Abstract
Coinfection with visceral leishmaniasis (VL) and human immunodeficiency virus (HIV) leads to frequent treatment failure, relapse, and death. In this retrospective analysis from eastern India (2005–2015), our primary objective was to ascertain the protective efficacy of secondary prophylaxis with monthly amphotericin B (AmB) given in patients with HIV–VL coinfection toward reducing relapse and mortality rates. The secondary objective was to compare clinical features, laboratory findings, and treatment outcomes in HIV–VL patients in contrast to VL monoinfection. Overall, 53 cases of HIV–VL and 460 cases of VL monoinfection were identified after excluding incomplete records. Initial cure rate was 96.23% in HIV–VL (27 received liposomal AmB and 26 AmB deoxycholate). All patients with initial cure (N = 51) were given antiretroviral therapy. Secondary prophylaxis (N = 27) was provided with monthly 1 mg/kg AmB (15 liposomal, 12 deoxycholate). No relapse or death was noted within 6 months in the secondary prophylaxis group (relapse: none versus 18/24 [75%]; mortality: none versus 11/24 [45.8%]; P < 0.001 for both). Secondary prophylaxis remained the sole significant predictor against death in multivariate Cox regression model (hazard ratio = 0.09 [95% confidence interval = 0.03–0.31]; P < 0.001). HIV–VL patients had higher 6-month relapse rate, less relapse-free 12-month survival, and higher mortality (P < 0.001 each) than VL monoinfection. In conclusion, it appears from this study that secondary prophylaxis with monthly AmB might be effective in preventing relapse and mortality in HIV–VL.
Introduction
Visceral leishmaniasis (VL) is endemic in India, a country that also contributes maximally to the global burden of the disease.1–3 India also ranks third as a country with gross number of people living with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), harboring almost 6% of the world population of HIV-infected people, with a prevalence of HIV around 0.27%.4,5
The problem of HIV and VL (HIV–VL) coinfection is recently being recognized as a major hurdle for disease control with significant public health implications: Leishmania has evolved strategies to survive and multiply within macrophages in HIV patients,6 engendering the possibility of drug resistance and treatment failure.7,8 The risk of developing VL is 100–2,300 times higher in HIV-infected patients than in HIV-negative individuals.9,10 Though randomized controlled trials (RCTs) are yet to be available in the arena of HIV–VL, it is generally accepted that worse outcome, higher relapse rate, mortality, drug toxicity, and resistance are common in this group of patients.10,11 Lack of RCT and high-quality evidence from well-designed studies in the field of HIV–VL coinfection makes treatment decisions and prognostications difficult.
In this retrospective analysis from eastern India (2005–2015), our primary objective was to ascertain the protective efficacy of secondary prophylaxis with monthly amphotericin B (AmB) given in patients with HIV–VL toward reducing relapse and mortality rate. The secondary objective was to compare clinical features, laboratory findings, and initial treatment outcomes in HIV–VL in comparison with VL monoinfection.
Materials and Methods
This retrospective observational study was conducted at the School of Tropical Medicine (STM), Kolkata, India, a tertiary care referral center for both VL and HIV cases for over 20 years. All patients admitted at the STM from January 2005 to February 2015 with VL were retrospectively included. Investigators' clinical records of patients managed under their care at inpatient and outpatient departments were analyzed anonymously. Hospital records of routinely collected data generated during inpatient management were analyzed with permission from the appropriate authorities. The hospital records that are stored as hard copies in the records department under supervision of the registrar of STM were sorted yearwise. Case records which showed an admission linked to the diagnosis or treatment of VL were extracted. These were further sorted based on availability of clear mention of the components of “case definition” for VL (vide infra). Approval of the Clinical Research Ethics Committee (CREC) of the STM was sought and obtained to undertake this study (CREC approval no. CREC-STM/307, date: January 9, 2016).
Diagnosis of Visceral Leishmaniasis.
Case definition of VL (all three were needed for inclusion)
-
1.
Symptoms and signs suggestive of VL (fever for more than 2 weeks and splenomegaly, from an endemic area)
-
2.
rK39 immunochromatographic strip test (ICT) (Kalazar Detect™; InBios International Inc., Seattle, WA) positivity
-
3.
Demonstrable amastigotes of Leishmania parasites (Leishman-Donovan bodies) in splenic or bone marrow aspirates.
Method of HIV diagnosis.
HIV testing at STM was performed with three parallel rapid diagnostic tests (CombAIDS RS Advantage ST, Span Diagnostics Pvt. Ltd., Udhna, Surat, Gujarat, India; Tri-Line [Pareekshak], Bhat Biotech Pvt. Ltd., Bangalore, Karnataka, India; and SD Bioline, Bio Standard Diagnostics Pvt. Ltd., Manesar, Gurgaon, Haryana, India) with positive results in all three required for diagnosis of HIV positivity. Discordant results were confirmed with Western Blot. Only records that clearly indicated HIV testing status were included for the study. HIV testing is undertaken on the basis of provider-initiated counseling and testing or direct walk-in client policy in our hospital.
