Abstract
Malaria serology through assaying for IgG against Plasmodium spp. antigens provides evidence into the infection history for an individual. The multiplex bead assay (MBA) allows for detection of IgG against multiple Plasmodium spp., and can be especially useful in many regions where Plasmodium falciparum is of primary clinical focus, but other species are co-endemic. Dried blood spots were collected from 805 Malian children attending 42 elementary schools in the regions of Mopti, Sikasso, Koulikoro, and Bamako capital district, and IgG assayed by MBA. As southern Mali is known to be holoendemic for P. falciparum, merozoite surface protein 1 19-kDa subunit (MSP-142) and apical membrane antigen 1 (AMA-1) antigens were included for serology against this parasite. Responses to these antigens both provided high estimates for lifetime exposure, with 730 (90%) children with IgG antibodies for MSP-142, 737 (91%) for AMA-1, and 773 (96%) positive for either or both. Also included was the antigen Plasmodium vivax MSP-119, against which 140 (17.4%) children were found to have antibodies. Increases in antibody titers with older age were clearly seen with the P. falciparum antigens, but not with the P. vivax antigen, likely indicating more of a sporadic, rather than sustained transmission for this species. The MBA provides effective opportunities to evaluate malaria transmission through serological analysis for multiple Plasmodium species.
Introduction
Malaria is caused by parasites within the protozoan genus Plasmodium that are transmitted by the mosquito genus Anopheles. The blood stages of both Plasmodium vivax and Plasmodium falciparum can cause severe illness and death by invading and destroying red blood cells (RBCs), and appropriate parasite identification and surveillance at the species level is needed to implement appropriate antimalarial drug strategies for a region. Plasmodium falciparum is the most prevalent and clinically relevant malaria on the African continent while P. vivax is generally found in populations more likely to carry the Duffy erythrocyte receptor that allows P. vivax attachment to RBCs.1 In 2000, 2.4 billion people in 106 countries and territories were at risk of malaria infection; however, because of growing resistance to drugs and insecticides, environmental changes, and human migration, these numbers in 2015 have increased to 3.2 billion people at risk in only 97 countries.2 Effective control efforts through insecticide-treated nets, indoor residual spraying, artemisinin-based combination therapies, and other interventions have substantially reduced P. falciparum cases and deaths, but have uncovered problems with a stubbornly persistent P. vivax, which has different biodynamics of infection.2,3
Mali, an economically poor country traversing Saharan and sub-Saharan Africa, is holoendemic for falciparum malaria in the southern part of the nation.4,5 In 2015, it was estimated that the parasite prevalence in some regions of southern Mali exceeded 80%,6 representing a large clinical burden for the area. As with other areas in Africa,7 infection with P. vivax is becoming more documented in Mali, and recent studies have shown infection with this parasite as both a single and mixed infection with P. falciparum.8,9
Serology can provide valuable information on the prevalence of exposure to malaria and can be helpful for characterizing parasite transmission dynamics in an area, and identifying where interventions are needed the most. As some Plasmodium spp. antigens are known to elicit an IgG response that can be detected for a long period, serological analysis of children can provide an estimate of lifetime exposure for these young individuals.10 To this end, we included P. falciparum and P. vivax antigens in a serology study that evaluated IgG responses by a multiplex bead assay (MBA), which has been used in other serological studies.11–13 Recombinant antigens included the P. vivax merozoite surface protein 1 19-kDa subunit (P. vivax MSP-119), the 42-kD subunit of P. falciparum MSP-1 (MSP-142), and P. falciparum apical membrane antigen 1 (AMA-1).
Materials and Methods
Study population.
The Ethics Committee of the National Institute of Public Health Research in Mali (02/2014/CE-INRSP) and the Institutional Review Board of Emory University reviewed and approved this study (IRB00060756). The trial was registered at ClinicalTrials.gov (NTC01787058). Data come from a cross-sectional serological study evaluating Ig G responses to antigens from a range of pathogens and vaccine-preventable diseases, which was nestled within a longitudinal impact evaluation of a school-based water, sanitation, and hygiene (WASH) program in Mali. Detailed methods and results from the impact evaluation are found elsewhere.14
Laboratory staff from the Centers for Disease Control and Prevention had no contact with children enrolled in the study nor any access to personal identifiers. A total of 805 Malian children, age range 4–17 years, in 42 elementary schools in the regions of Mopti, Sikasso, Koulikoro, and Bamako capital district provided dried blood spots (DBSs) for the study. The design for school enrollment and children sampling was formatted for a matched-control WASH study, as described previously.14 Whole blood specimens were collected onto a wheel with six circular filter paper extensions (TropBio Pty Ltd., Townsville, Australia), each designed to absorb 10 μL of whole blood. Between 1 and 3 months after collection and drying at room temperature, DBSs were stored at −20°C. Samples were collected between January and June 2014, which is the dry season in Mali.
Antigen coupling to beads.
