 67 h, respectively) and both produce gamma rays. We used the cell-free pBR322 plasmid assay to directly compare DNA damage induced by the radionuclides without complications due to cellular and subcellular barriers, DNA repair mechanisms and other cellular responses. For the first time, the levels of activity per cell required to achieve significant cytotoxic effects was calculated from viability and clonogenic assays using radiolabelled lipophilic complexes in three prostate and breast cancer cell lines, selected for their possible future use in in vivo models for radionuclide therapy of circulating tumor cells and micrometastases using Auger electron emitters.
2. Materials and methods
2.1. Radionuclide preparation
111In-chloride (111InCl3) (Mallinckrodt, Netherlands) was supplied at 111 MBq in 0.3 mL 0.05 M HCl. 67Ga-citrate (6.46 mM citrate, Mallinckrodt, Netherlands) was converted to 67Ga-chloride (67GaCl3) [29]. Briefly, 67Ga-citrate (82–160 MBq in 2.2 mL) was diluted to 5 mL with dH2O and passed through a Silica Light SEP-PAK column (Waters, US) at 1 mL/min. After washing with 5 mL dH2O, trapped 67Ga was eluted with 0.1 M HCl (Sigma, UK) and collected in 0.5 mL fractions. Fractions with the highest activity concentration 67Ga-chloride (200–800 MBq/mL, fractions 6 or 7) were used.
2.2. Cell-free DNA damage by 111In and 67Ga
125 ng pBR322 (10 μL supplied in 10 mM Tris–HCl (pH 8.0) with 1 mM ethylenediaminetetraacetic acid (EDTA), Sigma) was mixed with 1 MBq 111In-chloride, 67Ga-chloride or 67Ga-citrate and incubated up to 72 h at 4 °C (final EDTA concentration 0.1 mM). The final volume was 100 μL in Dulbecco's phosphate-buffered saline pH 7.4 (PBS; Thermo Fisher, UK).
Plasmids were co-incubated with 14 mM dimethylsulfoxide (DMSO), excess EDTA (5 mM) or diethylenetriamine pentaacetic acid (DTPA; 5 mM). Controls included untreated plasmid (no radionuclide), external irradiation (where radionuclides in a 50 mL tube were physically separated from plasmid in 1.5 mL microcentrifuge tube inside the 50 mL tube), and equivalent amounts of non-radioactive gallium- (0.69 pmol) and indium-chloride (0.58 pmol) at molar concentrations equivalent to 1 MBq radionuclide.
After treatment, 12.5 ng plasmid on a 0.8% agarose gel, run at 100 V for 25 min, was visualized with Gel Red™ (Biotium, USA) by UV transilluminator (Gel Doc-it, BioRad, UK).
2.3. Analysis of gel electrophoresis images
Images were analyzed by densitometry of each plasmid band (Fig. 1, Fig. 2, S1–3; supercoiled, circular and linear; Image J 1.48, NIH, USA). Background was measured and subtracted from band intensity. The fraction of supercoiled plasmid (undamaged) of total plasmid represents undamaged plasmid.
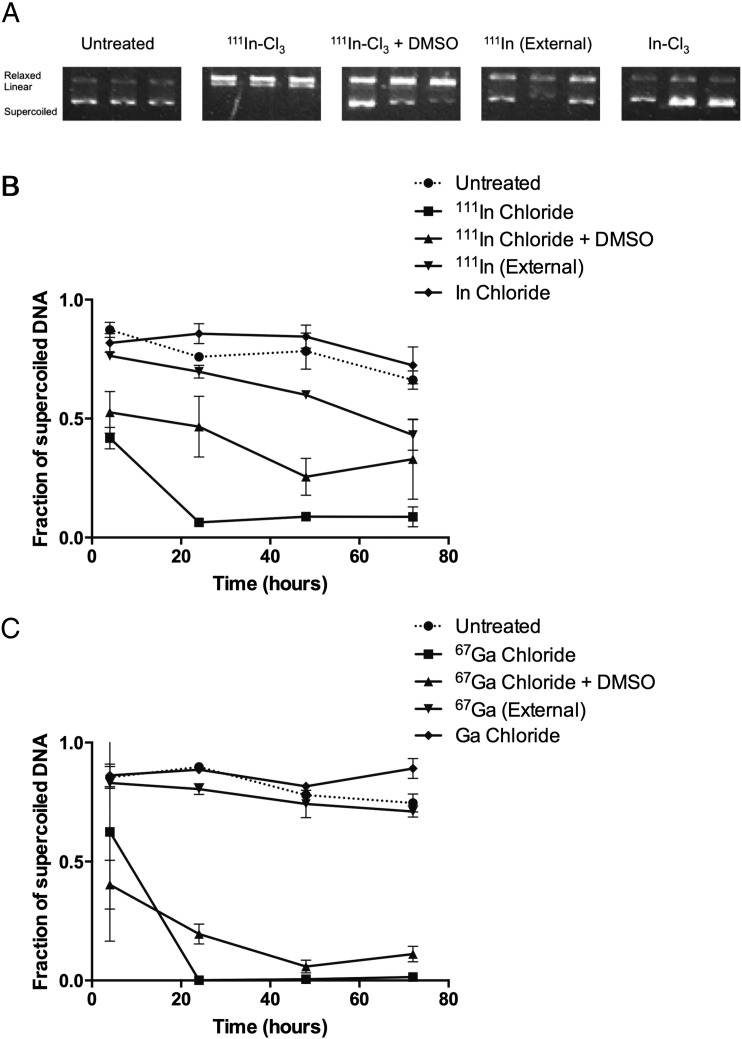
Fig. 1.
