Abstract
Noma is an orofacial gangrene affecting malnourished children and mainly observed in tropical countries, particularly sub-Saharan Africa. Epidemiological data on noma are scarce, but a current estimate of the global incidence is 30,000–40,000 cases per year, with a mortality rate of approximately 85% and a burden of disease calculated to be a loss of 1–10 million disability-adjusted life years. The etiology of noma is multifactorial with malnutrition as an ever present factor, often in combination with concomitant diseases, such as measles, malaria, and human immunodeficiency virus (HIV), and poor oral hygiene. The pathogenesis is a fast-spreading, noncontagious gangrenous infection occurring in the face, often preceded by acute necrotizing gingivitis, and stomatitis. Rare microbiological studies suggest an opportunistic infection caused by an imbalance in normal intraoral microorganisms. Prevention lies in food security, measles vaccination, prevention of malaria and HIV, including the early detection and treatment of necrotizing gingivitis and stomatitis. Early treatment with antibiotics may prevent gangrene or reduce its extent. Late treatment consists of surgical rehabilitation, which is often complex. However, access to medical care is very limited for noma patients due to the extremely poor conditions in which they live that are frequently located in remote rural areas. The authors support the United Nations Human Rights Council Resolution 19/7 adopted on March 22, 2012 “The right to food,” and advocate for the inclusion of noma on the list of neglected tropical diseases to encourage more medical and institutional attention for this often lethal or very mutilating infectious gangrene.
Introduction
Noma is an orofacial gangrene, mainly occurring in malnourished children debilitated by disease and living in the developing world. The mortality of noma is high and the survivors harbor such facial deformities that they are often rejected from society and family life. Although known since antiquity, the epidemiology, pathophysiology, and etiology of the disease remain a subject of debate.1
Medical History
Noma is an old companion of humankind. Described by classical and medieval authors, the disease was common in Europe and the United States for centuries. In 1649, noma was included in the first book about neglected diseases, Observationes Medicae de Affectibus Omissis, by Arnoldus Bootius. By the end of the 19th century, noma gradually disappeared from European and U.S. hospitals due to increasing improvements in the welfare of the population.2 In the rare noma cases that crossed the paths of bacteriologists in the 20th century, no conclusive findings about the causative microorganisms were made until Stewart concluded in 1912 that noma was not a specific infection, but rather an opportunistic one caused by normal oral flora.3 Shortly after the introduction of sulfonamides and penicillin in the 1940s, it appeared that treatment with antimicrobial drugs reduced the mortality of noma from around 85% to approximately 15%.4 By contrast, a clear concept with regards to reconstructive noma surgery was developed only 50 years ago.4
Epidemiology
The majority of reported noma patients are children 2–7 years of age living in sub-Saharan Africa. Noma cases occur primarily in countries with extreme poverty and low measles vaccination rates.5 The true global incidence of noma is unknown with estimates ranging from 30,000 to 140,000 individuals.6,7 However, the authors have good methodological reasons to support the opinion that an estimated incidence of 30,000–40,000 is closer to the actual reality. The lower incidence was based on a comparison of large numbers of noma and cleft lip patients in a referral hospital with a known incidence of cleft lip in that region, followed by simple extrapolation. The high incidence was based on a few interviews with hospital directors in areas where noma was prevalent, probably a rough guess. With a survival rate of 15% and a life expectancy of 40 more years, a global prevalence of 210,000 noma survivors is estimated. Many cases are not discovered due to weak health care systems, lack of knowledge about noma, stigma, and neglect. Children often die without a diagnosis, treatment, or even a record of their death, as the disease primarily affects the poorest children in remote areas where there is no reliable recording of births, diseases or deaths, and where health care workers do not recognize noma. Risk factors for noma are increasing due to rising economic disparities, higher food prices, more hunger and malnutrition, climatic changes, human immunodeficiency virus (HIV)/acquired immune deficiency syndrome, and national and international neglect. The mortality rate from noma is estimated to be 85% as most children receive no treatment. Data on noma incidence in three sub-Saharan countries reveals the contribution of noma to child mortality as ranging from < 0.5–> 3%, while worldwide, 0.5% of child mortality may result from noma.7–10 A common method to quantify the burden of a disease is the measurement of disability-adjusted life years (DALYs). Taking into account premature mortality and disability for surviving noma patients, a recent preliminary study by the Swiss Tropical and Public Health Institute, estimated the burden to be between 1–10 million DALYs.11 This is the first estimate of the global burden from noma and does not include the significant impacts to the household, the stigma, or the psychological effects.