The recombinant antigen P. vivax MSP-11915 was fused with glutathione-S-transferase (GST) to allow purification. The external domain of AMA-1 and MSP-142 antigens were produced at Walter Reed Army Institute of Research under previously published conditions.16 To couple antigens to polystyrene beads, the 1-ethyl-3-(3-dimethylaminopropyl) (Calbiochem, Woburn, MA) carbodiimide method was used to convert carboxyl groups on the surface of specifically classified spectral magnetic polystyrene microspheres (MagPlex Beads; Luminex Corporation, Austin, TX) to reactive esters. The esters readily react with available primary amino groups on the antigens to form covalent amide bonds between antigen and microspheres. The recombinant antigens were coupled to differently classified spectral activated beads (12.5 million) using 23 μg P. vivax MSP-119/GST, 23 μg AMA-1, and 17 μg MSP-142 in 50 mM 2-(N-morpholino)ethanesulfonic acid, 0.85% NaCl, pH 5.0. As a control, a different bead classification was coupled with 15 μg GST using the same conditions to ensure no nonspecific binding of human IgG to the GST fusion protein on the P. vivax antigen. Successful coupling for P. vivax MSP-119 (fused to GST) was determined by test runs using an in-house polyclonal IgG anti-GST. In addition, completed couplings of P. falciparum and P. vivax antigens to beads were validated by reactivity to know positive sera pools. Blank wells and positive and negative sera were included on each assay plate for the study as controls.
DBS elution and serology data acquisition.
One filter paper extension (10 μL dried whole blood) from each child was placed in 0.5 mL of elution buffer consisting of phosphate-buffered saline (PBS) with 0.5% bovine serum albumin, 0.3% Tween 20, 0.1% sodium azide, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, and 0.1% casein and allowed to elute overnight at 4°C with gentle shaking. Afterward, the elution was further diluted 1:4 with the same elution buffer that contained sufficient amounts of crude and unclarified Escherichia coli extract (final at 3 μg/mL). The E. coli extract adsorbs E. coli-specific IgG that could react with residual E. coli proteins coupled to beads. After overnight storage at 4°C, the final dilutions, representing a serum dilution of approximately 1:400, were exposed to antigen-coupled beads in a 96-well formatted plate for 1.5 hours at room temperature with each well containing about 1,500 antigen-coupled beads per spectral classification. Bound antigen-specific IgG was detected on the coupled beads using biotin-linked antihuman IgG monoclonal antibodies (1:500 antihuman IgG1-3; Southern Biotech, Birmingham, AL; 1:2,500 antihuman IgG4; Sigma, St. Louis, MO). IgG was quantitatively reported on the antigens by streptavidin-linked R-phycoerythrin (1:200; Invitrogen, Waltham, MA) as previously described.11 Between steps, the magnetic beads were washed three times with 0.05% Tween 20 in PBS using a Bio-Plex Pro II Wash Station (Bio-Rad, Hercules, CA). Mean data from duplicate wells were obtained from the median fluorescence intensity (MFI; ranging from channels 1 to 32,766) from each well, which were acquired using a Bio-Plex 100 reader with Bio-Plex Manager 6.1 software (Bio-Rad). Background (bg) from a DBS blank was subtracted from the MFI (MFI-bg). For all antigens, the inter-plate percent coefficient of variation for positive controls on all plates was < 16%, and beads coupled with GST showed no appreciable reactivity from the 805 children. The median and ranges of signal intensities for each antigen for all samples in the survey are shown in Supplemental Table 1. In addition to the malaria antigens listed above, samples were tested for antibodies to enteric infections, neglected tropical diseases, and other vector-borne diseases. These results will be reported elsewhere.
Seropositivity cutoff determination.
Cutoffs for all antigens were determined using 86 serum specimens from U.S. adults who had not traveled outside the country, and, thus, had no chance of malaria exposure. All serum specimens were assayed at 1:400 dilution to reflect the same approximate serum dilution of the Mali samples. Outliers greater than 5 standard deviations (SDs) above the mean were eliminated (N = 2 data points for MSP-142, 2 for AMA-1, and 0 for P. vivax MSP-119), and data from the remaining specimens were used to calculate the mean + 3 SD as the seropositivity cutoff. Cutoff MFI-bg values were determined to be 312 for MSP-142, 237 for AMA-1, and 393 for P. vivax MSP-119.
Prevalence of positive IgG responses to each antigen per school.
Location and prevalence of positive IgG responses per school was plotted for each antigen using ArcGIS 10.3.1 mapping software (Environmental Systems Research Institute, Redlands, CA).
Statistics.
Modeling for correlations in antibody titers was done by the PROC CORR procedure within SASv9.3 (SAS Institute Inc., Cary, NC) and increases in antibody levels with increasing age were performed by using the PROC GENMOD procedure.
Results
Study area and schools.