A: Representative image of pBR322 on an agarose gel following treatment with a radionuclide. Here, pBR322 was incubated with 1 MBq 111In-chloride (111In-Cl3 or as external radiation (111In (external)) for 72 h in the presence or absence of DMSO or cold indium chloride (InCl3). B and C: Fraction of supercoiled (undamaged) plasmid, as measured from gels such as A. Plasmids were incubated with either 111InCl3 (B) or 67GaCl3 (C). Data points are average ± standard deviation (SD; n = 2–3). Relaxed bands represent single strand breaks; linear bands are double strand breaks.
Fig. 2.
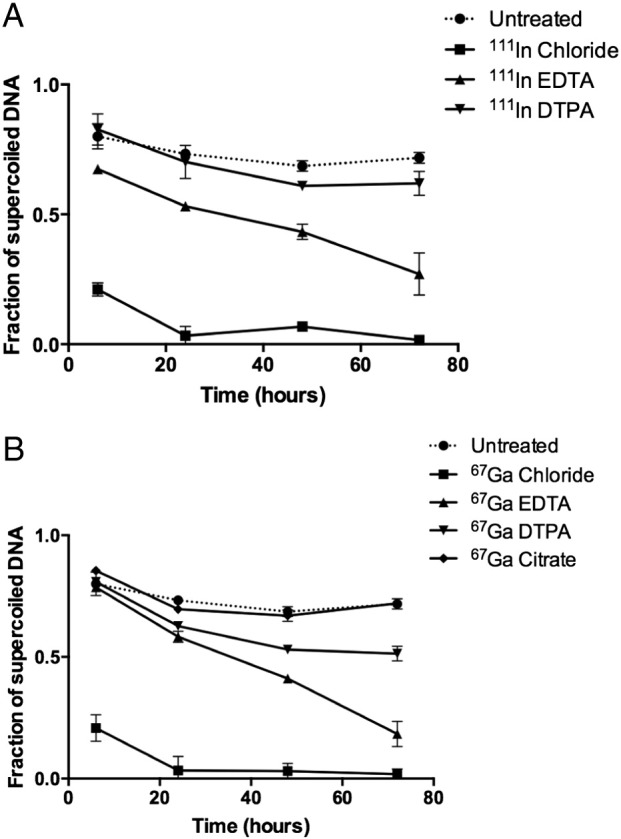
Effect of chelators on plasmid damage treated with 1 MBq 111In-chloride (A) or 67Ga-chloride (B). The amount of supercoiled DNA (undamaged) was measured from gels where plasmid was incubated with the radionuclide in the presence or absence of chelators EDTA, DTPA and citrate for up to 72 h. Data points are average ± SD (n = 2–3).
2.4. 111In- and 67Ga-oxinate complex synthesis
Lipophilic complexes were prepared from 67Ga citrate as described previously [30]. 67Ga citrate (Mallinckrodt Medical Inc., Netherlands; 20–50 MBq, 0.1–1 mL) was added to 50 μg oxine (8-hydroxyquinoline; dissolved in ethanol at 1 mg/mL; Sigma). The solution was extracted into dichloromethane (Sigma, USA) and this fraction separated and evaporated to dryness and reconstituted in 2% ethanol in saline.
Tropolone (2-hydroxy-2,4,6-cycloheptatriene-1-one) (Sigma) was dissolved in ethanol (1 mg/mL). MPO (2-mercaptopyridine-N-oxide) (Sigma) was dissolved in distilled water at 1 mg/mL. 67Ga citrate (Mallinckrodt Medical Inc., Netherlands) was added at 20–50 MBq (0.1–1 mL) to the ligand solution (50 μg tropolone and 500 μg MPO). The resulting solutions were extracted into dichloromethane (Sigma, USA) and measured for labeling yield following drying.
Up to 75 MBq 111In-chloride (0.1–0.2 mL), adjusted to pH 6 with acetate buffer, was added to oxine (1 mg/mL in 2% ethanol) and vortexed for 5 min. 111In-oxine complex was extracted into chloroform, evaporated and reconstituted in 2% ethanol in saline [31].
Radiochemical yield of radiometal complexes was measured in a dose calibrator.
2.5. Cell culture
Human prostate cancer cells DU145, courtesy of Dr. Florian Kampmeier [32], and breast cancer cells HCC1954, were grown in RPMI-1640 at 37 °C in a humidified atmosphere with 5% CO2. Human breast cancer cells MDA-MB-231 were grown in Dulbecco's Modified Eagle Medium (DMEM; with high glucose 4.5 g/L; PAA, Austria). Media were supplemented with L-glutamine (1.5 mM; PAA Laboratories, Austria), 10% fetal bovine serum (Invitrogen) and penicillin (50 I.U./mL)/streptomycin (50 μg/mL) (Invitrogen). Prior to use, cells were trypsinised and washed twice with PBS.
2.6. Cellular uptake and retention of radionuclide oxine complexes
Cells (106) in suspension were incubated with 0.1 MBq 67Ga- or 111In-oxine in 1 mL PBS for 1 h at 37 °C and 5% CO2, then pelleted and washed twice with PBS. Cell-bound (pellet) and free (supernatant) activity was measured by gamma counter.
For cellular retention studies, cells were treated and washed as above and plated in a 6-well plate for three days. At different times, medium was collected, cells washed, and the amount of cell-bound versus free activity measured. The percentage of cell-bound activity retained within the cell at time points after 1 h (set at 100%) was measured.