Etiological Considerations
Noma has a multifactorial etiology. A prerequisite is malnutrition, often related to extreme poverty, but other factors are concomitant diseases and poor oral hygiene resulting in gingivitis. Malnutrition is closely correlated with immunity, which may lead to a nutritionally acquired immune deficiency syndrome.12 This condition is particularly prevalent in children who were preterm and low birth weight babies. Depending on the region in Africa, these infants represent up to 25% of births.13 The age of the onset of noma corresponds nutritionally to a period of vulnerability coinciding with the period of weaning, with underweight infants particularly at risk. Importantly, weaning corresponds also to the age of the onset of debilitating and immunosuppressive diseases, such as the first malaria crisis or measles.14
Social factors play a role in the onset of noma, particularly in large families where the mother has grand multiparity and an inadequate nutritional status. The link between the number of past pregnancies and chronic malnutrition in children with noma suggests that it could be related to maternal malnutrition during pregnancy. This may increase the risk for prematurity or low birth weight infants, thus promoting a vicious cycle of repetitive infections and chronic malnutrition and opening the door for noma onset. Various diseases have been described in association with noma, such as malaria, typhus, measles, chickenpox, tuberculosis, and HIV infection.4,15,16 Measles and malaria are the most frequently reported illnesses that precede noma.4,5,17 These diseases often occur during the weaning period and have serious immunosuppressive effects.
Insufficient oral hygiene can facilitate the onset of a necrotizing gingivitis characterized by the presence of some periodontal pathogens and sometimes resulting in the development of noma.18–20
Pathogenesis
Noma is a gangrenous disease affecting young children that rapidly destroys the soft and hard tissues of the face and leads to a massive loss of substance with obvious esthetic prejudice and functional sequelae.1 This destructive process is divided into several stages for clinical reasons.
Clinical Signs Preceding Noma
Noma does not start as a necrotizing process. It is preceded by a small intraoral ulcer, an aphtous lesion or, frequently, by acute necrotizing gingivitis (ANG).20,21 ANG is characterized by spontaneous bleeding, ulceration of the gingival papillae, pain and sometimes, greyish pseudomembranes (Figure 1A ).22,23 In Africa, there is a high prevalence of ANG in children that ranges from 15% to 60%, depending on the region and the degree of poverty.21,24,25 ANG is facilitated by the lack of dental hygiene, but there is also evidence that malnutrition alone can lead to changes in the oral microflora and to ANG.26 In general, ANG can be treated by improving oral hygiene, but if the child is undernourished and dental hygiene with follow-up cannot be achieved, antibiotics are recommended.23,27 Without treatment, ANG may evolve into a necrotizing stomatitis and result in the destruction of the attached gingival mucosa, the surrounding oral mucosa, and the underlying bone (Figure 1B). This stage requires antibiotic treatment. If no treatment is undertaken, the risk of progression toward noma is very high.19
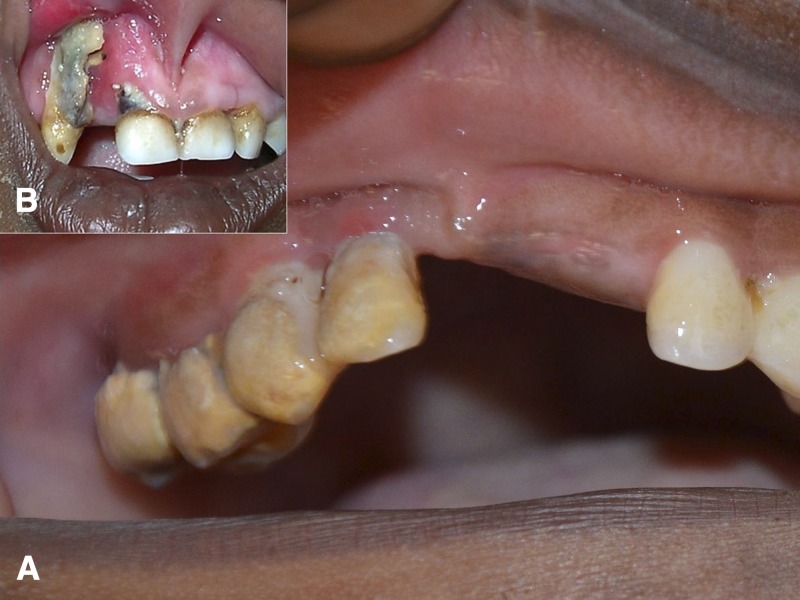
Figure 1.
Acute necrotizing gingivitis (A) and necrotizing stomatitis (B). (A) Acute necrotizing gingivitis (ANG) is considered as an important precursor of noma and is characterized by spontaneous bleeding, ulceration of the papillae, and gingival pain. In this patient, ANG of the upper right dental arch is accompanied by an abundance of calculus and plaque, greyish pseudomembranes and disappearance of gingival papillae. (B) Upper right dental arch: necrotizing stomatitis with destruction of gingival papillae and attached mucosa. Presence of greyish pseudomembranes and exposure of alveolar bone. The lesion shows destruction of gingival mucosa and underlying necrotic bone, a likely precursor of noma.
Acute Noma: Stage of Edema and Halitosis
The starting point of noma is defined when facial edema appears in addition to intraoral necrotizing stomatitis (Figure 2 ), accompanied by a typical halitosis often considered as pathognomonic. This stage is short and lasts only a few days.