Treatment and follow-up.
The initial therapy for both VL monoinfection and HIV–VL coinfection were recorded. Primary outcome was the rate of VL relapses within 6 months of treatment. Secondary outcome measures were relapse-free 12-month survival and mortality during follow-up. Initial cure was defined as clinical and parasitological cure within 30 days of treatment initiation. Clinical cure was defined as absence of fever and any other constitutional symptoms along with regression of splenomegaly (≥ 50%) and improvement of hematological parameters (hemoglobin ≥ 10 gm/dL) within 30 days of follow-up. To assess parasitological cure, splenic aspirate (or bone marrow aspirate in whom spleen was not palpable) was performed on or before day 30 after therapy initiation. Relapse was defined as reappearance of signs or symptoms suggestive of leishmaniasis after initial cure, followed by identification of LD bodies in splenic aspirate (or bone marrow aspirate if spleen is not palpable or less than 5 cm palpable) within or after 6 months. Relapse-free 12-month survival was defined as hospital records showing regular 12 months of follow-up without any suggestion of relapse or requirement of retreatment for VL after initial cure. Treatments offered for relapses of VL and deaths due to VL at STM after initial cure were noted.
We usually assess suspected and confirmed VL cases with serum and urine rK39 ICTs. All hospital records with rK39 positivity were noted and analyzed.
Secondary prophylaxis.
Records of HIV–VL patients who were offered secondary prophylaxis were documented in terms of drugs and doses used and the duration of secondary prophylaxis.
Exclusion criteria.
The records with following deficits were excluded: any record not fulfilling any one of the three criteria for diagnosis of VL, records which did not clearly indicate whether testing for HIV was undertaken, records which did not provide data regarding CD4 cell count at baseline or at 6 months of follow-up of HIV–VL cases, and records of those patients lost to follow-up within 12 months of initial cure. Those patients who were lost to follow-up or could not be contacted through telephone were also excluded.
The following data were recorded: age, gender, occurrence, and number of relapses of VL previously; clinical symptoms and signs pertaining to VL; HIV-positive or negative status, tuberculosis coinfection at any point of time after initial entry in STM records; hemoglobin (gm/dL), total leukocyte count (/μL), and platelet count (/μL) at baseline only; CD4 cell count (/μL) at baseline and at 6 months of follow-up after initial cure in HIV–VL coinfected patients; initial cure rate, relapses within first 6 months of follow-up after initial cure, and relapse-free 12-month survival after initial cure, and mortality rate.
Statistical methods.
Continuous data were presented as mean ± SD and categorical data as number (proportion). Comparison of means was tested with Student's t test or Mann–Whitney U test and comparison of proportions were undertaken with χ2 test or Fisher's exact test, as was deemed appropriate.
Three separate analyses were done. First was comparison of clinical features, diagnostic and treatment outcome of patients with VL monoinfections versus those with HIV–VL coinfections. Second was treatment outcome of patients with HIV–VL coinfection within 12 months of follow-up after initial cure. And the last was survival analysis of patients with HIV–VL with death as dependent variable. In survival analysis, variables associated with increased mortality from univariate analysis with P < 0.2 were selected for entry into multivariate Cox regression model, taking into account effects of several potential factors simultaneously and then hazard ratios (HRs) were obtained. All statistical analyses were done using SPSS, version 16 (SPSS Inc., Chicago, IL).
Results
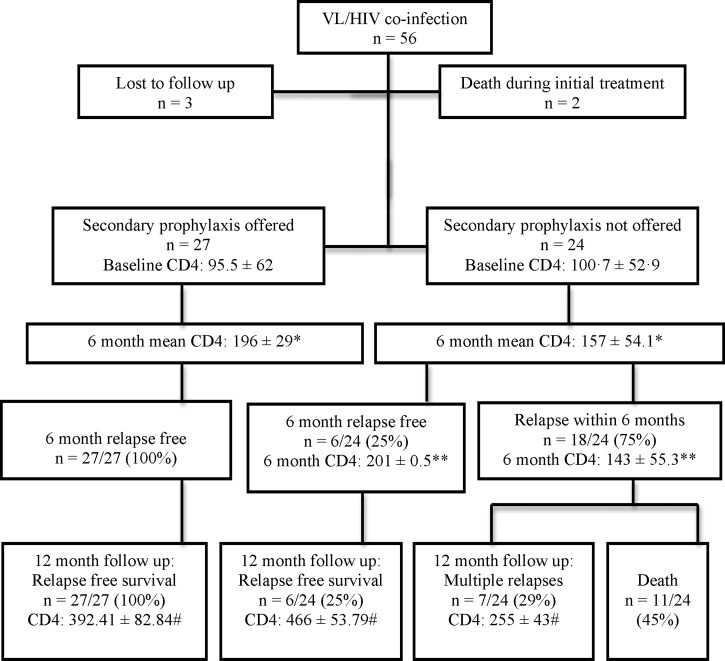
Records of 53 patients with HIV–VL coinfection were included (Figure 1 ) with 41 males (77%, 95% confidence interval [CI] = 64.5–86.6) and mean age 34 ± 8.8 years (median = 34 years, interquartile range = 12 years). Mean CD4 count at presentation was 98.1 ± 58.6/μL. Among these patients, 22 (41%, 95% CI = 29.3–54.9) had prior documented VL infections. Patients who presented with relapses had a mean of 1.5 relapses of VL before the diagnosis of HIV.