2.7. Viability assay
Cells (2.5 × 105) were incubated with 67Ga- (2–25 MBq/mL) or 111In-oxine (0.5–30 MBq/mL) in medium (250 μL total) at 37 °C for 1 h. Oxine and ethanol concentrations were 7 μM and 1%, respectively. Controls included 67Ga-citrate and 111In-chloride (no significant uptake in cells) and decayed oxine complexes at levels equivalent to complete decay of 20 MBq/mL samples.
Following incubation, cells were centrifuged, washed and seeded in medium in 6-well plates for 3 days at 37 °C in 5% CO2. Cells were then washed, trypsinised and counted for viability by trypan blue exclusion.
2.8. Clonogenic survival
Cells were treated as in the viability assay, but after treatment and washing, 800–2500 cells were seeded in 6-well plates at 37 °C in 5% CO2 for 10–14 days. Medium was replaced every 3 days. Colonies (>50 cells) were fixed, stained with methanol/1% crystal violet (Sigma, 1:1) and counted. The results were plotted as the surviving percentage relative to untreated values, with the latter set at 100%.
2.9. Statistical analysis
For plasmid studies, data were analyzed by 2-way ANOVA. Student and paired t-tests were used to compare preparations at any one particular time point or the results from the oxine studies, respectively. Statistical analyses were carried out with GraphPad Prism 5 (version 5.04, GraphPad Software Inc., USA).
3. Results
3.1. Cell free plasmid DNA damage by 111In and 67Ga
Incubation of pBR322 supercoiled DNA with 111In and 67Ga (0.1-1 MBq) led to single and double DNA strand breaks, i.e. conversion of supercoiled plasmid to either relaxed or linear plasmid, respectively. Plasmid integrity (i.e. the fraction of plasmid remaining in the supercoiled form) decreased as radioactivity increased; Fig. 1A; Fig. S1). As this activity produced significant damage without assay saturation, it was deemed suitable for comparative studies. DNA damage was detected as early as 4 h post incubation (Fig. 1B–C) and after 24 h of incubation, the supercoiled DNA fractions were reduced to 0.001 ± 0.002 and 0.06 ± 0.01 for 67Ga and 111In respectively (p = 0.002 compared to untreated). In contrast, untreated controls (0.76 to 0.90) and non-radioactive In-chloride (5.8 nM; 0.89 ± 0.002) or Ga-chloride (6.9 nM; 0.86 ± 0.04) controls produced little DNA damage.
Plasmid DNA was partially protected against 111In-induced damage, and less so from 67Ga, by co-incubation with DMSO (Figs. 1 and S2). At 24 h, DMSO co-incubation led to supercoiled fractions of 0.47 ± 0.13 and 0.20 ± 0.04, for 1 MBq 111In and 67Ga, respectively.
External irradiation (i.e. radionuclide separated from the plasmid by the walls of a plastic tube; so that only gamma photons were incident upon the plasmid-containing solution) produced relatively little DNA damage for 67Ga (p > 0.05 at 48 h compared to untreated controls). External 111In produced significantly more DNA damage than untreated controls (p < 0.05 at 48 h), but significantly less than internal 111In-chloride with and without DMSO (p < 0.001 and p < 0.001 at 48 h, respectively).
The addition of chelating agents EDTA, DTPA and citrate provided partial protection of DNA against damage by both radionuclides (Figs. 2, S3). At 72 h, 67Ga-chloride plus EDTA or DTPA (5 mM) gave a supercoiled fraction of 0.18 ± 0.05 or 0.51 ± 0.03, respectively, compared to 0.02 ± 0.02 for 67Ga-chloride only (Fig. 2B). Similarly, 67Ga-citrate produced less DNA damage (supercoiled fraction: 0.72 ± 0.02) than 67Ga-chloride. Incubation with 111In-chloride plus additional EDTA or DTPA led to supercoiled fractions of 0.27 ± 0.08 or 0.62 ± 0.05, respectively, compared to 0.02 ± 0.02 for 111In-chloride alone (Fig. 2A).
3.2. Radionuclide oxine synthesis
Radiolabelling yields for 67Ga-oxine, -tropolone, and -MPO were 92%, 80%, and 25%, respectively, and 98% for 111In-oxine.