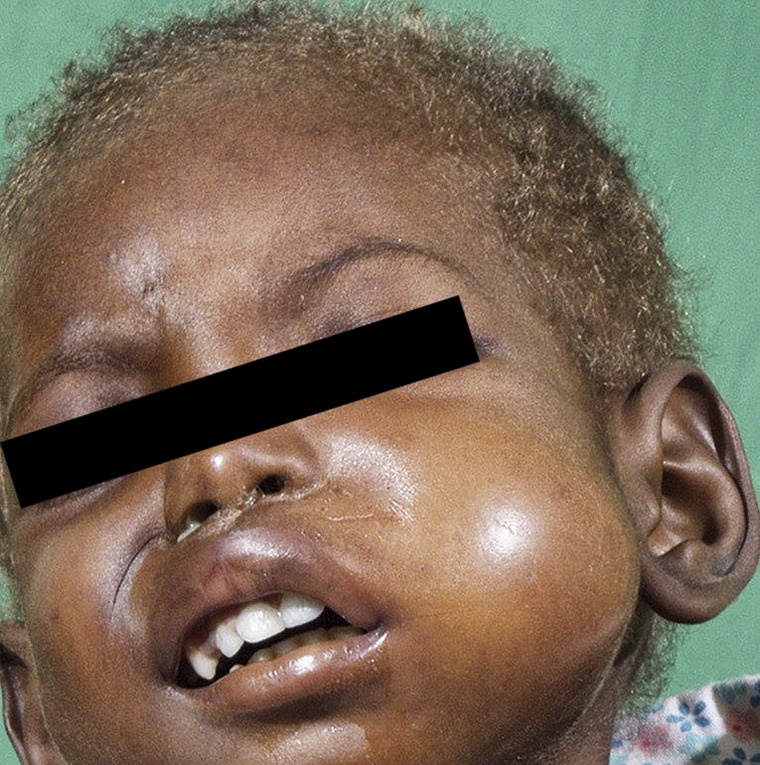
Figure 2.
Acute noma: edema stage in a child with discolored and brittle hair, a secondary sign of malnutrition.
Acute Noma: Stage of Necrosis
Following the appearance of necrotizing stomatitis and facial edema, a necrotizing infection extends rapidly within a few days into the intraoral mucosa, the facial muscles, the skin, the maxilla, and the mandible. A bluish discoloration of the skin indicates underlying necrosis coming to the surface (Figure 3 ). A peculiarity of this gangrene is that it appears to have a self-limiting character ending in a well-demarcated necrosis. On some occasions, the body appears to be able to resist and halt the expansion of the gangrene at a certain stage. Interestingly, some children do not receive treatment and develop relatively small lesions, while others experience huge facial destruction despite appropriate medical treatment. This may be due to differences in the degree of impairment of their immune systems.
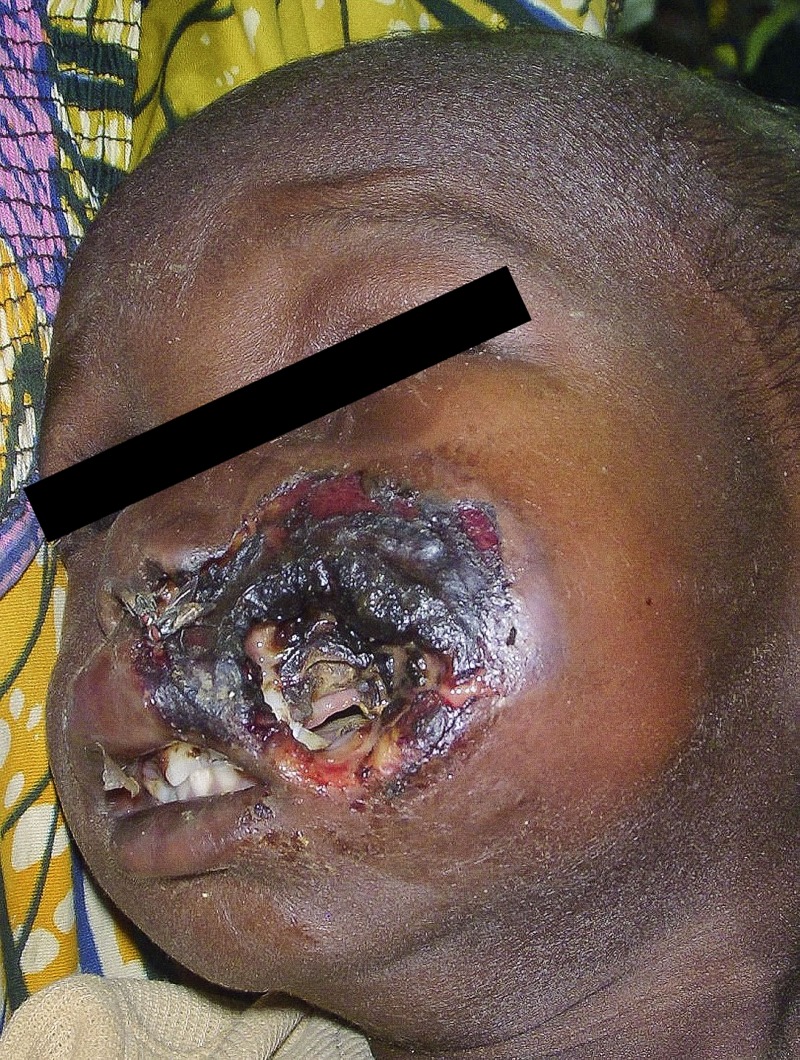
Figure 3.
