Abstract
Understanding variations in malaria transmission and exposure is critical to identify populations at risk and enable better targeting of interventions. The indigenous Batwa of southwestern Uganda have a disproportionate burden of malaria infection compared with their non-indigenous neighbors. To better understand the individual- and community-level determinants of malaria exposure, a seroepidemiological study was conducted in 10 local council cells in Kanungu District, Uganda, in April 2014. The Batwa had twice the odds of being seropositive to two Plasmodium falciparum–specific antigens, apical membrane antigen-1 and merozoite surface protein-119, compared with the non-indigenous Bakiga (odds ratio = 2.08, 95% confidence interval = 1.51–2.88). This trend was found irrespective of altitude level and after controlling for cell location. Seroconversion rates in the Batwa were more than twice those observed in the Bakiga. For the Batwa, multiple factors may be associated with higher exposure to malaria and antibody levels relative to their non-indigenous neighbors.
Considerable heterogeneity in malaria transmission levels and disease burden can be found within and between populations in endemic areas, due to variations in local vector populations, socioeconomic factors, and individual susceptibility.1,2 Understanding this variation is important to enable better deployment of interventions to reach the populations most at risk.
Malaria transmission intensity is commonly measured using the entomological inoculation rate (EIR; the number of infectious bites per person per year) and/or parasite rate (the number of infected individuals per population). However, in low-transmission settings, these metrics lack sensitivity due to low numbers of infected mosquitos and humans.3 Serological techniques are increasingly used in the assessment of malaria transmission intensity and provide credible estimates of transmission that correlate well with EIR.4 At an individual level, antibody responses can provide an alternative measure against which to assess risk of exposure to infection.5,6
This study presents the results of a seroepidemiological study investigating exposure to malaria in the indigenous Batwa and neighboring non-indigenous populations in Kanungu District, located in southwestern Uganda, bordering the Democratic Republic of Congo. The district is largely rural and is characterized by mesoendemic malaria transmission with intermittent epidemics of disease.7,8 Rainfall is bimodal with peaks in April and October.
The Batwa are traditional hunter-gatherers from Uganda, Burundi, Rwanda, and eastern Congo.9 There are approximately 6,700 Batwa individuals residing in southwestern Uganda, comprising the easternmost population of central Africa's pygmy population.10 In 1992, the Batwa in Uganda were evicted from their traditional forest homelands due to the formation of Bwindi Impenetrable National Park.11 The dominant non-indigenous ethnic group in the region, the Bakiga, are traditional agriculturalists, originally migrating from Rwanda to Uganda before the time of European colonization in the early 1900s and settling in the southwestern region of the country.12
Approval for this study was granted from the research ethics boards of McGill University (Ref: A11-M120-13B), the London School of Hygiene and Tropical Medicine (Ref: 7761), the Ottawa Hospital Research Network (Ref: 20150201-01H), and the University of Guelph (Ref: 14MR002). The study design is consistent with the Canadian Tri-Council's policies and requirements of the Ethical Conduct of Research Involving Human Subjects. Informed consent was obtained verbally from all participants and village chairpersons.
Community-based cross-sectional surveys were conducted as described in detail elsewhere.13,14 In April 2014, 136 indigenous Batwa households and 201 non-indigenous Bakiga households were surveyed in the 10 local councils (hereafter referred to as “cells”) in Kanungu District, comprising all of Kanungu's 10 Batwa settlements and their neighboring Bakiga villages, resulting in a total of 543 and 731 individuals, respectively. The response rate was 94.9% and 99.4% among the Batwa and Bakiga, respectively. Ages ranged from < 1 to 96 years (mean = 20.4 years) among the Batwa and < 1 to 87 years (mean = 22.7 years) among the Bakiga. Population samples were comparable in age and sex, although the Batwa population had slightly more men (44.9% versus 38.9%, χ2 = 4.26, P = 0.039) and was slightly younger (50.5% versus 46.8% under 15 years of age, χ2 = 13.1, P = 0.011) compared with the Bakiga. Ownership and usage of insecticide-treated bednets were lower among Batwa individuals (33.8% versus 47.2%, χ2 = 22.3, P < 0.001, and 21.3% versus 40.3%, χ2 = 52.5, P < 0.001, respectively). The majority of individuals in both populations lived in houses with iron sheet roofing: 88.8% among the Batwa and 90.0% among the Bakiga (P = 0.49).