3.3. Binding and retention of radionuclide oxine complexes
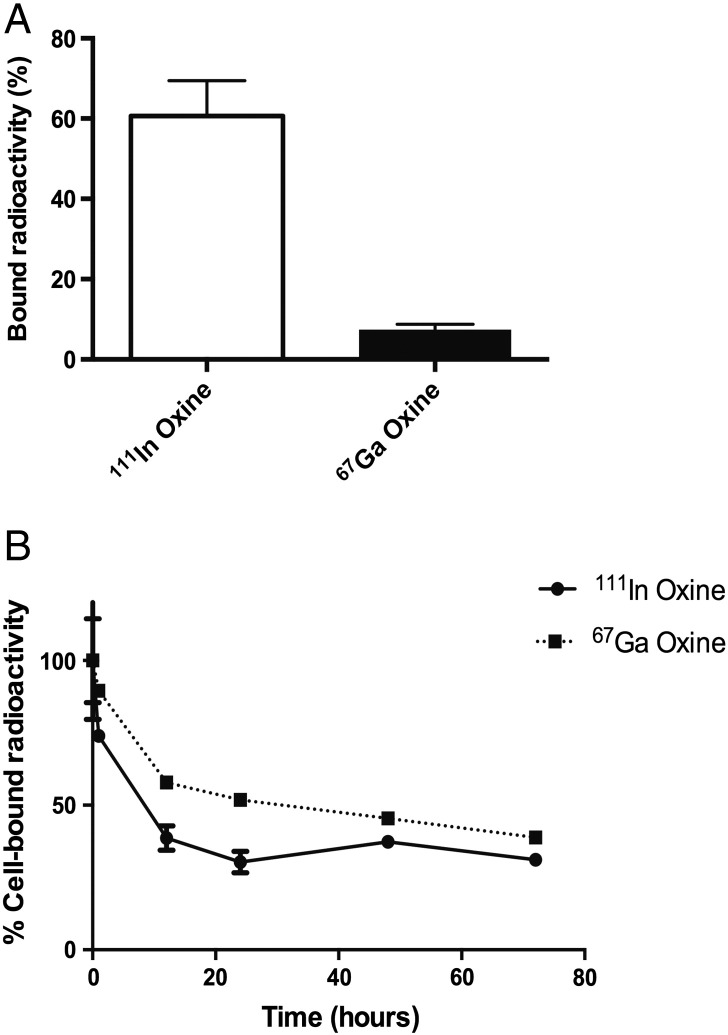
67Ga-oxine gave the highest cell binding (Fig. S4); all subsequent studies were carried out with the oxine complex. In DU145 cells, a one-hour incubation with 111In-oxine or 67Ga-oxine allowed radionuclide binding at 60.6 ± 8.8% or 7.5 ± 1.3%, respectively (Fig. 3A). This decreased with time with only 31.2 ± 1.4% and 38.8% ± 0.7% of the initially-bound 111In and 67Ga, respectively, retained 72 h after a one-hour incubation period (Fig. 3B). Similar results were found in cell lines MDA-MB-231 and HCC1954 (Figs. S5, S6). The different cell labeling efficiencies of 111In-oxine and 67Ga-oxine raise the issue of whether to discuss cellular toxicity in relation to the radioactivity to which the cells are exposed in total (i.e. the radioactivity added to the culture) or the radioactivity that is accumulated in the cells (referred to as “cell-bound” activity henceforth). Both are discussed together in the following paragraphs.
Fig. 3.
Cell binding (A) and retention (B) of 0.1 MBq 111In-oxine and 67Ga-oxine in 106 DU145 cells following an initial one-hour incubation. 100% in panel B refers to the maximum bound radioactivity at one hour (A). Data are average ± SD (n = 3/group).
3.4. Trypan blue viability assay
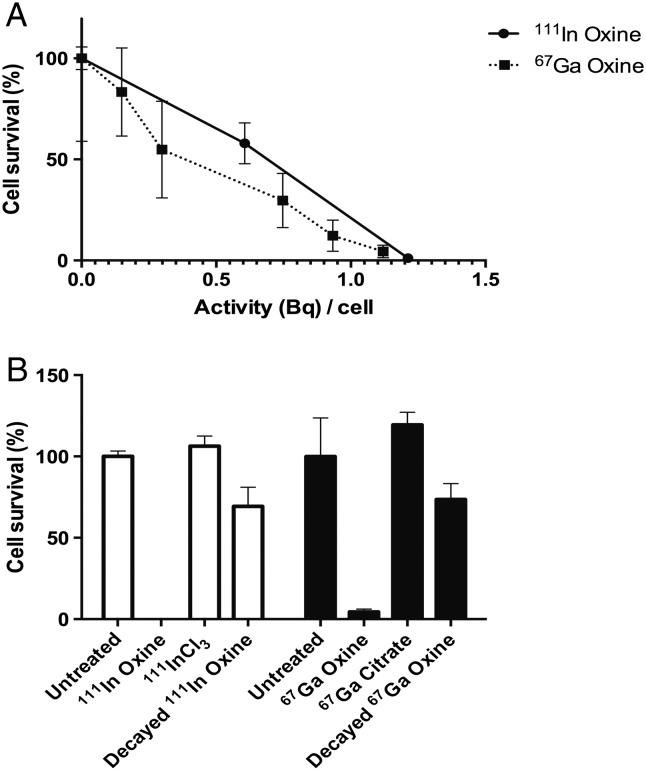
A50 is defined as the cell-bound activity causing 50% reduction in viability relative to untreated cells (100%). Three days after an initial one-hour incubation period with 67Ga-oxine, the A50 was approximately 1 Bq/cell (Table 1, Fig. 4A). Cell-bound 67Ga activity required to reduce viability to 17.4 ± 6.6% was approximately 1.5 Bq/cell; this required incubation with 15 MBq/mL 67Ga-oxine (Fig. 4B). At this concentration, 67Ga-citrate, which was not taken up significantly in cells, caused only 53% loss in viability (Fig. 4B). A similar loss in viability occurred in the control sample incubated with decayed 67Ga-oxine.
Table 1.
Cell-bound activity per cell (Bq/cell) required for a 50% (A50) and 90% (A10) reduction in viability and clonogenicity in DU145 cells compared to untreated cells (determined by interpolation of data in Figs. 4A and 5A).
| A50 (Bq/cell) |
A10 (Bq/cell) |
|||
|---|---|---|---|---|
| 67Ga-oxine | 111In-oxine | 67Ga-oxine | 111In-oxine | |
| Viability (Trypan blue) | 1.0 | 1.0 | 1.5 | N/A |
| Clonogenic | 0.3 | 0.7 | 1.0 | 0.9 |
No A10 value exists for 111In-oxine (viability assay), as loss of membrane integrity was not achieved in more than 75% of cells.
Fig. 4.