Acute noma: necrosis stage. Bluish discoloration of the skin is a sign of underlying necrosis and is visible in this patient through a small hole of already sloughed skin.
Acute Noma: Stage of Sloughs and Healing
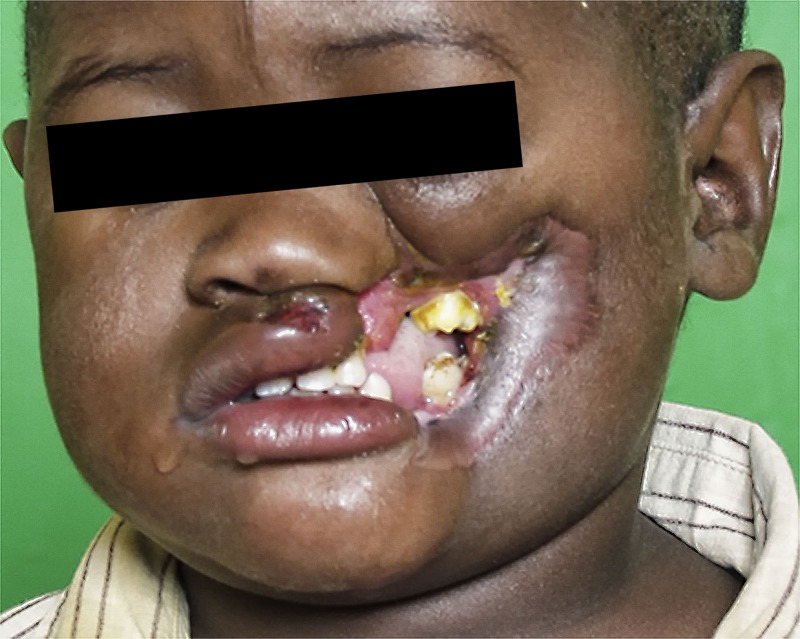
After demarcation of the gangrene, the necrotic tissue starts to slough (Figure 4 ). At this stage, many patients die due to sepsis. If they do survive and the body has eliminated most necrotic tissue by sloughing, suppuration or sequestration, signs of wound healing appear. During this stage, granulation tissue forms, wound contracture occurs, and both mucosa and epithelium start to grow from the margins of the wound over the granulating surface. Depending on the tissue defect and the health of the patient, this healing process may take weeks or many months. The wound healing process may lead to intraoral constricting bands hampering normal mouth opening (trismus) and, in severe cases, to fibrous or even bony ankylosis of the temporomandibular joint. The dramatic result of this condition is the fact that it will impair the nutrition of children who are already undernourished.
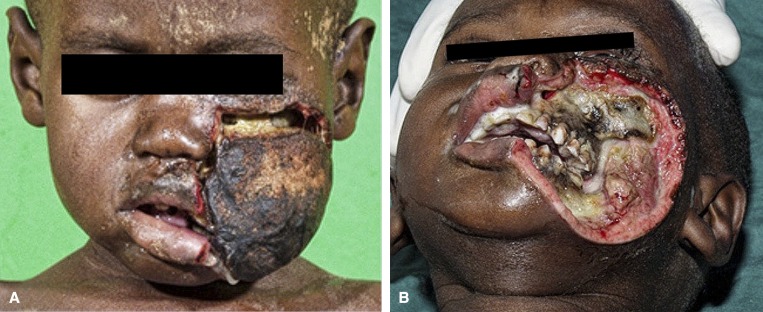
Figure 4.
Acute noma: slough and healing. After this slough (A) the first granulation tissue can be observed at the border of the wound as a sign of wound healing (B). Parts of the maxilla and mandible will sequestrate during the following months.
Noma Sequelae
Only approximately 15% of children survive acute noma.7–9 Most survivors present with facial deformities and trismus or ankylosis of the mandible, resulting in eating problems, oral incontinence, speech difficulties, and social isolation (Figure 5 ). At a later age, these contractures often lead to growth disturbances and result in further facial disfigurement and functional impairment.28 The psychological impact of surviving noma can be easily understood, but it has been rarely studied.29,30
Figure 5.
Noma sequelae. In this patient (same as in Figure 4), the process of healing has resulted in severe wound contracture with ankylosis of the mandible, leading to eating and speech problems, including facial disfigurement.