Figure 1.
Baseline and follow-up data of HIV–VL coinfected patients. * Comparison of mean CD4 counts at 6 months between the groups of patients who did or did not receive secondary prophylaxis; P value (Mann–Whitney U test) = 0.015. ** Comparison of mean CD4 counts at 6 months within the group of patients who did not receive secondary prophylaxis, between the patients who did or did not experience relapses within the first 6 months of follow-up; P value (Student's t test) of = 0.007. # Comparison of 12-month mean CD4 counts among the surviving patients; P value (analysis of variance) ≤ 0.001, Tukey's post hoc all P values are < 0.001 except post hoc comparison between the patients who received secondary prophylaxis vs. patients with a 12-month relapse-free survival despite not having secondary prophylaxis (P = 0.085). HIV–VL = human immunodeficiency virus–visceral leishmaniasis.
Clinical features of HIV–VL coinfection.
The clinical features of HIV–VL coinfection were fever (100%), weight loss (98%), pallor (75%), hepatomegaly (91%), and splenomegaly (100%). Fifteen (28.30%) patients with HIV–VL presented with atypical clinical features—gastrointestinal in seven (13.2%), respiratory in six (11.3%), and bleeding in two (3.8%) (Table 1).
Table 1.
Distribution of typical and atypical clinical symptoms and signs in HIV–VL coinfected patients
| Symptoms and signs | N (%) | 95% confidence interval | |
|---|---|---|---|
| Fever | 53 (100) | 93.24–100 | |
| Weight loss | 52 (98) | 90.06–99.67 | |
| Pallor | 40 (75) | 62.43–85.07 | |
| Hepatomegaly | 48 (91) | 79.75–95.9 | |
| Splenomegaly | 53 (100) | 93.24–100 | |
| Atypical features | 15 (28) | 17.97–41.57 | |
| Distribution of atypical features | Diarrhea | 7 (13) | 6.55–24.84 |
| Cough | 6 (11) | 5.29–22.58 | |
| Shortness of breath | 2 (4) | 1.04–12.75 | |
| Menorrhagia | 2 (4) | 1.04–12.75 | |
| Per-rectal bleeding | 1 (2) | 0.33–9.94 | |
HIV–VL = human immunodeficiency virus–visceral leishmaniasis.
Comparison of clinical features, laboratory diagnostics, and treatment outcomes between patients with VL monoinfection and HIV–VL coinfection.
We accessed 460 VL-monoinfected patients' case records with complete information. The mean age was 28 ± 14 years with 225 males (48%, 95% CI = 44.4–53.5). Patients with HIV–VL had significantly lower level of hemoglobin compared with monoinfected patients. When tested with serum samples, rK39 ICTs were positive in 50 (94.3%, 95% CI = 84.6–98.1) coinfected compared with 454 (98.7%, 95% CI = 97.2–99.4) monoinfected VL patients, with trend toward lower serum sample positivity in HIV–VL (P = 0.056). However, results of rK39 ICT positivity with urine samples in HIV–VL coinfected patients were similar to monoinfected patients (98.1%, 95% CI = 90.0–99.7 versus 99.4%, 95% CI = 98.1–99.8; P = 0.33). Initial cure rates were comparable in both groups (HIV–VL versus VL, respectively, 51/53 [96.2%, 95% CI = 87.3–98.9] versus 456/460 [99.1%, 95% CI = 97.8–99.7], P = 0.12) with same regimen—AmB deoxycholate (1 mg/kg for alternate days up to a total dose of 20 mg/kg) (HIV–VL: 26 patients, VL monoinfection: 335 patients) or liposomal AmB (7.5 mg/kg on two consecutive days for a total 15 mg/kg),12 (HIV–VL: 27 patients, VL monoinfection: 125 patients). Coinfected patients with initial cure had significantly more relapses within first 6 months of follow-up (18/51 [35.3%, 95% CI = 3.6–49] versus 30/456 [6.6%, 95% CI = 4.7–9.2], respectively, P < 0.001) and significantly less relapse-free 12-month survival (33/51 [64.7%, 95% CI = 50.9–76.4] versus 418/456 [91.7%, 95% CI = 88.8–93.9], respectively, P < 0.001). Mortality rate of patients with HIV–VL coinfection (11/51, 21.6%, 95% CI = 12.5–34.6) was significantly higher than VL monoinfected patients (2/456, 0.4%, 95% CI = 0.12–1.6) with P < 0.001 (Table 2).