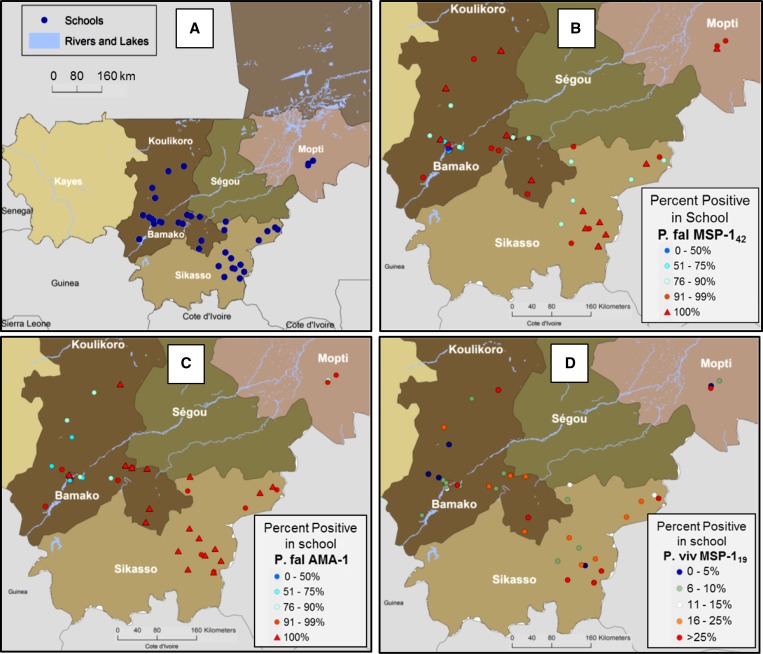
As shown in Figure 1A , 42 schools were included in the study, representing a large part of southern Mali. Four Mali regions were included: Mopti with four schools, Sikasso with 16, Koulikoro with 20, and the Bamako capital district with two schools. An average of 19 children were sampled from each school (range: 14–21), with 28 schools having exactly 20 children sampled.
Figure 1.
Map of southwestern Mali showing school locations and malaria antibody prevalence by school. (A) Schools within the regions of Bamako (N = 2), Mopti (N = 4), Sikasso (N = 16), and Koulikoro (N = 20) were all included in the study. (B) Percent of children in each school sampled with IgG antibodies against the Plasmodium falciparum antigen merozoite surface protein (MSP-142). (C) Percent of children in each school sampled with IgG antibodies against the P. falciparum antigen apical membrane antigen 1 (AMA-1). (D) Percent of children in each school sampled with IgG antibodies against the Plasmodium vivax antigen MSP-119.
Prevalence of antigen positivity by school.
Of all children in the study, a high percentage were found to be positive for P. falciparum antibodies with 90% of all children having IgG antibodies against MSP-142, 91% of children with antibodies to AMA-1, and 96% positive for either or both antigens. This was also reflected in mapping of the percent positive children within each of the 42 schools as shown in Figure 1B and C. Of 42 schools, 20 (47.6%) had 100% of children with MSP-142 antibodies and 16 (38.1%) had 100% of children with AMA-1 antibodies. Overall, 32 (76.2%) of the 42 schools had 100% of children positive for MSP-142 or AMA-1 antibodies. Due to the saturation of historical P. falciparum infection, it was difficult to assess any geospatial patterning for the transmission intensity of this parasite. However, a few schools around the urban center of Bamako showed relatively lower percentages of children positive for P. falciparum antigens. In addition, elevation of the school was plotted against antibody prevalence, but no correlation was observed (data not shown).
Serology for P. vivax revealed much different findings, with only 17.5% of all Malian children sampled having antibodies against this parasite. As shown in Figure 1D, this varied widely, with school positivity rates of 0–36%, and showed a higher number of positive children in the southernmost region of Sikasso. Interestingly, only two of the 42 schools showed a complete absence of children seropositive for P. vivax, indicating low-level and dispersed transmission for this parasite. Again, plotting school elevation against antibody prevalence within the school was found to provide no correlation (data not shown).