Microbiology
Bacteria have been assumed to play a role due to the rapidity of the evolution of the disease and to the smell of the lesion itself. Different species have been described, such as Borrelia vincentii and Fusiformis fusiformis.31 The last century saw different schools of thought regarding the etiology of noma. Among the various theories (i.e., the malnutrition and vitamin deficiency theory and the debilitating theory, particularly related to measles), the microbiological theory supposed an interaction between viruses and bacteria with herpesviruses preparing the field for an unknown “noma bacterium” as the trigger organism for the development of the disease.32,35
Recent studies using genomic approaches showed an imbalance in the normal oral flora with an overall loss of bacterial diversity. More precisely, acute noma seems to be characterized by the diminution of Capnocytophaga and Fusobacteria genera and by the increase of Prevotella genus.15,36,37 Indeed, Prevotella intermedia was already reported in previous studies undertaken with classical cultures.35 Prevotella intermedia is a well-known periodontal pathogen in adults, but it has been detected also in the primary dentition of small children.38,39 It is frequently recovered from various oral purulent infections in children and also in secondary nosocomial infections.40,41 Of note, P. intermedia is always associated with other pathogens and it has never been reported as a monoinfecting agent. Therefore, the proliferation of P. intermedia in noma lesions seems to be the consequence of changes in local ecological conditions due to the development of the disease. These findings invalidate the existence of a single noma pathogen and tend to reconsider noma as a multifactorial, opportunistic disease developing in a relatively normal oral flora in children suffering from chronic malnutrition.15
Preventive Measures
Primary prevention of noma can be achieved by economic development, but only if this development ensures that parents can feed their children adequately. Food security programs may be less desirable, but they are effective. However, these preventive measures are political and public health issues. Primary medical prevention of noma consists of measles vaccination programs and the prevention and treatment of concomitant diseases, such as HIV and malaria. Secondary prevention, including the detection and treatment before visible symptoms is difficult and requires surveillance programs in areas of famine and poverty where limited resources are already stretched to the limit by other health priorities.
Tertiary prevention of the disease consists of methods to reduce the negative impact of symptomatic disease, such as disability or death, through treatment and rehabilitation.
Noma is not a common disease, but typically occurs with other neglected diseases and malnutrition. Noma should not be targeted alone, in contrast with vertical programs aiming to eradicate specific health conditions, such as the nearly successful eradication of guinea worm infection. Rather, noma should be included in neglected disease, poverty, malnutrition, and health education programs.
Diagnosis and Treatment
Treatment of acute noma.
A medical history of a recent illness combined with the beginning of facial swelling with a foul-smelling discharge from the mouth of a malnourished child is strongly indicative. There are a few clinical pictures that may have a similar appearance and should be considered, such as Buruli ulcer, mumps or angioedema, a tooth abscess, herpetic stomatitis, or local cellulitis. Of note, it is only in this initial, short, nonspecific phase that treatment with antibiotics has the potential to prevent the development of facial gangrene. The development of facial gangrene a few days later will remove any trace of doubt concerning the diagnosis, but antibiotic treatment is unable to limit the extension of the lesion at that time. Treatment consists of three main elements: antibiotics (amoxicillin and metronidazole); hydration and nutritional support; and treatment of concomitant disease/s and deficiencies to prevent death. The choice of these antibiotics is empirical, and not based on culture results and determination of resistance. These facilities are not present in areas where noma is prevalent. Little is known about the presence of multidrug resistant microorganisms in the extremely poor communities where noma is found. Frequent concomitant diseases are pulmonary and gastrointestinal infections, malaria, and HIV, particularly in southern Africa.15,42–44 Local wound care is mandatory with frequent wound dressing, the simple removal of slough with scissors, and extraction of sequesters. Treatment of acute noma is an issue related to secondary health care.
Surgical treatment of noma sequelae.
At this stage, the differential diagnosis includes leprosy, leishmaniasis, cutaneous tuberculosis, squamous cell carcinoma, cleft lip, yaws and trauma, which may present as facial deformities mimicking noma. Surgical rehabilitation of noma sequelae is directed at the functional and cosmetic improvement of the face. The basic principle is to release the trismus or ankylosis and to replace lost tissue by transferring local tissue flaps and, when the loss is substantial, from other parts of the body. This requires tertiary health care in specialized health care facilities. At present, this is mainly provided by nongovernmental humanitarian organizations or European hospitals.28,45–47 The social impact of surgical rehabilitation is rewarding with better chances for education, employment and marriage after surgery.29,30
Noma: A Neglected Disease and a Violation of Human Rights
Noma remains a neglected disease in tropical medicine, in local health policies and in neglected tropical disease programs. None of the many institutional reports, for example, from the World Health Organization (WHO), has included noma in their estimations of the global burden of diseases, although a recent first and rough estimation of the burden of the disease of noma (1–10 million DALYs) qualifies the disease to be fully recognized as a neglected tropical disease.48 The continued existence of noma is evidence that the most vulnerable children lack the basic right to food. In 2012, the United Nations Human Rights Council Advisory Committee cited children affected by noma as an example of the effects of severe malnutrition and childhood diseases as follows: “Noma, the face of poverty, represents the worst violations of the rights of the child.”49,50
Conclusions
Noma is a noncontagious infectious disease with a relatively high burden of disease and should be part of neglected tropical disease programs. The neglect of noma is a major humanitarian concern that requires the attention of national and international organizations. Noma is a biological indicator of extreme poverty, malnutrition, and human rights violations affecting the most vulnerable children. The vicious cycle of noma neglect begins with WHO and continues without awareness or monitoring, which keeps the disease unknown throughout the world. Therefore, noma should be on the WHO list of Neglected Tropical Diseases. Wherever noma is found, primary health care is needed to improve nutrition, immunizations, exclusive breastfeeding, hygiene, sanitation, and dental care. Global monitoring and research should be supported. The stakeholders, their responsibilities and actions are summarized in Table 1.