Table 2.
Comparison of demographics, clinical, diagnostic, and therapy-related results in patients with HIV–VL vs. VL monoinfection
| Variables at baseline | Parameters | VL | HIV–VL | P value |
|---|---|---|---|---|
| N = 460 | N = 53 | |||
| Age in years | Mean ± SD | 28 ± 14 | 34 ± 8 | 0.67 |
| Male gender | N (%) | 225 (48.9) | 41 (77.4) | < 0.001 |
| Liver palpable below right costal margin (in cm) | Mean ± SD | 3.4 ± 2.1 | 3.9 ± 1.8 | 0.41 |
| Spleen palpable below left costal margin (in cm) | Mean ± SD | 9.4 ± 3.9 | 9.7 ± 3.3 | 0.37 |
| Hemoglobin at baseline (gm/dL) | Mean ± SD | 7.8 ± 1.4 | 7.5 ± 1.7 | 0.01 |
| Total leukocyte count (/μL) | Mean ± SD | 2,457 ± 1,905 | 2,722 ± 1,486 | 0.11 |
| Platelet count at baseline (/μL) | Mean ± SD | 1.3 ± 0.5 | 1.3 ± 0.7 | 0.71 |
| rK39 positivity in serum | N (%) | 454 (98.7) | 50 (94.3) | 0.056* |
| rK39 positivity in urine | N (%) | 457 (99.3) | 52 (98.1) | 0.33* |
| Initial cure rate | N (%) | 456 (99.1) | 51 (96.2) | 0.12 |
| After initial cure is achieved | ||||
| Variables after initial cure | Parameters | VL | HIV–VL | P value |
| N = 456 | N = 51 | |||
| One or more relapses of VL within the first 6 months of follow-up | N (%) | 30 (6.5) | 18 (34) | < 0.001 |
| Relapse-free survival at 12 months | N (%) | 418 (90.9) | 33 (64.7) | < 0.001 |
| Mortality | N (%) | 2 (0.4) | 11 (21.6) | < 0.001 |
HIV–VL = human immunodeficiency virus–visceral leishmaniasis; SD = standard deviation.
Comparison of proportions done with Fisher's exact test.
Treatment and secondary prophylaxis in patients with HIV–VL coinfection (Figure 1).
Coinfected patients were treated with AmB deoxycholate or liposomal AmB. Two patients who presented with two previous relapses did not respond to initial treatment with AmB deoxycholate and died in hospital. All patients were given antiretroviral treatment (ART) (fixed drug combination containing tenofovir 300 mg + lamivudine 300 mg + efavirenz 600 mg daily) following AmB total dose. Mean time to start ART from study entry in patients receiving AmB deoxycholate was 33 ± 10 days and 13 ± 3 days for patients receiving liposomal AmB .
Twenty-seven patients (52.9%, 95% CI = 39.5–65.9) received secondary prophylaxis (15 patients with intravenous liposomal AmB 1 mg/kg monthly and 12 patients with intravenous AmB deoxycholate 1 mg/kg monthly). Secondary prophylaxis was stopped after 6 months in 25 patients when CD4 count rose above 200/μL. It had to be continued beyond 6 months in two patients (till 7th and 8th months, respectively) till CD4 rose above 200/μL. There was no statistically significant difference between baseline CD4 counts in patients with or without secondary prophylaxis (95.5 ± 62 versus 100.7 ± 52.9, respectively, P = 0.7). However, at 6 months, patients on secondary prophylaxis had higher mean CD4 counts (196 ± 29.01 versus 157 ± 54.1, respectively, P = 0.015). Among patients not on secondary prophylaxis, those who did not experience relapses within first 6 months (N = 6) had higher mean CD4 counts than those who did in the first 6 months (N = 18) (201 ± 0.5 versus 143 ± 55.3, P = 0.017).
Primary and secondary outcomes.
None in the secondary prophylaxis group versus 18/24 patients (75%, 95% CI = 55.1–88) without secondary prophylaxis had a relapse within the first 6 months of follow-up (P < 0.001). All patients with secondary prophylaxis (27/27) versus 6/24 (25%, 95% CI = 12–44.9) without secondary prophylaxis achieved 12-month relapse-free survival (P < 0.001). None in the secondary prophylaxis group versus 11/24 patients (45.8%, 95% CI = 27.9–64.9) without secondary prophylaxis died during follow-up (P < 0.001), the mean duration of survival before death being 10.4 ± 2.5 months (Figure 1).
Survival analysis.