Relationship of MFI-bg signals from different antigens.
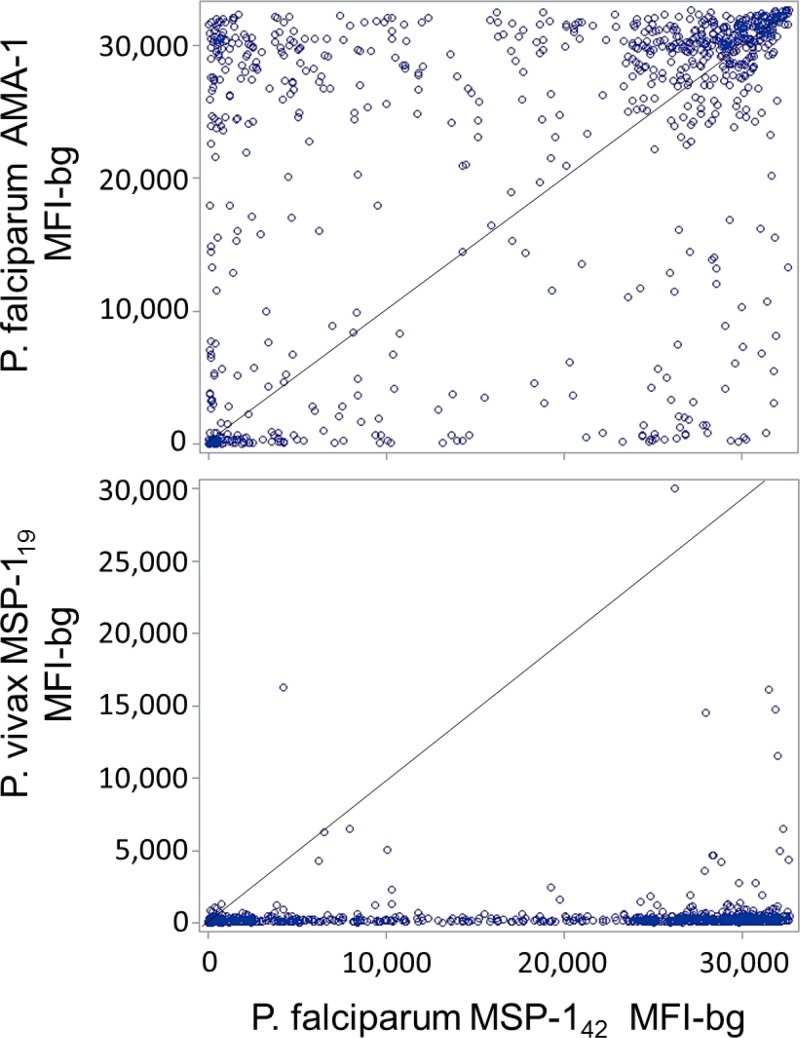
The P. falciparum antigens MSP-1 and AMA-1 have been used extensively in malaria serological studies,11,17–20 and are both thought to induce IgG antibody responses which would be present for long periods of time following infection.10,21 For this cohort of Malian children, we found the MFI-bg signal intensities for each of these antigens to be highly skewed, with the distinct possibility of having a high titer for one of these antigens and not for the other (Figure 2 ). However, the antibody signal intensities for these two antigens were found to be correlated for the overall population of children (Spearman's rank correlation coefficient: ρ = 0.493, P < 0.0001). Testing for the relationship between P. falciparum MSP-142 and P. vivax MSP-119 found a statistically significant rank correlation (ρ = 0.299, P < 0.001), but antibody intensity varied, with the P. vivax antigen showing fewer high titers.
Figure 2.
Scatterplots of median fluorescence intensity with background subtracted (MFI-bg) signal for antigen Plasmodium falciparum merozoite surface protein (MSP-142) with the P. falciparum apical membrane antigen 1 (AMA-1) and Plasmodium vivax MSP-119 antigens from all the children in the study (N = 805). Scatterplots show MFI-bg signal between the two P. falciparum antigens, and between the P. falciparum and P. vivax MSP-1 homologs. Diagonal line represents y = x reference line.
Distribution of MFI-bg signal for different antigens.
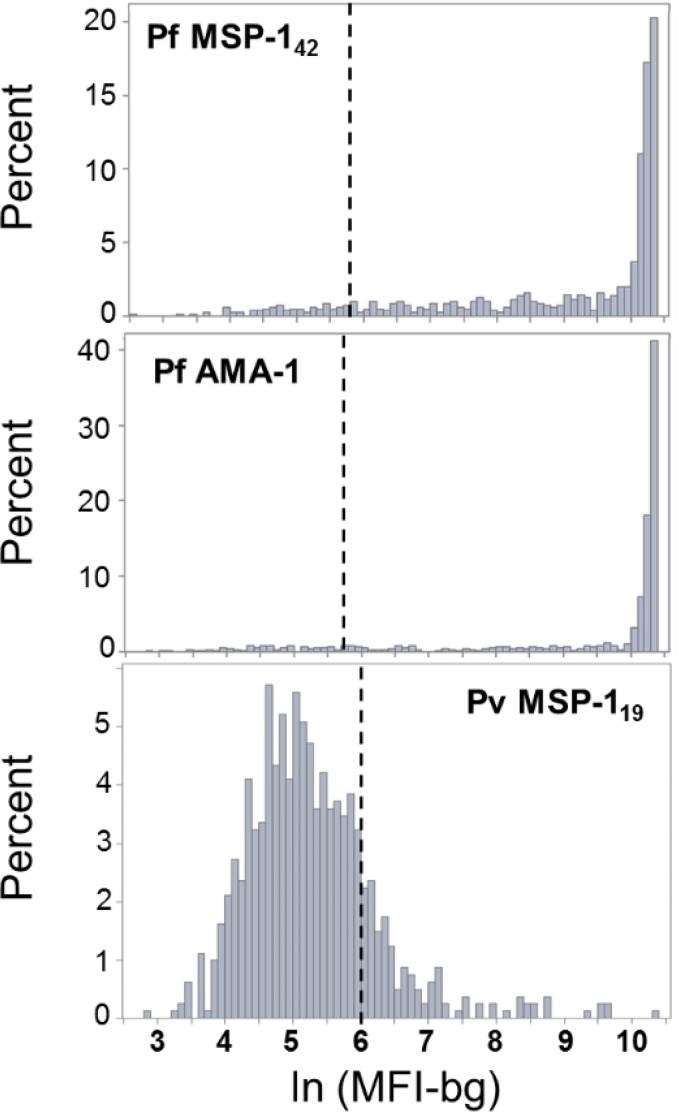
For the sample dilution and experimental conditions used for the MBA, the serology for P. falciparum antigens showed a strong positive skew, with many of the observations on the upper end of the Bio-Plex machine's fluorescence detection capacity (Figure 3 ). As explained in Methods section, seropositivity cutoff determination for the P. falciparum antigens provided a cutoff value that was well outside the highest density of the MFI-bg distribution, and showed strong evidence for very few children in this Malian population as not having IgG antibodies against P. falciparum. In contrast, the distribution of the P. vivax MSP-119 MFI-bg signal provided a much more symmetric distribution, and indicated the majority of children as not having antibodies against P. vivax. Overall, antibody titers were lower for the children considered positive for P. vivax antibodies, but some children showed MFI-bg values in the high thousands (Figures 2 and 3), possibly indicating recent, or multiple lifetime, P. vivax infections for these few individuals.