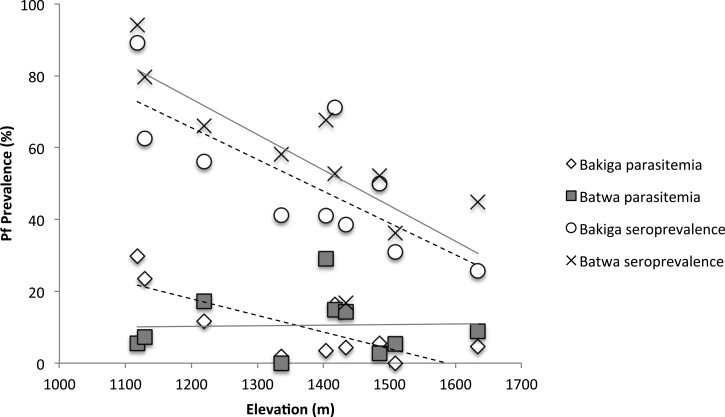
Malaria prevalence by CareStart™ malaria rapid diagnostic tests (mRDTs) was 14.9% among the Batwa compared with 9.26% among the Bakiga (χ2 = 33.0 P < 0.001). In univariable regression analyses, age, ethnicity, bednet ownership, and household roof material were associated with mRDT positivity. These variables were included in a mixed-effects logistic regression along with two cell-level factors, elevation and normalized differential vegetation index (NDVI). In the final model, after controlling for age and cell-level clustering, there were no significant effects of sex, bednet ownership, roof material, or NDVI on mRDT positivity. However, a significant altitude–ethnicity interaction effect was found. In population-specific models, higher elevation was significantly associated with lower odds of mRDT positivity among the Bakiga (odds ratio [OR] = 0.19, 95% confidence interval [CI] = 0.11–0.35, P < 0.001 at elevations 1,301–1,500 m; OR = 0.11, 95% CI = 0.04–0.33, P < 0.001 at elevations > 1,500 m). In contrast, among the Batwa, the effect was less clear, with no altitude effect at 1,301–1,500 m (OR = 1.16, 95% CI = 0.73–1.82, P = 0.534) and lower odds of mRDT positivity at elevations > 1,500 m (OR = 0.39, 95% CI = 0.20–0.71, P = 0.003) (Figure 1 ).
Figure 1.
Prevalence of malaria infection measured by malaria rapid diagnostic test (mRDT) and Plasmodium falciparum seroprevalence among the Batwa (solid line) and Bakiga (dashed line) living at different altitudes in Kanungu District, Uganda, in 2014.
Antibodies were eluted from filter paper blood spots and assayed by enzyme-linked immunosorbent assay, as previously described.5 All Batwa (N = 543) and Bakiga (N = 731) samples were tested for the presence of human antibodies (IgG) against merozoite surface protein-119 (MSP-119) and apical membrane antigen-1 (AMA-1) of Plasmodium falciparum. To define seropositive individuals, the optical density data were fitted using maximum likelihood methods to establish seronegative and seropositive Gaussian distributions. For each antigen, the cutoff was set as the mean titer of the seronegative distribution plus three standard deviations.15 Overall, seroprevalence of antibodies to AMA-1 was higher than MSP-119 in both study populations. Individuals with current P. falciparum infection measured by mRDT were significant more likely than mRDT-negative individuals to be seropositive (76.0% versus 50.5%, OR = 3.11, 95% CI = 2.02–4.78, P < 0.001) and had higher antibody responses to both MSP-119 and AMA-1 (F = 13.2, P < 0.001 and F = 6.60, P = 0.01, respectively).