The profile of patients who died had the following characteristics significantly different from the survivors: lower hemoglobin level at baseline, lower mean CD4 cell count at 6 months of follow-up, lower proportion of patients receiving secondary prophylaxis, and higher proportion of patients experiencing at least one relapse within the first 6 months of follow-up (Tables 3 and 4).
Table 3.
Comparison of demographics, clinical, and laboratory results of patients with HIV–VL coinfection who survived vs. those who died
| Variables | Parameters | HIV–VL patients who died | HIV–VL patients who survived | P value |
|---|---|---|---|---|
| N = 11 | N = 40 | |||
| Age in years | Mean ± SD | 35 ± 12 | 34 ± 7 | 0.67 |
| Male gender | N (%) | 9 (81.8) | 31 (77.5) | 0.56 |
| Liver palpable below right costal margin (in cm) | Mean ± SD | 4.3 ± 1.8 | 3.8 ± 1.9 | 0.41 |
| Spleen palpable below left costal margin (in cm) | Mean ± SD | 10.3 ± 3.6 | 9.3 ± 3.1 | 0.37 |
| Hemoglobin at baseline (gm/dL) | Mean ± SD | 6.5 ± 1.4 | 7.8 ± 1.6 | 0.01 |
| Total leukocyte count (/μL) | Mean ± SD | 2136 ± 724 | 2955 ± 1588 | 0.11 |
| Platelet count at baseline (/μL) | Mean ± SD | 1.2 ± 0.7 | 1.3 ± 0.6 | 0.71 |
| CD4 cell count at baseline (/μL) | Mean ± SD | 92.9 ± 62.6 | 103.4 ± 56.8 | 0.59 |
| CD4 cell count at 6 months (/μL) | Mean ± SD | 119.1 ± 40.1 | 191.2 ± 33.4 | < 0.001 |
| Patients who received secondary prophylaxis | N (%) | 0 | 27 (67.5) | < 0.001 |
| Tuberculosis coinfection | N (%) | 0 | 7 (17.5) | 0.32 |
| One or more relapses of VL within the first 6 months of follow-up | N (%) | 11 (100%) | 7 (17.5) | < 0.001 |
HIV–VL = human immunodeficiency virus–visceral leishmaniasis; SD = standard deviation.
Table 4.
Result of multivariable Cox regression with death as the dependent variable
| Variables | B | SE | Wald | P value | HR | 95% confidence interval for HR |
|---|---|---|---|---|---|---|
| Hemoglobin concentration at baseline | 0.11 | 0.15 | 0.63 | 0.43 | 1.12 | 0.84–1.48 |
| Total leukocyte count at baseline | 0.00 | 0.00 | 0.07 | 0.78 | 1.00 | 1.0–1.0 |
| Secondary prophylaxis received or not | −2.36 | 0.61 | 15.12 | < 0.001 | 0.09 | 0.03–0.31 |
| CD4 cell count at 6 months of follow-up | −0.003 | 0.01 | 0.24 | 0.63 | 1.00 | 0.98–1.01 |
| Relapse within the first 6 months | 0.65 | 0.66 | 0.97 | 0.32 | 1.91 | 0.52–6.95 |
B = Beta coefficient; HR = hazard ration; SE = standard error.
Table 4 summarizes the results of the multivariate Cox regression for prediction of mortality in patients with HIV–VL. The following variables had P value < 0.2 in univariate analysis (Table 3) and were included in the Cox regression: hemoglobin level at baseline, total leukocyte count at baseline, CD4 cell count at 6-month follow-up, secondary prophylaxis, and relapse within the first 6 months of follow-up. Controlling for all other variables, secondary prophylaxis remained the sole significant predictor (HR = 0.09 [95% CI = 0.03–0.31] against death, P < 0.001) of survival.
Discussion
This study highlights the importance of secondary prophylaxis in reducing relapses and mortality in patients with HIV–VL coinfection. This result is of vital importance as there are no well-designed studies in this area, especially from India, and our current understanding of HIV–VL coinfection is still in its infancy. Although HIV seropositivity among Indian patients with VL is around 1.5–6.3%13–15 (5.6% from a recent cohort in Bihar, India),16 clinical, diagnostic, and importantly, treatment outcome–related parameters are not well described in literature.
Most of our patients had presented with typical clinical features of VL. However, 28.3% had atypical clinical features, majority being gastrointestinal, pulmonary, and bleeding manifestation. Most important clinical features were high relapse rate (34% in first 6 months) and mortality rate (almost 25%). Similar results were also seen in a recent study by Burza and others from Bihar, India.10 These essentially demonstrate that the natural history of VL with HIV as a backdrop is significantly different from VL monoinfection despite good compliance with ART.