Figure 3.
Histograms for signal intensities of the three antigens used in the study. The distribution of median fluorescence intensity with background subtracted (MFI-bg) signal for the children in the study is plotted as log-transformed on the x axis. Hashed vertical lines signify the seropositivity cutoff value for each antigen. Note that the histogram for each antigen has a unique y-axis scale.
Malaria antibody levels in relation to child age.
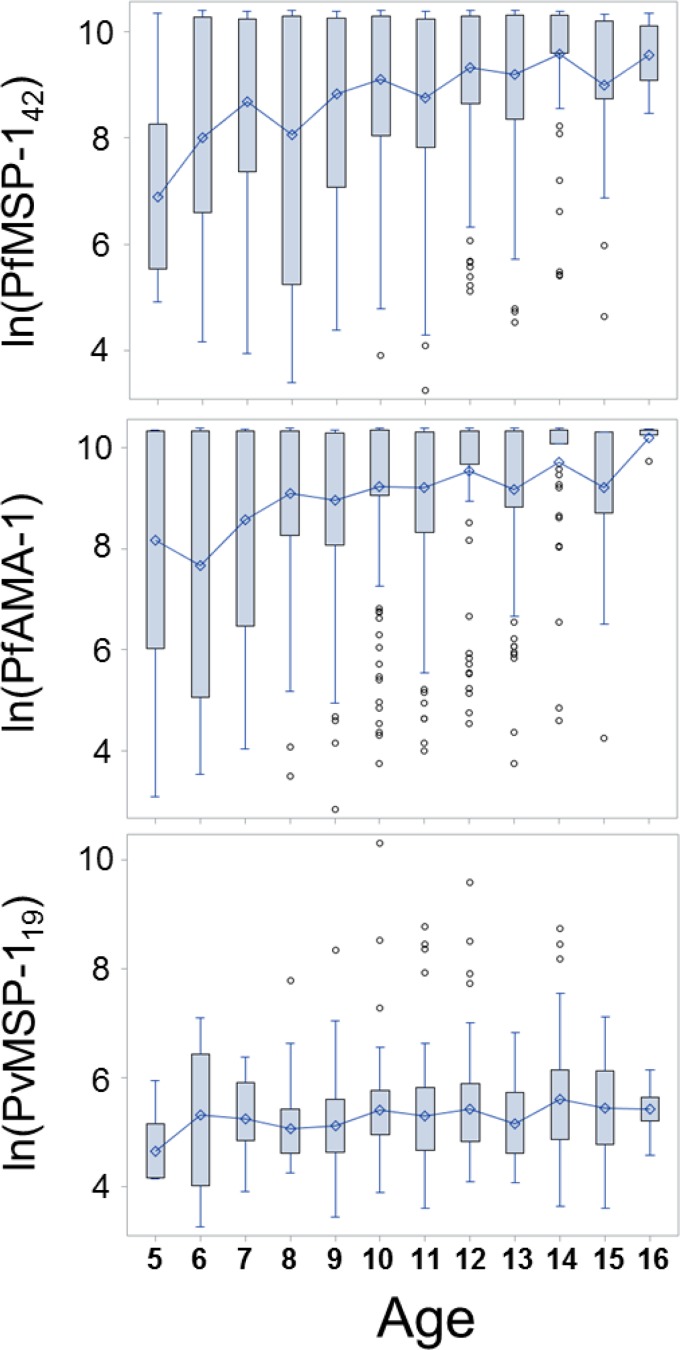
At birth, children in malaria-endemic regions tend to have significant titers of maternal antimalarial antibodies, but these generally are lost by approximately the 6th month of life.22,23 In high-transmission settings, children are almost inevitably exposed to malaria at a young age, and begin developing IgG antibodies based on their own adaptive immune responses. As shown in Figure 4 for this sample of Malian children aged 5–16 years, we found a substantial increase in the mean P. falciparum antibody levels as age increased. Older children in this setting have an almost certainty of being exposed to P. falciparum multiple times throughout their lives, and this is reflected by the increase in titers for these antigens. Modeling for antibody increase over time revealed an increase for every year of life of 756 MFI-bg units for MSP-142 (Wald χ2 = 9.69, P = 0.002) and 616 MFI-bg units for AMA-1 (Wald χ2 = 6.46, P = 0.011).
Figure 4.
Connecting boxplots of ln(MFI-bg) for all antigens by age of participants in the study. Boxplots signify the mean (diamond) and display the interquartile range with whiskers extending 1.5 times the interquartile range (IQR) above and below. Outliers displayed as circles outside of 1.5× IQR. Bins connected at means between age divisions. For each, age(n): 5(4), 6(16), 7(23), 8(27), 9(35), 10(85), 11(74), 12(80), 13(51), 14(46), 15(16), 16(5).