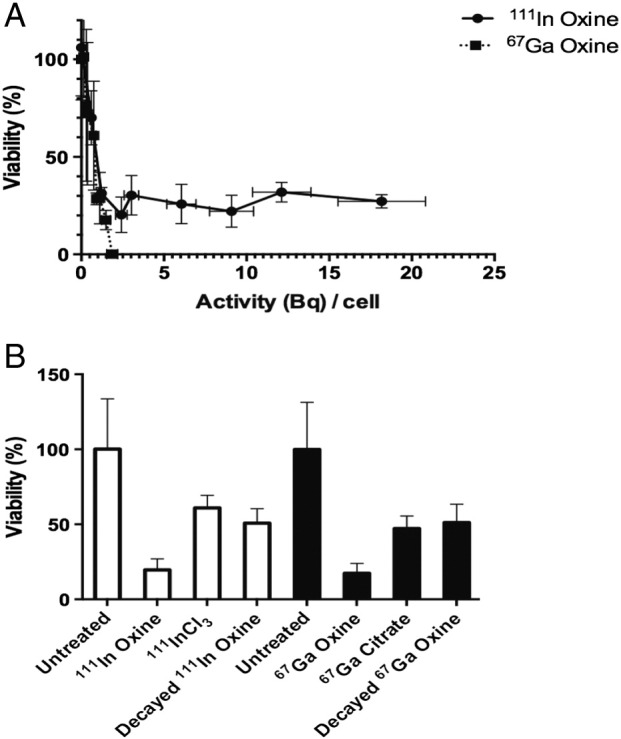
Viability (trypan blue) of DU145 cells treated with 111In-oxine or 67Ga-oxine at 72 h. A: Viability with increasing cell-bound activities (Bq) per cell. B: Controls for radionuclide oxine treatment: untreated cells, non-cell-bound radioactivity and decayed oxine complexes, standardized at 15 MBq/mL, inducing cellular uptake of 9.09 ± 1.33 Bq/cell for 111In-oxine and 1.12 ± 0.20 Bq/cell for 67Ga-oxine groups. Data are average ± SD (n = 3/group).
Qualitatively similar results were obtained with 111In; the A50 was approximately 1 Bq/cell. However, even at cell-bound activities up to 19 Bq/cell, viability did not drop below 20%. As for 67Ga, the controls showed a significant level of toxicity caused by decayed 111In-oxine similar to that caused by 111In-chloride, which did not bind significantly to cells. Interestingly, 67Ga-oxine-induced toxicity at 15 MBq/mL was the same as that caused by the same concentration of 111In-oxine, despite this concentration of 111In-oxine yielding almost 10-fold higher activity per cell. Non-cell bound 111In-chloride caused toxicity (viability around 50%). A similar level of toxicity resulted from the purely chemical effect of decayed 111In-oxine.
Qualitatively similar results for both 67Ga and 111In were found in cell lines MDA-MB-231 and HCC1954 (Figs. S7 and S8 and Table S1).
3.5. Clonogenic survival assay
A one-hour incubation period with 67Ga-oxine (15 MBq/mL) with cellular uptake as little as 1.1 Bq/cell was enough to diminish clonogenic survival to 4.4% ± 3.1% compared to untreated controls (Fig. 5A). Replacing 67Ga-oxine with 67Ga-citrate at this same concentration, with minimal cellular uptake, caused no significant loss in clonogenicity compared to untreated controls (Fig. 5B). Qualitatively similar results were obtained for 111In demonstrating that neither radionuclide affected clonogenicity significantly unless bound to the cell (Fig. 5B). Fully decayed radioactive 67Ga-oxine and 111In-oxine added to the incubation medium led to a significant decrease in relative clonogenic survival (to 74 ± 17% and 69 ± 20% for decayed 67Ga-oxine and 111In-oxine, respectively, see Fig. 5B) compared to untreated controls. However this chemical toxicity was much less than the toxicity of their non-decayed counterparts, indicating that the radioactivity was by far the major contributor to the observed toxic effect. Qualitatively similar results were found in cell lines MDA-MB-231 and HCC1954 (Figs. S9 and S10 and Table S2).
Fig. 5.
Clonogenic assay of DU145 cells treated with 111In-oxine or 67Ga-oxine. A: Clonogenicity with increasing cell-bound activities (Bq) per cell. B: Controls for radionuclide oxine treatment: untreated cells, non-cell-bound radioactivity and decayed oxine complexes, standardized to treatment at 15 MBq/mL, achieving uptake of 9.09 ± 1.33 Bq/cell for 111In-oxine and 1.12 ± 0.20 Bq/cell for 67Ga-oxine groups. Data are average ± SD (n = 3/group). Clonogenicity for 111In-oxine at 0.28 ± 0.48% is not visible on the graph.
4. Discussion
The plasmid data presented here suggest the involvement of different mechanisms of DNA damage. These include ionization and formation of free radicals along the tracks of Auger electrons, local ionization events caused directly by the residual ion after Auger electron emission (short range effects), free radicals diffusing significant distances from the Auger electron track and residual ion, and minor ionization and free radicals caused by gamma photons (long range effects).
DNA damage produced by 67Ga was significantly reduced by chelation with EDTA, DTPA or citrate and incubation with hydroxyl radical scavenger DMSO. The protective effect of chelating the radionuclides with EDTA, DTPA or citrate, on DNA may be a distance effect; assuming unchelated positively-charged In3+ and Ga3+ bind directly to negatively charged DNA, as has previously been shown for 111In [33]. Complexing 67Ga with EDTA, forming a negatively charged 1:1 6-coordinate complex, would completely envelope the 67Ga atom, preventing metal coordination by plasmid DNA [34]. However, 111In forming a 1:1 complex with EDTA would leave some possibility for DNA to coordinate directly to the radiometal which would remain coordinatively unsaturated because of its larger ionic radius [35]. DTPA offers more protection than EDTA against DNA damage by 111In (Fig. 2); this may be because being octadentate it more completely fills the coordination sphere of indium than EDTA does [36]. The degree of protection (EDTA < DTPA < citrate2) is also in line with the negative charge of the resulting complex: (1-, 2-, 5-).