Regarding diagnostics, an important ancillary result is utility of rK39 ICT in diagnosis of VL in people living with HIV/AIDS (positivity in serum 94.3%; positivity in urine 98.1%). We previously reported utility of urine rK39 test as one of the simplest noninvasive, field-adaptive, reliable test for diagnosis of VL with 100% sensitivity and 86.33% specificity.17 In the setting of HIV–VL, Indian studies reported good efficacy of rK39 ICT for diagnosis of VL.18 Our results indicate that rK39 works well in both serum and urine samples in Indian patients with VL with or without HIV. There was a statistically insignificant trend toward lower serum sample positivity in HIV–VL than in monoinfected patients with VL. It should be noted that rK39 ICT works better in Indian subcontinent compared with east African patients with VL.19 The utility of urine rK39 as a minimally invasive technique might make it an attractive screening tool under field conditions.
The major challenges of HIV–VL are those of relapses and mortality. Almost one-third of our coinfected patients underwent at least one relapse within first 6 months and almost two-thirds within 1st year. Relapse rates ranging from 25% to 61%, depending on varying definitions used, have been reported previously.10,20–24 A recent systematic review identified the following factors as predictors of relapse: failure of rise of CD4+ cells at follow-up, lack of secondary prophylaxis, and previous history of relapse of VL.24
Secondary prophylaxis has previously been tried in HIV–VL especially in Europe and Africa. The agents used for secondary prophylaxis were pentavalent antimony (24 patients),25,26 liposomal AmB (14 patients),26,27 AmB lipid complex (eight patients),28 and pentamidine (75 patients, mostly from Ethiopia, uncontrolled).26,29,30 No systematic study on secondary prophylaxis has been conducted in India, one of the largest contributors of the global burden of leishmaniasis. Despite uniformly low sample size and inconsistent designs in these studies, a high trend of relapse in coinfection is evident from these results. Regarding secondary prophylaxis antimonials, AmB and pentamidine appeared to render protection.24–26,30 The doses used for liposomal AmB varied from 3 mg/kg/month (five patients),27 with statistically insignificant protection against relapse to 200–350 mg monthly doses (nine patients),26 with statistically significant protection against relapse. Considering average body weight of a typical VL patient in India being around 30 kg and 75th percentile being around 40 kg,31 doses of 200–350 mg would amount to around 5–10 mg/kg/month, raising issues of toxicity and cost. Moreover VL caused by Leishmania infantum in Europe is traditionally treated with larger doses of liposomal AmB in comparison with Leishmania donovani in the Indian subcontinent. Considering toxicity, cost, and susceptibility, a dose of 1 mg/kg/month AmB for secondary prophylaxis has been in use in our institution (STM, Kolkata, India).
Initially, our patients with HIV–VL were managed conventionally (AmB + ART), but the problem of repeated relapses and mortality was evident to us within the first few years. In the present study, we therefore adopted the policy of secondary prophylaxis, on compassionate ground, which was subsequently adopted by few other physicians at the STM. HIV–VL coinfected patients were admitted to STM in different historical periods with similar clinical features. However, we could not find any other particular factor as indication for commencing secondary prophylaxis, apart from the intention to prevent relapses and mortality in the HIV–VL coinfected patients. Hence, majority of our patients who received secondary prophylaxis were from the later part of the retrospective study period.
The largest reported cohort of HIV–VL from India demonstrated low hemoglobin level and concurrent infection with tuberculosis as independent risk factors for mortality, with early ART reducing the risk of mortality and relapse in multivariate model.10 Another recent report from Bihar, India, identified failure to initiate ART and concurrent tuberculosis as independent risk factors for mortality and poor outcome but could not identify any factors associated with relapse.32 However, none of these groups offered secondary prophylaxis against VL. Tuberculosis occurred in 13.7% in our coinfected patients, but was not found to be a predictor of mortality. We demonstrated that secondary prophylaxis remained the singularly significant factor in reducing relapses and mortality after multivariate survival analysis (Cox regression).
Our study has certain limitations: low sample size, retrospective design, and heterogeneity of AmB preparations used for treatment, and possible bias toward more stringent follow-up in patients with HIV–VL coinfection.
In conclusion, this study demonstrates that initial treatment with AmB (usual total dose of 15–20 mg/kg) followed by ART and secondary prophylaxis with monthly AmB 1 mg/kg till CD4 rises above 200/μL can effectively prevent relapse and decrease mortality in patients infected with HIV–VL in India. We believe that the results of this study will provide a major edge in the battle against HIV–VL coinfection, though further prospective randomized control trials are required in this area.
ACKNOWLEDGMENTS
We are grateful to Indrajit Chatterjee, Consultant Physician in Geriatric and General (Internal) Medicine, Glan Clwyd Hospital, Wales, United Kingdom, for kindly consenting to review the manuscript for English language usage, context, and overall editing. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Disclaimer: Part of the results of this study were presented at MSF Scientific Day, New Delhi, India, 2015.