This same trend was not observed in the antibody levels for the P. vivax MSP-119 antibodies, likely due to the overall low probability of exposure. Modeling provided an estimated increase of 14 MFI-bg units for every year of life, but this was nonsignificant (Wald χ2 = 0.18, P = 0.67). Throughout this age range of children, high antibody levels appeared to be random across ages, suggesting stochastic exposure and largely the absence of repeated exposures as children age.
Discussion
Many areas of Africa still remain holoendemic for P. falciparum, and this parasite represents an enormous public health and economic concern for many nations.6 Due to the affliction of falciparum malaria, surveillance for other Plasmodium spp. in these nations has largely been neglected, even though some areas are co-endemic with all four of the human malarias.8 With the identification of the P. vivax Duffy-binding protein as the putative mediator for RBC invasion, this led to the logical assumption that much of the African population would not be susceptible to infection by this parasite as a large percentage of the population has a Duffy-negative phenotype. However, P. vivax infection has been identified in Duffy-negative individuals, providing evidence that this parasite is more common than initially thought.1 In Mali, it is estimated around 80–95% of the population is Duffy-negative,24 and estimates for the transmission of vivax malaria do not exist,6 though previous microscopy evidence has been gathered from multiple sites in the country.9
Serological studies for malaria assess the concept of historical transmission rather than prevalence estimates through active case detection. In areas of the world co-endemic for multiple human malarias, the MBA provides a cost-effective and practical way to estimate transmission histories for multiple species. In addition, by the nature of having serology data on all antigens collected simultaneously, the MBA provides malaria research groups increased potential to gather data from studies conducted by other disease groups. In this study, we found that schoolchildren in southern Mali showed nearly ubiquitous carriage of IgG antibodies against P. falciparum antigens MSP-142 and AMA-1, typically at high titers which would indicate both repeated and recent lifetime exposures. In a geospatial context, no differences were noted in the prevalence of children in schools having antibodies against P. falciparum, with the exception of a few schools around the urban area of Bamako. As a data sample, the relationship between P. falciparum MSP-142 and AMA-1 antibody carriage was found to be significant, but a high titer was occasionally seen in a child for one antigen but a negligible titer for the other (Figure 2). For southern Mali as a whole, levels of antibodies against the P. falciparum proteins MSP-142 and AMA-1 were found to be very high, concordant with previous studies showing high parasite prevalence in this area.4,25 These data revealed a clear increase in the levels of antibodies to the P. falciparum antigens as children mature, as repeated exposure is likely. This aligns with previous studies showing IgG against P. falciparum antigens as an important factor in the development of clinical immunity.26,27
Within this southern part of Mali, serological evidence for the transmission profile of P. vivax was quite different, with a much smaller percentage of children harboring IgG antibodies against the P. vivax antigen MSP-119. Overall, evidence for previous P. vivax exposure was shown in 17.3% of all children sampled, and displayed a much more heterogeneous endemicity pattern among the schools. Schools in the southern most region of Sikasso tended to show higher percentages of children with P. vivax antibodies, with multiple schools in this region showing over 25% of children with lifetime exposure to this parasite. As the mosquito vector would be shared between P. vivax and P. falciparum, previous reports showing high P. falciparum parasite rates among young children in this area4 would indicate a robust regional vector population for the lifecycle of both parasites to persist. It was interesting to find only two of 42 schools sampled in southern Mali with no children harboring anti-P. vivax antibodies. This would allude to widespread low-transmission but a persistent rate of P. vivax that has been found by other studies.28,29 Evidence for this notion was also supported by the finding that IgG antibody levels for the P. vivax antigen did not increase with age, indicating a more stochastic nature of P. vivax exposure, rather than repeated exposures as one ages.
With the predominance of P. falciparum, asymptomatic nature of P. vivax, potential intra-host competition between the two parasites, and paucity of the Duffy receptor, it is not surprising that P. vivax infections have been overlooked in this area of Mali. Though not as clinically relevant in many settings, it is becoming more appreciated that P. vivax lifecycle can persist in some Duffy-negative African populations,7,9,30 and serological surveillance through the MBA offers a prime opportunity for inexpensive and robust identification of regions where this parasite may be endemic.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the students, parents, and teachers who allowed us to conduct this work, as well as the Government of Mali. We would also like to thank the research team, including Abdoulaye Sow, Seydou Samaké, Salif Ismaïla Traoré, Fatoumata Habib Traoré, Karim Diamoutene, Yacouba Sogore, Alpha Oumar Haidara, as well as Niélé Hawa Diarra and Samba Diop from the University of Bamako. We also thanks the UNICEF, WaterAid, CARE, Oxfam, and Save the Children teams for their support, specifically Jérémie Toubkiss, Yagouba Diallo, Seydou Niafo, Touréba Keïta, Assitan Sogoré, Salimata Togola, Fatoumata Haïdara, Mamadou Diallo, Zoumana Cissé, Ousmane Haïdara, and Thierno Bocoum.
Disclaimer: The funder had no involvement in the design, collection, analysis, or interpretation of the data or in the preparation of this manuscript.