Free radical scavengers such as DMSO are unlikely to protect against the short-range effects. Previous studies with free radical scavengers have focused on 125I, where DMSO reduced strand breakage by 40% if 125I was not bound to DNA. When bound to DNA, damage induced by 125I was not diminished by DMSO [37]. Also, non-plasmid-bound 99mTcO4− caused several fold lower induction of single strand breakage in the presence of DMSO [38], [39].
Overall, direct incubation of radionuclides with plasmid DNA in a cell-free system is useful to understand DNA damage by radionuclide emissions and decay only. However, in these experiments radionuclides can directly bind DNA, thus overestimating the potential damage compared to the cellular environment, where direct binding to DNA is less likely.
Results obtained in cell studies showed that 111In and 67Ga induced high clonogenic toxicity only if incorporated into the cell; external radionuclides and other variables had little effect. Nonetheless decayed radionuclides, producing amongst other compounds zinc and cadmium, did influence both viability and clonogenicity. External irradiation via gamma emissions produced more DNA damage for 111In-chloride than 67Ga-chloride due perhaps to higher gamma ray exposure rate constants. Surprisingly, cell viability was decreased for non-internalized 111In (60.9 ± 8.4%) and 67Ga (47.2 ± 8.4%) compared to untreated cells, while clonogenic toxicity was not. This highlights that they measure different aspects of cytotoxicity and are complementary rather than alternative methods.
The clonogenic toxicity of incorporated radioactivity is similar for the two radionuclides and for all three cell lines, with an A10 of approximately 1 Bq/cell. This similarity should be interpreted cautiously, since the cellular toxicity of Auger electron emitters is likely to be highly dependent on the intracellular distribution of the radionuclides, which we have not determined and which may not be the same for the two radionuclides. Nevertheless this figure may be a useful guide to how much radioactivity must be incorporated into cancer cells in vivo for effective targeted radionuclide therapy (tRT) and could be used to assess feasibility of clinical tRT.
It should be noted that 67Ga-oxine is not a very effective method of incorporating 67Ga into cells. Results were, however, consistent with previous trends, including radiolabeling yields for oxine with 67Ga [30] and cell labeling numbers of 111In-oxine [31] and 67Ga-oxine [40]. Efficient cell labeling with 111In is probably due to 111In-oxine diffusing into the cell cytoplasm and dissociating whereupon 111In binds intracellular macromolecules and is trapped within the cell [31]. In leukocytes, 111In-oxine also partly localizes to the nucleus [41]. If 67Ga-oxine forms a more stable complex [40], the radionuclide may diffuse in but become trapped less readily due to slower dissociation. In order to achieve comparable cellular uptake (Bq/cell), the radioactive concentration of 67Ga-oxine was increased compared to 111In-oxine.
Future studies should focus on targeted approaches as well as in vivo therapeutic studies comparing 67Ga with 111In as well as beta emitters such as 177Lu. Interestingly, the higher energy Auger electrons emitted by 67Ga compared to other Auger electron-emitting radionuclides may provide an advantage by relaxing the requirement for 67Ga to be localized in specific sub-cellular compartments (in particular the nucleus) in order to be effective as a therapeutic. The critical observation that 67Ga (and similarly 111In) has to be cell bound to be effective suggests that future targeting studies can focus on the feasibility in vivo of achieving target uptake of around 1 Bq per cancer cell required for effective cell killing.
5. Conclusion
67Ga damages plasmid DNA in a manner that may be dependent on distance to the DNA, which in turn may be affected by the chemical form of the radionuclide. Neither 67Ga nor 111In showed substantial toxicity unless incorporated into the cells. The threshold cellular uptake of 67Ga to achieve substantial cell kill is of the order of 1 Bq per cell. 67Ga deserves further evaluation for radionuclide therapy, especially in the context of a theranostic pairing with 68Ga.
Acknowledgments
MF bin Othman was funded by the Malaysian Ministry of Education. Research was supported by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under grant number WT 088641/Z/09/Z", the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London, and the King's College London and UCL Comprehensive Cancer Imaging Centre. Funded by the CRUK and EPSRC in association with the MRC and DoH (England). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
This is a free access article and can also be viewed on the journal’s Web site (www.nucmedbio.com). Complimentary access to this article is available until the next issue publishes online.
Supplementary data to this article can be found online at doi:10.1016/j.nucmedbio.2016.10.008.