Table 1.
Stakeholders responsibilities and actions
| Organization | Responsibility | Actions |
|---|---|---|
| WHO, World Bank, FAO, UNICEF | International priority | Inclusion with neglected diseases and global burden of disease programs |
| Epidemiology and surveillance | Global monitoring system | |
| Interagency cooperation | ||
| Global and tropical disease programs | Education | Include noma |
| Etiological research | Support research | |
| NGOs, donors | Education and awareness raising | Report noma cases and survivors |
| Etiological research | Support primary care prevention | |
| Surgery and rehabilitation | Support etiological research | |
| Support noma survivors for surgery and rehabilitation | ||
| National MoH | National noma action plan | Primary health care and public health |
| Primary health care | Training and education of health workers for early diagnosis and treatment | |
| Appoint noma action person | ||
| Elimination of discrimination of noma survivors | ||
| Improve access to reconstructive surgery |
FAO = Food and Agriculture Organization; NGO = nongovernmental organization; UNICEF = United Nations Children's Fund; WHO = World Health Organization.
If the end of neglect of this preventable childhood disease will lead to the global eradication of noma is a bigger question related to the ever increasing number of human beings on this planet and the concomitant increasing scarcity of resources.
ACKNOWLEDGMENTS
We warmly thank Rosemary Sudan for editorial assistance.
Footnotes
Authors' addresses: M. Leila Srour, Health Frontiers, Vientiane, Laos, E-mail: leila@butterflychildren.org. Klaas Marck, Department of Plastic Surgery, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands, E-mail: k.marck@chello.nl. Denise Baratti-Mayer, Service of Plastic and Reconstructive Surgery, Department of Surgery, Geneva University Hospitals, Geneva, Switzerland, E-mail: gesnoma@bluewin.ch.
References
- 1.Baratti-Mayer D, Pittet B, Montandon D, Bolivar I, Bornand JE, Hugonnet S, Jaquinet A, Schrenzel J, Pittet D, Geneva Study Group on Noma Noma: an “infectious” disease of unknown aetiology. Lancet Infect Dis. 2003;3:419–431. doi: 10.1016/s1473-3099(03)00670-4. [DOI] [PubMed] [Google Scholar]
- 2.Marck KW. A history of noma, the “face of poverty”. Plast Reconstr Surg. 2003;111:1702–1707. doi: 10.1097/01.PRS.0000055445.84307.3C. [DOI] [PubMed] [Google Scholar]
- 3.Stewart MJ. Observations on the histopathology of cancrum oris. J Pathol. 1912;16:221–225. [Google Scholar]
- 4.Tempest MN. Cancrum oris. Br J Surg. 1966;53:949–969. doi: 10.1002/bjs.1800531109. [DOI] [PubMed] [Google Scholar]
- 5.Enwonwu CO, Falkler WA, Jr, Phillips RS. Noma (cancrum oris) Lancet. 2006;368:147–156. doi: 10.1016/S0140-6736(06)69004-1. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Geneva: World Health Organization; 1998. Le noma aujourd'hui. Un problème de santé publique. Rapport sur une consultation d'experts. [Google Scholar]
- 7.Fieger A, Marck KW, Busch R, Schmidt A. An estimation of the incidence of noma in north-west Nigeria. Trop Med Int Health. 2003;8:402–407. doi: 10.1046/j.1365-3156.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- 8.Barmes DE, Enwonwu CO, Leclercq MH, Bourgeois D, Falkler WA. The need for action against oro-facial gangrene (noma) Trop Med Int Health. 1997;2:1111–1114. doi: 10.1046/j.1365-3156.1997.d01-220.x. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois DM, Diallo B, Frieh C, Leclercq MH. Epidemiology of the incidence of oro-facial noma: a study of cases in Dakar, Senegal, 1981–1993. Am J Trop Med Hyg. 1999;61:909–913. doi: 10.4269/ajtmh.1999.61.909. [DOI] [PubMed] [Google Scholar]
- 10.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C, Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;5:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 11.Haesen S, Furst T, Utzinger J. Noma: epidemiology and global burden of a neglected disease. Joint conference of the International Society for Environmental Epidemiology (ISEE), the International Society of Exposure Science (ISES) and the International Society of Indoor Air Quality and Climate (ISIAQ) Basel, Switzerland: 2013. Abstract: 4226, ID: P-1-14-13. [Google Scholar]
- 12.Chandra RK. 1990 McCollum Award lecture. Nutrition and immunity: lessons from the past and new insights into the future. Am J Clin Nutr. 1991;53:1087–1101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- 13.UNICEF Low Birthweight. Country, Regional and Global Estimates. 2004. http://www.unicef.org/publications/files/low_birthweight_from_EY.pdf Available at. Accessed July 24, 2016.