Footnotes
Financial support: This work was supported by Dubai Cares Foundation.
Authors' addresses: Eric Rogier and Patrick J. Lammie, Division of Parasitic Diseases and Malaria, Centers for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: wwx6@cdc.gov and pjl1@cdc.gov. Delynn M. Moss, Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: dmm3@cdc.gov. Anna N. Chard, Victoria Trinies, and Matthew C. Freeman, Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, GA, E-mails: achard@emory.edu, vtrinies@gmail.com, and mcfreem@emory.edu. Seydou Doumbia, Malaria Research and Training Center, Faculty of Medicine and Odontostomatology, University of Sciences, Techniques and Technologies of Bamako (USTTB), Bamako, Mali, E-mail: sdoumbi@icermali.org.
References
- 1.Zimmerman PA, Ferreira MU, Howes RE, Mercereau-Puijalon O. Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv Parasitol. 2013;81:27–76. doi: 10.1016/B978-0-12-407826-0.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Achieving the Malaria MDG Target: Malaria Essentials. 2015. http://apps.who.int/iris/bitstream/10665/184521/1/9789241509442_eng.pdf Accessed January 21, 2016.
- 3.Richter J, Franken G, Holtfreter MC, Walter S, Labisch A, Mehlhorn H. Clinical implications of a gradual dormancy concept in malaria. Parasitol Res. 2016;115:2139–2148. doi: 10.1007/s00436-016-5043-0. [DOI] [PubMed] [Google Scholar]
- 4.Toure M, Sanogo D, Dembele S, Diawara SI, Oppfeldt K, Schioler KL, Haidara DB, Traore SF, Alifrangis M, Konradsen F, Doumbia S. Seasonality and shift in age-specific malaria prevalence and incidence in Binko and Carriere villages close to the lake in Selingue, Mali. Malar J. 2016;15:219. doi: 10.1186/s12936-016-1251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulibaly D, Travassos MA, Kone AK, Tolo Y, Laurens MB, Traore K, Diarra I, Niangaly A, Daou M, Dembele A, Sissoko M, Guindo B, Douyon R, Guindo A, Kouriba B, Sissoko MS, Sagara I, Plowe CV, Doumbo OK, Thera MA. Stable malaria incidence despite scaling up control strategies in a malaria vaccine-testing site in Mali. Malar J. 2014;13:374. doi: 10.1186/1475-2875-13-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Mali, World Malaria Report 2015. 2015. http://www.who.int/malaria/publications/country-profiles/profile_mli_en.pdf?ua=1 Accessed March 13, 2016.
- 7.Ba H, Duffy CW, Ahouidi AD, Deh YB, Diallo MY, Tandia A, Conway DJ. Widespread distribution of Plasmodium vivax malaria in Mauritania on the interface of the Maghreb and west Africa. Malar J. 2016;15:80. doi: 10.1186/s12936-016-1118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams J, Njie F, Cairns M, Bojang K, Coulibaly SO, Kayentao K, Abubakar I, Akor F, Mohammed K, Bationo R, Dabira E, Soulama A, Djimde M, Guirou E, Awine T, Quaye SL, Ordi J, Doumbo O, Hodgson A, Oduro A, Magnussen P, Ter Kuile FO, Woukeu A, Milligan P, Tagbor H, Greenwood B, Chandramohan D. Non-falciparum malaria infections in pregnant women in west Africa. Malar J. 2016;15:53. doi: 10.1186/s12936-016-1092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernabeu M, Gomez-Perez GP, Sissoko S, Niambele MB, Haibala AA, Sanz A, Thera MA, Fernandez-Becerra C, Traore K, Alonso PL, Bassat Q, Del Portillo HA, Doumbo O. Plasmodium vivax malaria in Mali: a study from three different regions. Malar J. 2012;11:405. doi: 10.1186/1475-2875-11-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, Liewsaree W, Riley EM, Hafalla JC. Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold BF, Priest JW, Hamlin KL, Moss DM, Colford JM, Jr, Lammie PJ. Serological measures of malaria transmission in Haiti: comparison of longitudinal and cross-sectional methods. PLoS One. 2014;9:e93684. doi: 10.1371/journal.pone.0093684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammie PJ, Moss DM, Brook Goodhew E, Hamlin K, Krolewiecki A, West SK, Priest JW. Development of a new platform for neglected tropical disease surveillance. Int J Parasitol. 2012;42:797–800. doi: 10.1016/j.ijpara.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Priest JW, Moss DM, Arnold BF, Hamlin K, Jones CC, Lammie PJ. Seroepidemiology of Toxoplasma in a coastal region of Haiti: multiplex bead assay detection of immunoglobulin G antibodies that recognize the SAG2A antigen. Epidemiol Infect. 2015;143:618–630. doi: 10.1017/S0950268814001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinies V, Garn JV, Chang HH, Freeman MC. The impact of a school-based water, sanitation, and hygiene program on absenteeism, diarrhea, and respiratory infection: a matched-control trial in Mali. Am J Trop Med Hyg. 2016;94:1418–1425. doi: 10.4269/ajtmh.15-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putaporntip C, Jongwutiwes S, Sakihama N, Ferreira MU, Kho WG, Kaneko A, Kanbara H, Hattori T, Tanabe K. Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc Natl Acad Sci USA. 2002;99:16348–16353. doi: 10.1073/pnas.252348999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heppner DG, Jr, Kester KE, Ockenhouse CF, Tornieporth N, Ofori O, Lyon JA, Stewart VA, Dubois P, Lanar DE, Krzych U, Moris P, Angov E, Cummings JF, Leach A, Hall BT, Dutta S, Schwenk R, Hillier C, Barbosa A, Ware LA, Nair L, Darko CA, Withers MR, Ogutu B, Polhemus ME, Fukuda M, Pichyangkul S, Gettyacamin M, Diggs C, Soisson L, Milman J, Dubois MC, Garcon N, Tucker K, Wittes J, Plowe CV, Thera MA, Duombo OK, Pau MG, Goudsmit J, Ballou WR, Cohen J. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23:2243–2250. doi: 10.1016/j.vaccine.2005.01.142. [DOI] [PubMed] [Google Scholar]
- 17.Rogier E, Wiegand R, Moss D, Priest J, Angov E, Dutta S, Journel I, Jean SE, Mace K, Chang M, Lemoine JF, Udhayakumar V, Barnwell JW. Multiple comparisons analysis of serological data from an area of low Plasmodium falciparum transmission. Malar J. 2015;14:436. doi: 10.1186/s12936-015-0955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WM, Lemnge MM, Cox J, Reyburn H, Riley EM. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Bretscher MT, Supargiyono S, Wijayanti MA, Nugraheni D, Widyastuti AN, Lobo NF, Hawley WA, Cook J, Drakeley CJ. Measurement of Plasmodium falciparum transmission intensity using serological cohort data from Indonesian schoolchildren. Malar J. 2013;12:21. doi: 10.1186/1475-2875-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ondigo BN, Hodges JS, Ireland KF, Magak NG, Lanar DE, Dutta S, Narum DL, Park GS, Ofulla AV, John CC. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis. 2014;210:1123–1132. doi: 10.1093/infdis/jiu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duah NO, Miles DJ, Whittle HC, Conway DJ. Acquisition of antibody isotypes against Plasmodium falciparum blood stage antigens in a birth cohort. Parasite Immunol. 2010;32:125–134. doi: 10.1111/j.1365-3024.2009.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kangoye DT, Mensah VA, Murungi LM, Nkumama I, Nebie I, Marsh K, Cisse B, Bejon P, Osier FH, Sirima SB, MVVC Infant Immunology Study Group Dynamics and role of antibodies to Plasmodium falciparum merozoite antigens in children living in two settings with differing malaria transmission intensity. Vaccine. 2016;34:160–166. doi: 10.1016/j.vaccine.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg R. Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol. 2007;23:193–196. doi: 10.1016/j.pt.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Sissoko MS, van den Hoogen LL, Samake Y, Tapily A, Diarra AZ, Coulibaly M, Bouare M, Gaudart J, Knight P, Sauerwein RW, Takken W, Bousema T, Doumbo OK. Spatial patterns of Plasmodium falciparum clinical incidence, asymptomatic parasite carriage and Anopheles density in two villages in Mali. Am J Trop Med Hyg. 2015;93:790–797. doi: 10.4269/ajtmh.14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rafiou A, Francine C, Ibrahim S, Sonon P, Dechavanne C, Djilali-Saiah A, Cottrell G, Le Port A, Massougbodji A, Remarque EJ, Luty AJ, Sanni A, Garcia A, Migot-Nabias F, Milet J, Courtin D. Plasmodium falciparum infection and age influence parasite growth inhibition mediated by IgG in Beninese infants. Acta Trop. 2016;159:111–119. doi: 10.1016/j.actatropica.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Murungi LM, Sonden K, Llewellyn D, Rono J, Guleid F, Williams AR, Ogada E, Thairu A, Farnert A, Marsh K, Draper SJ, Osier FH. Targets and mechanisms associated with protection from severe Plasmodium falciparum malaria in Kenyan children. Infect Immun. 2016;84:950–963. doi: 10.1128/IAI.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahid S, Stresman GH, Kamal SS, Sepulveda N, Kleinschmidt I, Bousema T, Drakeley C. Heterogeneous malaria transmission in long-term Afghan refugee populations: a cross-sectional study in five refugee camps in northern Pakistan. Malar J. 2016;15:245. doi: 10.1186/s12936-016-1305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripura R, Peto TJ, Chalk J, Lee SJ, Sirithiranont P, Nguon C, Dhorda M, von Seidlein L, Maude RJ, Day NP, Imwong M, White NJ, Dondorp AM. Persistent Plasmodium falciparum and Plasmodium vivax infections in a western Cambodian population: implications for prevention, treatment and elimination strategies. Malar J. 2016;15:181. doi: 10.1186/s12936-016-1224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, Koros J, Owour B, Luckhart S, Wirtz RA, Barnwell JW, Rosenberg R. Evidence for transmission of Plasmodium vivax among a Duffy antigen negative population in western Kenya. Am J Trop Med Hyg. 2006;75:575–581. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.