Footnotes
Authors' addresses: Rama P. Goswami, Ayan Basu, Yogiraj Ray, and Mehebubar Rahman, Department of Tropical Medicine, School of Tropical Medicine, Kolkata, India, E-mails: drrpgoswami@gmail.com, ayanbasustm@gmail.com, jaggs.nbmc@gmail.com, and rmehbub@gmail.com. Rudra P. Goswami, Department of Rheumatology, Institution of Post Graduate Medical Education and Research, Kolkata, India, E-mail: rudra.goswami@gmail.com. Santanu K. Tripathi, Department of Clinical and Experimental Pharmacology, School of Tropical Medicine, Kolkata, India, E-mail: tripathi.santanu@gmail.com.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh SP, Reddy DC, Rai M, Sundar S. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop Med Int Health. 2006;11:899–905. doi: 10.1111/j.1365-3156.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 3.Directorate of National Vector Borne Disease Control Programme . National Roadmap for Kala-Azar Elimination in India. Delhi, India: 2014. [Google Scholar]
- 4.National AIDS Control Organization . State Fact Sheets. Delhi, India: Department of AIDS Control, Ministry of Health and Family Welfare, Government of India; 2014. [Google Scholar]
- 5.UN Joint Programme on HIV/AIDS (UNAIDS) The Gap Report. 2014. http://www.refworld.org/docid/53f1e1604.html Available at. Accessed September 25, 2015.
- 6.Alexander J, Brombacher F. T helper1/T helper2 cells and resistance/susceptibility to Leishmania infection: is this paradigm still relevant? Front Immunol. 2012;3:80. doi: 10.3389/fimmu.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mock DJ, Hollenbaugh JA, Daddacha W, Overstreet MG, Lazarski CA, Fowell DJ, Kim B. Leishmania induces survival, proliferation and elevated cellular dNTP levels in human monocytes promoting acceleration of HIV co-infection. PLoS Pathog. 2012;8:e1002635. doi: 10.1371/journal.ppat.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvis JN, Lockwood DN. Clinical aspects of visceral leishmaniasis in HIV infection. Curr Opin Infect Dis. 2013;26:1–9. doi: 10.1097/QCO.0b013e32835c2198. [DOI] [PubMed] [Google Scholar]
- 9.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burza S, Mahajan R, Sinha PK, van Griensven J, Pandey K, Lima MA, Sanz MG, Sunyoto T, Kumar S, Mitra G, Kumar R, Verma N, Das P. Visceral leishmaniasis and HIV co-infection in Bihar, India: long-term effectiveness and treatment outcomes with liposomal amphotericin B (AmBisome) PLoS Negl Trop Dis. 2014;8:e3053. doi: 10.1371/journal.pntd.0003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-Leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7:e2195. doi: 10.1371/journal.pntd.0002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami RP, Goswami RP, Das S, Satpati A, Rahman M. Short-course treatment regimen of Indian visceral leishmaniasis with an Indian liposomal amphotericin B preparation (Fungisome™) Am J Trop Med Hyg. 2016;94:93–98. doi: 10.4269/ajtmh.14-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha PK, Rabidas VN, Pandey K, Verma N, Gupta AK, Ranjan A, Das P, Bhattacharya SK. Visceral leishmaniasis and HIV coinfection in Bihar, India. J Acquir Immune Defic Syndr. 2003;32:115–116. doi: 10.1097/00126334-200301010-00017. [DOI] [PubMed] [Google Scholar]
- 14.Thakur CP, Narayan S, Ranjan A. Kala-azar (visceral leishmaniasis) and HIV coinfection in Bihar, India: is this combination increasing? J Acquir Immune Defic Syndr. 2003;32:572–573. doi: 10.1097/00126334-200304150-00017. [DOI] [PubMed] [Google Scholar]
- 15.Mathur P, Samantaray JC, Vajpayee M, Samanta P. Visceral leishmaniasis/human immunodeficiency virus co-infection in India: the focus of two epidemics. J Med Microbiol. 2006;55:919–922. doi: 10.1099/jmm.0.46574-0. [DOI] [PubMed] [Google Scholar]
- 16.Burza S, Mahajan R, Sanz MG, Sunyoto T, Kumar R, Mitra G, Lima MA. HIV and visceral leishmaniasis coinfection in Bihar, India: an underrecognized and underdiagnosed threat against elimination. Clin Infect Dis. 2014;59:552–555. doi: 10.1093/cid/ciu333. [DOI] [PubMed] [Google Scholar]
- 17.Goswami RP, Bairagi B, Kundu PK. K39 strip test: easy, reliable and cost-effective field diagnosis for visceral leishmaniasis in India. J Assoc Physicians India. 2003;51:759–761. [PubMed] [Google Scholar]
- 18.Goswami RP, Goswami RP, Das S, Ray Y, Rahman M. Testing urine samples with rK39 strip as the simplest non-invasive field diagnosis for visceral leishmaniasis: an early report from eastern India. J Postgrad Med. 2012;58:180–184. doi: 10.4103/0022-3859.101378. [DOI] [PubMed] [Google Scholar]