In univariable regression analyses, age, ethnicity, current infection status measured by mRDT, and bednet ownership were associated with seropositivity. In a mixed-effects logistic regression, after adjusting for age, infection status, elevation, and clustering by cell, the Batwa had a 2-fold higher odds of being seropositive compared with the Bakiga (OR = 2.08, 95% CI = 1.51–2.88). The mixed-effects model showed no significant effect of sex, bednet ownership or NDVI, and no significant altitude–ethnicity interaction. Increasing elevation was significantly associated with reduced odds of seroprevalence among both the Batwa and Bakiga (OR = 0.24, 95% CI = 0.10–0.57, P = 0.001 at elevations 1,301–1,500 m; OR = 0.11, 95% CI = 0.04–0.33, P < 0.001 at elevations > 1,500 m). The Batwa had consistently higher seroprevalence than Bakiga living at the similar elevations (Figure 1).
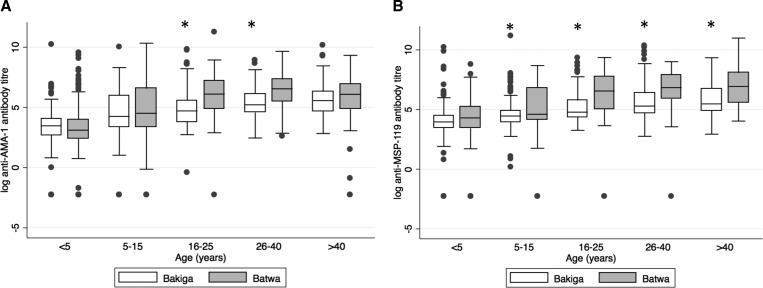
Mean MSP-119 and AMA-1 specific antibody titers were significantly lower in the Bakiga compared with the Batwa (F = 28.37, P < 0.001 and F = 5.76, P = 0.016, respectively). A comparison of antibody titers by age group is shown in Figure 2 . Reverse cumulative distribution curves of untransformed AMA-1 and MSP-119 antibody titers were generated to allow for visual inspection of variability and central tendencies of antibody data.16 These demonstrated a higher proportion of individuals with elevated antibody titers among the Batwa for both antigens.
Figure 2.
Logarithmic antibody titers by age group in the Batwa (N = 543) and Bakiga (N = 731) in Kanungu District, Uganda, in 2014 for (A) anti-AMA-1 and (B) anti-MSP-119. An asterisk indicates that significant differences exist between populations within the same age group. Sample size by age group for Batwa: < 5 years (N = 125), 6–15 years (N = 146), 16–25 years (N = 87), 26–40 years (N = 77), > 40 years (N = 104), and Bakiga: < 5 years (N = 157), 6–15 years (N = 174), 15–25 years (N = 94), 26–40 years (N = 156), > 40 years (N = 126). AMA-1 = apical membrane antigen-1; MSP-119 = merozoite surface protein-119.
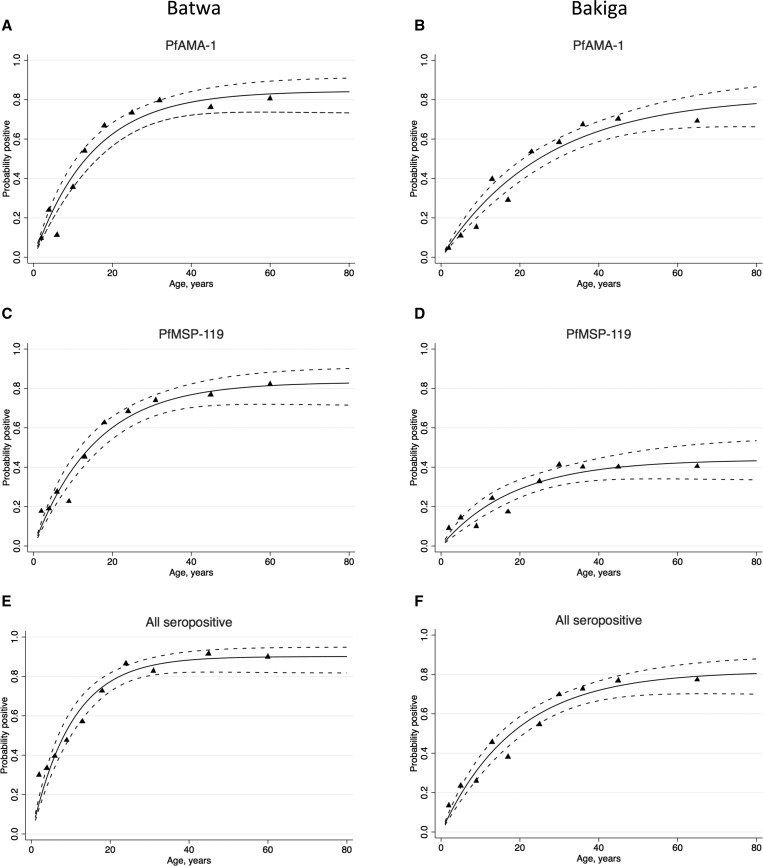
A simple reversible catalytic model was fitted to the seroprevalence data using maximum likelihood methods.4 The resulting age–seroprevalence curves illustrate the cumulative rate of exposure to P. falciparum antigens in each population (Figure 3 ). Seroconversion rates for AMA-1 and MSP-119 in the Batwa were more than 2-fold higher than those in the Bakiga (overall λ = 0.088, 95% CI = 0.071–0.109, versus λ = 0.044, 95% CI = 0.035–0.054).
Figure 3.