- 14.Enwonwu CO, Phillips RS, Ferrell CD. Temporal relationship between the occurrence of fresh noma and the timing of linear growth retardation in Nigerian children. Trop Med Int Health. 2005;10:65–73. doi: 10.1111/j.1365-3156.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 15.Baratti-Mayer D, Gayet-Ageron A, Hugonnet S, François P, Pittet-Cuenod B, Huyghe A, Bornand JE, Gervaix A, Montandon D, Schrenzel J, Mombelli A, Pittet D, Geneva Study Group on Noma (GESNOMA) Risk factors for noma disease: a 6-year, prospective, matched case-control study in Niger. Lancet Glob Health. 2013;1:e87–e96. doi: 10.1016/S2214-109X(13)70015-9. [DOI] [PubMed] [Google Scholar]
- 16.Chidzonga MM, Mahomva L. Noma (cancrum oris) in human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV and AIDS): clinical experience in Zimbabwe. J Oral Maxillofac Surg. 2008;66:475–485. doi: 10.1016/j.joms.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein A. Noma. Am J Dis Child. 1940;59:219–237. [Google Scholar]
- 18.Sheiham A. The epidemiology of chronic periodontal disease in western Nigerian schoolchildren. J Periodontal Res. 1968;3:257–267. doi: 10.1111/j.1600-0765.1968.tb01928.x. [DOI] [PubMed] [Google Scholar]
- 19.Taiwo JO. Oral hygiene status and necrotizing ulcerative gingivitis in Nigerian children. J Periodontol. 1993;64:1071–1074. doi: 10.1902/jop.1993.64.11.1071. [DOI] [PubMed] [Google Scholar]
- 20.Horning GM. Necrotizing gingivostomatitis: NUG to noma. Compend Contin Educ Dent. 1996;17:951–954. 6, 7–8 passim; quiz 64. [PubMed] [Google Scholar]
- 21.Idigbe EO, Enwonwu CO, Falkler WA, Ibrahim MM, Onwujekwe D, Afolabi BM, Savage KO, Meeks VI. Living conditions of children at risk for noma: Nigerian experience. Oral Dis. 1999;5:156–162. doi: 10.1111/j.1601-0825.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 22.Giddon DB, Zackin SJ, Goldhaber P. Acute necrotizing ulcerative gingivitis in college students. J Am Dent Assoc. 1964;68:380–386. [PubMed] [Google Scholar]
- 23.Goldhaber P, Giddon DB. Present concepts concerning the etiology and treatment of acute necrotizing ulcerative gingivitis. Int Dent J. 1963;14:468–496. [Google Scholar]
- 24.Malberger E. Acute infectious oral necrosis among young children in the Gambia, West-Africa. J Periodontal Res. 1967;2:154–162. doi: 10.1111/j.1600-0765.1967.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 25.Enwonwu CO. Epidemiological and biochemical studies of necrotizing ulcerative gingivitis and noma (cancrum oris) in Nigerian children. Arch Oral Biol. 1972;17:1357–1371. doi: 10.1016/0003-9969(72)90169-0. [DOI] [PubMed] [Google Scholar]
- 26.Sawyer DR, Nwoku AL, Rotimi VO. Comparison of oral microflora between well-nourished and malnourished Nigerian children. ASDC J Dent Child. 1986;53:439–443. [PubMed] [Google Scholar]
- 27.Holmstrup PWJ. Necrotizing Periodontal Disease. In: Lindhe J, Lange NP, Karring T, editors. Clinical Periodontology and Implant Dentistry. 3rd edition. Oxford, United Kingdom: Blackwell; 1997. pp. 258–278. [Google Scholar]
- 28.Pittet B, Rüegg E, Baratti-Mayer D, Jaquinet A. EMC: Techniques chirurgicales: Chirurgie Plastique, Reconstructrice et Esthétique. Paris: Elsevier-Masson; 2015. Le traitement chirurgical des séquelles de noma; pp. 1–18. [Google Scholar]
- 29.Leuzinger S. Suivi social des enfants atteints de noma ayant été transférés en Suisse en vue d'une prise en charge chirurgicale. University of Geneva thesis, Geneva: Switzerland; 2011. [Google Scholar]
- 30.Lafferty NE. Liverpool: 2012. Changing the face of Africa. School of Tropical Medicine 2012 Thesis. [Google Scholar]
- 31.Emslie RD. Cancrum oris. Dent Pract. 1963;13:481–495. [Google Scholar]
- 32.Enwonwu CO. Questions and answers on orofacial gangrene (NOMA)/Summary of notes from NOMA symposium at IADR98. Nice, France: International Association of Dental Research; 1998. [Google Scholar]