Appendix A. Supplementary data
Supplementary material
References
- 1.Bartold S.P., Donohoe K.J., Fletcher J.W., Haynie T.P., Henkin R.E., Silberstein E.B. Procedure guideline for gallium scintigraphy in the evaluation of malignant disease. J Nucl Med. 1997;38:990–994. [PubMed] [Google Scholar]
- 2.Biersack H.J., Freeman L.M. Springer Berlin Heidelberg; 2008. Clinical nuclear medicine. [Google Scholar]
- 3.Motohashi H. The relationship between Ga-67 uptake and transferrin receptors in cultured cells. Kanagawa Shigaku. 1990;24:618–629. [PubMed] [Google Scholar]
- 4.Luttropp C.A., Jackson J.A., Jones B.J., Sohn M.H., Lynch R.E., Morton K.A. Uptake of gallium-67 in transfected cells and tumors absent or enriched in the transferrin receptor. J Nucl Med. 1998;39:1405–1411. [PubMed] [Google Scholar]
- 5.Weiner R.E. The mechanism of 67Ga localization in malignant disease. Nucl Med Biol. 1996;23:745–751. doi: 10.1016/0969-8051(96)00119-9. [DOI] [PubMed] [Google Scholar]
- 6.Weiner R.E., Avis I., Neumann R.D., Mulshine J.L. Transferrin dependence of Ga(NO3)3 inhibition of growth in human-derived small cell lung cancer cells. J Cell Biochem Suppl. 1996;24:276–287. doi: 10.1002/jcb.240630523. [DOI] [PubMed] [Google Scholar]
- 7.Howell R.W. Radiation spectra for auger-electron emitting radionuclides: report no. 2 of AAPM nuclear medicine task group no. 6. Med Phys. 1992;19:1371–1383. doi: 10.1118/1.596927. [DOI] [PubMed] [Google Scholar]
- 8.Buchegger F., Perillo-Adamer F., Dupertuis Y.M., Delaloye A.B. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging. 2006;33:1352–1363. doi: 10.1007/s00259-006-0187-2. [DOI] [PubMed] [Google Scholar]
- 9.Jonkhoff A.R., Huijgens P.C., Versteegh R.T., van Dieren E.B., Ossenkoppele G.J., Martens H.J. Gallium-67 radiotoxicity in human U937 lymphoma cells. Br J Cancer. 1993;67:693–700. doi: 10.1038/bjc.1993.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonkhoff A.R., Huijgens P.C., Versteegh R.T., van Lingen A., Ossenkoppele G.J., Drager A.M. Radiotoxicity of 67-gallium on myeloid leukemic blasts. Leuk Res. 1995;19:169–174. doi: 10.1016/0145-2126(94)00130-3. [DOI] [PubMed] [Google Scholar]
- 11.Jonkhoff A.R., Plaizier M.A., Ossenkoppele G.J., Teule G.J., Huijgens P.C. High-dose gallium-67 therapy in patients with relapsed acute leukaemia: a feasibility study. Br J Cancer. 1995;72:1541–1546. doi: 10.1038/bjc.1995.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel R.B., Brechbiel M.W., Mattes M.J. A comparison of 4 radionuclides conjugated to antibodies for single-cell kill. J Nucl Med. 2003;44:632–640. [PubMed] [Google Scholar]
- 13.Ochakovskaya R., Osorio L., Goldenberg D.M., Mattes M.J. Therapy of disseminated B-cell lymphoma xenografts in severe combined immunodeficient mice with an anti-CD74 antibody conjugated with (111)indium, (67)gallium, or (90)yttrium. Clin Cancer Res. 2001;7:1505–1510. [PubMed] [Google Scholar]
- 14.Govindan S.V., Goldenberg D.M., Elsamra S.E., Griffiths G.L., Ong G.L., Brechbiel M.W. Radionuclides linked to a CD74 antibody as therapeutic agents for B-cell lymphoma: comparison of auger electron emitters with beta-particle emitters. J Nucl Med. 2000;41:2089–2097. [PubMed] [Google Scholar]
- 15.Berry D.J., Ma Y., Ballinger J.R., Tavare R., Koers A., Sunassee K. Efficient bifunctional gallium-68 chelators for positron emission tomography: tris(hydroxypyridinone) ligands. Chem Commun (Camb) 2011;47:7068–7070. doi: 10.1039/c1cc12123e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boros E., Ferreira C.L., Yapp D.T., Gill R.K., Price E.W., Adam M.J. RGD conjugates of the H2dedpa scaffold: synthesis, labeling and imaging with 68Ga. Nucl Med Biol. 2012;39:785–794. doi: 10.1016/j.nucmedbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Eisenwiener K.P., Prata M.I., Buschmann I., Zhang H.W., Santos A.C., Wenger S. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjug Chem. 2002;13:530–541. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira C.L., Lamsa E., Woods M., Duan Y., Fernando P., Bensimon C. Evaluation of bifunctional chelates for the development of gallium-based radiopharmaceuticals. Bioconjug Chem. 2010;21:531–536. doi: 10.1021/bc900443a. [DOI] [PubMed] [Google Scholar]
- 19.Knetsch P.A., Zhai C., Rangger C., Blatzer M., Haas H., Kaeopookum P. [(68)Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging alphavbeta3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nucl Med Biol. 2015;42:115–122. doi: 10.1016/j.nucmedbio.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma M.T., Cullinane C., Imberti C., Baguna Torres J., Terry S.Y., Roselt P. New tris(hydroxypyridinone) bifunctional chelators containing isothiocyanate groups provide a versatile platform for rapid one-step labeling and PET imaging with 68Ga3+ Bioconjug Chem. 2016;27:309–318. doi: 10.1021/acs.bioconjchem.5b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Notni J., Pohle K., Wester H.J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. Eur J Nucl Med Mol Imaging Res. 2012;2:28. doi: 10.1186/2191-219X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxboel J., Brandt-Larsen M., Schjoeth-Eskesen C., Myschetzky R., El-Ali H.H., Madsen J. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and (64)Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl Med Biol. 2014;41:259–267. doi: 10.1016/j.nucmedbio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Ramogida C.F., Cawthray J.F., Boros E., Ferreira C.L., Patrick B.O., Adam M.J. H2CHXdedpa and H4CHXoctapa-chiral acyclic chelating ligands for 67/68Ga and 111 In radiopharmaceuticals. Inorg Chem. 2015;54:2017–2031. doi: 10.1021/ic502942a. [DOI] [PubMed] [Google Scholar]