- 19.Goswami RP, Rahman M, Guha SK. Utility of K39 strip test in visceral leishmaniasis (VL) and HIV co-infected patients: an early report from eastern India. J Assoc Physicians India. 2007;55:154–155. [PubMed] [Google Scholar]
- 20.Villanueva JL, Alarcón A, Bernabeu-Wittel M, Cordero E, Prados D, Regordán C, Alvar J. Prospective evaluation and follow-up of European patients with visceral leishmaniasis and HIV-1 coinfection in the era of highly active antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2000;19:798–801. doi: 10.1007/s100960000364. [DOI] [PubMed] [Google Scholar]
- 21.Molina I, Falcó V, Crespo M, Riera C, Ribera E, Curran A, Carrio J, Diaz M, Villar del Saz S, Fisa R, López-Chejade P, Ocaña I, Pahissa A. Efficacy of liposomal amphotericin B for secondary prophylaxis of visceral leishmaniasis in HIV-infected patients. J Antimicrob Chemother. 2007;60:837–842. doi: 10.1093/jac/dkm294. [DOI] [PubMed] [Google Scholar]
- 22.Berenguer J, Cosin J, Miralles P, Lopez JC, Padilla B. Discontinuation of secondary anti-Leishmania prophylaxis in HIV-infected patients who have responded to highly active antiretroviral therapy. AIDS. 2000;14:2946–2948. doi: 10.1097/00002030-200012220-00020. [DOI] [PubMed] [Google Scholar]
- 23.Casado JL, Lopez-Velez R, Pintado V, Quereda C, Antela A, Moreno S. Relapsing visceral leishmaniasis in HIV-infected patients undergoing successful protease inhibitor therapy. Eur J Clin Microbiol Infect Dis. 2001;20:202–205. doi: 10.1007/s100960100457. [DOI] [PubMed] [Google Scholar]
- 24.Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis. 2011;5:e1153. doi: 10.1371/journal.pntd.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribera E, Ocaña I, de Otero J, Cortes E, Gasser I, Pahissa A. Prophylaxis of visceral leishmaniasis in human immunodeficiency virus-infected patients. Am J Med. 1996;100:496–501. doi: 10.1016/s0002-9343(97)89503-4. [DOI] [PubMed] [Google Scholar]
- 26.Pintado V, Martin-Rabadan P, Rivera ML, Moreno S, Bouza E. Visceral leishmaniasis in HIV-infected and non-HIV-infected patients: a comparative study. Medicine (Baltimore) 2001;80:54–73. doi: 10.1097/00005792-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A, Bossi L, Germagnoli L, Lazzarin A, Uberti-Foppa C, Cinque P. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41:5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Vélez R, Videla S, Márquez M, Boix V, Jiménez-Mejías ME, Górgolas M, Arribas JR, Salas A, Laguna F, Sust M, Cañavate C, Alvar J, Spanish HIV-Leishmania Study Group Amphotericin B lipid complex versus no treatment in the secondary prophylaxis of visceral leishmaniasis in HIV-infected patients. J Antimicrob Chemother. 2004;53:540–543. doi: 10.1093/jac/dkh084. [DOI] [PubMed] [Google Scholar]
- 29.Delgado Fernández M, García Ordoñez MA, Martos Pérez F, Reguera Iglesias JM, Jiménez Oñate F, Colmenero Castillo JD. The clinical and evolutional characteristics of visceral leishmaniasis in patients with HIV infection. Med Interna. 1997;14:506–510. [PubMed] [Google Scholar]
- 30.Diro E, Ritmeijer K, Boelaert M, Alves F, Mohammed R, Abongomera C, Ravinetto R, De Crop M, Fikre H, Adera C, Colebunders R, van Loen H, Menten J, Lynen L, Hailu A, van Griensven J. Use of pentamidine as secondary prophylaxis to prevent visceral leishmaniasis relapse in HIV infected patients, the first twelve months of a prospective cohort study. PLoS Negl Trop Dis. 2015;9:e0004087. doi: 10.1371/journal.pntd.0004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harhay MO, Olliaro PL, Vaillant M, Chappuis F, Lima MA, Ritmeijer K, Costa CH, Costa DL, Rijal S, Sundar S, Balasegaram M. Who is a typical patient with visceral leishmaniasis? Characterizing the demographic and nutritional profile of patients in Brazil, east Africa, and south Asia. Am J Trop Med Hyg. 2011;84:543–550. doi: 10.4269/ajtmh.2011.10-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan R, Das P, Isaakidis P, Sunyoto T, Sagili KD, Lima MA, Mitra G, Kumar D, Pandey K, Van Geertruyden JP, Boelaert M, Burza S. Combination treatment for visceral leishmaniasis patients coinfected with human immunodeficiency virus in India. Clin Infect Dis. 2015;61:1255–1262. doi: 10.1093/cid/civ530. [DOI] [PMC free article] [PubMed] [Google Scholar]