- 33.Contreras A, Falkler WA, Jr, Enwonwu CO, Idigbe EO, Savage KO, Afolabi MB, Onwujekwe D, Rams TE, Slots J. Human Herpesviridae in acute necrotizing ulcerative gingivitis in children in Nigeria. Oral Microbiol Immunol. 1997;12:259–265. doi: 10.1111/j.1399-302x.1997.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 34.Falkler WA, Jr, Enwonwu CO, Idigbe EO. Microbiological understandings and mysteries of noma (cancrum oris) Oral Dis. 1999;5:150–155. doi: 10.1111/j.1601-0825.1999.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 35.Falkler WA, Jr, Enwonwu CO, Idigbe EO. Isolation of Fusobacterium necrophorum from cancrum oris (noma) Am J Trop Med Hyg. 1999;60:150–156. doi: 10.4269/ajtmh.1999.60.150. [DOI] [PubMed] [Google Scholar]
- 36.Bolivar I, Whiteson K, Stadelmann B, Baratti-Mayer D, Gizard Y, Mombelli A, Pittet D, Schrenzel J, Geneva Study Group on Noma (GESNOMA) Bacterial diversity in oral samples of children in niger with acute noma, acute necrotizing gingivitis, and healthy controls. PLoS Negl Trop Dis. 2012;6:e1556. doi: 10.1371/journal.pntd.0001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huyghe A, Francois P, Mombelli A, Tangomo M, Girard M, Baratti-Mayer D, Bolivar I, Pittet D, Schrenzel J, Geneva Study Group on Noma Microarray analysis of microbiota of gingival lesions in noma patients. PLoS Negl Trop Dis. 2013;7:e2453. doi: 10.1371/journal.pntd.0002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamma JJ, Diamanti-Kipioti A, Nakou M, Mitsis FJ. Profile of subgingival microbiota in children with primary dentition. J Periodontal Res. 2000;35:33–41. doi: 10.1034/j.1600-0765.2000.035001033.x. [DOI] [PubMed] [Google Scholar]
- 39.Kononen E. Development of oral bacterial flora in young children. Ann Med. 2000;32:107–112. doi: 10.3109/07853890009011759. [DOI] [PubMed] [Google Scholar]
- 40.Brook I. Aerobic and anaerobic microbiology of infections after trauma in children. J Accid Emerg Med. 1998;15:162–167. doi: 10.1136/emj.15.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brook I. Microbiology of nosocomial sinusitis in mechanically ventilated children. Arch Otolaryngol Head Neck Surg. 1998;124:35–38. doi: 10.1001/archotol.124.1.35. [DOI] [PubMed] [Google Scholar]
- 42.Nath S, Jovic G. Cancrum oris: management, incidence, and implications of human immunodeficiency virus in Zambia. Plast Reconstr Surg. 1998;102:350–357. doi: 10.1097/00006534-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Naidoo S, Chikte UM. Noma (cancrum oris): case report in a 4-year-old HIV-positive South African child. SADJ. 2000;55:683–686. [PubMed] [Google Scholar]
- 44.Rowe D, McKerrow N, Uys A, Winstanley T. Cancrum oris (noma) in a malnourished HIV-child from rural Kwazulu-Natal. South Afr J HIV Med. 2004;8:45–46. [Google Scholar]
- 45.Pittet B, Mahajan AL, Alizadeh N, Schlaudraff KU, Fasel J, Montandon D. The free serratus anterior flap and its cutaneous component for reconstruction of the face: a series of 27 cases. Plast Reconstr Surg. 2006;117:1277–1288. doi: 10.1097/01.prs.0000208297.02556.a5. [DOI] [PubMed] [Google Scholar]
- 46.Bouman MA, Marck KW, Griep JE, Marck RE, Huijing MA, Werker PM. Early outcome of noma surgery. J Plast Reconstr Aesthet Surg. 2010;63:2052–2056. doi: 10.1016/j.bjps.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Bos K, Marck K. The Surgical Treatment of Noma. Alphen aan den Rijn, the Netherlands: Uitgeverij Belvédére/Medidact; 2006. [Google Scholar]
- 48.Srour ML, Marck KW, Baratti-Mayer D. Noma: neglected, forgotten and a human right issue. Int Health. 2015;7:149–150. doi: 10.1093/inthealth/ihv001. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler J. Preliminary Study on Severe Malnutrition and Childhood Diseases with Children Affected by Noma as an Example. 2011. http://righttofood.org/wp-content/uploads/2012/09/A-HRC-AC-7-CRP-2.pdf Available at. Accessed December 28, 2016.
- 50.Ziegler J. Noma—the Devastating Disease. http://www.righttofood.org/work-of-jean-ziegler-at-the-un/noma/ Available at. Accessed December 28, 2016.