- 24.Simecek J., Notni J., Kapp T.G., Kessler H., Wester H.J. Benefits of NOPO as chelator in gallium-68 peptides, exemplified by preclinical characterization of (68)Ga-NOPO-c(RGDfK) Mol Pharm. 2014;11:1687–1695. doi: 10.1021/mp5000746. [DOI] [PubMed] [Google Scholar]
- 25.Waldron B.P., Parker D., Burchardt C., Yufit D.S., Zimny M., Roesch F. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with gallium-68. Chem Commun. 2013;49:579–581. doi: 10.1039/c2cc37544c. [DOI] [PubMed] [Google Scholar]
- 26.Cornelissen B., Waller A., Target C., Kersemans V., Smart S., Vallis K.A. 111In-BnDTPA-F3: an auger electron-emitting radiotherapeutic agent that targets nucleolin. Eur J Nucl Med Mol Imaging Res. 2012;2:9. doi: 10.1186/2191-219X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornelissen B., Darbar S., Kersemans V., Allen D., Falzone N., Barbeau J. Amplification of DNA damage by a gammaH2AX-targeted radiopharmaceutical. Nucl Med Biol. 2012;39:1142–1151. doi: 10.1016/j.nucmedbio.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Reilly R.M., Kiarash R., Cameron R.G., Porlier N., Sandhu J., Hill R.P. 111In-labeled EGF is selectively radiotoxic to human breast cancer cells overexpressing EGFR. J Nucl Med. 2000;41:429–438. [PubMed] [Google Scholar]
- 29.Ščasnár V., van Lier J. The use of SEP-PAK Sl cartridges for the preparation of gallium chloride from the citrate solution. Eur J Nucl Med Mol Imaging. 1993;20:273. doi: 10.1007/BF00170012. [DOI] [PubMed] [Google Scholar]
- 30.Ballinger J.R., Boxen I. Gallium-67-labelled red blood cells as a blood-pool marker for dual-isotope imaging. Int J Rad Appl Instrum B. 1992;19:79–81. doi: 10.1016/0883-2897(92)90188-5. [DOI] [PubMed] [Google Scholar]
- 31.Thakur M.L., Segal A.W., Louis L., Welch M.J., Hopkins J., Peters T.J. Indium-111-labeled cellular blood components: mechanism of labeling and intracellular location in human neutrophils. J Nucl Med. 1977;18:1022–1026. [PubMed] [Google Scholar]
- 32.Kampmeier F., Williams J., Maher J., Mullen G., Blower P. Design and preclinical evaluation of a 99mTc-labelled diabody of mAb J591 for SPECT imaging of prostate-specific membrane antigen (PSMA) Eur J Nucl Med Mol Imaging Res. 2014;4:13. doi: 10.1186/2191-219X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terry S.Y., Vallis K.A. Relationship between chromatin structure and sensitivity to molecularly targeted auger electron radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83:1298–1305. doi: 10.1016/j.ijrobp.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung W.-S., Chung Y.K., Shin D.M., Kim S.-D. Crystal- and solution-structure characteristics of ethylenediaminetetraacetatoaluminate(III) and gallate(III) Bull Chem Soc Jpn. 2002;75:1263–1267. [Google Scholar]
- 35.Ilyukhin A.B., Malyarik M.A., Petrosyants S.P., Davidovich R.L., Samsonova I.N. Coordination numbers seven and eight in the complexes of triwalent indium with diethylenetriaminepentaacetic acid and crystal structures of Na2[InHdtpa] · (ClO4) · 378H2O, C(NH2)3[InHdtpa] · 2H2O, (CH3)4 N[InHdtpa] · 3H2O and ((NH2)3)2[Indtpa] · 2H2O. Zh Neorg Khim. 1995;40:1125–1136. [Google Scholar]
- 36.Maecke H.R., Riesen A., Ritter W. The molecular structure of indium-DTPA. J Nucl Med. 1989;30:1235–1239. [PubMed] [Google Scholar]
- 37.Balagurumoorthy P., Chen K., Adelstein S.J., Kassis A.I. Auger electron-induced double-strand breaks depend on DNA topology. Radiat Res. 2008;170:70–82. doi: 10.1667/RR1072.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kotzerke J., Punzet R., Runge R., Ferl S., Oehme L., Wunderlich G. 99mTc-labeled HYNIC-DAPI causes plasmid DNA damage with high efficiency. PLoS One. 2014;9:e104653. doi: 10.1371/journal.pone.0104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runge R., Oehme L., Kotzerke J., Freudenberg R. The effect of dimethyl sulfoxide on the induction of DNA strand breaks in plasmid DNA and colony formation of PC Cl3 mammalian cells by alpha-, beta-, and auger electron emitters 223Ra, 188Re, and 99mTc. Eur J Nucl Med Mol Imaging Res. 2016:6. doi: 10.1186/s13550-016-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yano Y., Budinger T.F., Ebbe S.N., Mathis C.A., Singh M., Brennan K.M. Gallium-68 lipophilic complexes for labeling platelets. J Nucl Med. 1985;26:1429–1437. [PubMed] [Google Scholar]
- 41.Puncher M.R., Blower P.J. Frozen section microautoradiography in the study of radionuclide targeting: application to indium-111-oxine-labeled leukocytes. J Nucl Med. 1995;36:499–505. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material