Age–seroprevalence curves for anti–Plasmodium falciparum antibody responses corresponding to (A) Batwa AMA-1 and (B) Bakiga AMA-1 seropositive individuals; (C) Batwa MSP-119 and (D) Bakiga MSP-119 individuals; (E) all Batwa and (F) all Bakiga seropositive individuals in Kanungu District, Uganda, in 2014. Triangles represent observed data, and the dotted blue line represents the upper and lower 95% confidence intervals for the predicted seroprevalence curve. Seroconversion rates: (A) λ = 0.057, (B) λ = 0.031, (C) λ = 0.053, (D) λ = 0.024, (E) λ = 0.088, (F) λ = 0.044. AMA-1 = apical membrane antigen-1; MSP-119 = merozoite surface protein-119.
These data strongly suggest that the indigenous Batwa of Kanungu, Uganda, are a population at heightened risk of malaria exposure and infection. In comparison to the neighboring non-indigenous Bakiga population, after accounting for age, the Batwa had twice the odds of seropositivity and double the rate of seroconversion based on malaria-specific antibodies.
Differences in elevation partly explain the between-cell differences in seroprevalence, with a trend towards lower seroprevalence at higher altitudes. This suggests that higher seroprevalence reflects increased exposure to infected vectors at lower altitudes. However, higher seroprevalence of antibodies to AMA-1 and MSP-119 antigens was observed among the Batwa relative to the Bakiga irrespective of altitude level, indicating that other population-specific determinants for malaria exposure may exist, and that certain factors may increase the Batwa's vulnerability to malaria exposure relative to their Bakiga neighbors. In addition to higher seroprevalence among the Batwa, the magnitude of antibody titers indicates that the Batwa are producing a significantly higher immune response to both MSP-119 and AMA-1 in relation to their non-indigenous neighbors. This could be explained by a lack of prior exposure to malaria parasites due to their previous habitation within the forest, resulting in the Batwa being immunologically naive to Plasmodium infection. Alternatively, genetic differences may play a role in the Batwa's production of such a strong immunological response to malarial infection, similar to the Fulani tribe of Burkina Faso,17–19 though the higher prevalence of infections among the Batwa suggests that this explanation, at least as a protective effect, is less likely.
This study has several limitations. The survey design did not permit georeferencing individual household locations; therefore, no spatial analysis or household-level entomological assessment was possible to investigate patterns of transmission within individual cells. Further immunological and genetic studies would be needed to investigate whether the Batwa's antibody response confers protective immunity or is genetically mediated; however, this was beyond the scope of the present study.
For the Batwa, the causes of higher malaria exposure and antibody levels are likely multifactorial. Further characterization of malaria transmission in Batwa and Bakiga communities through spatial analysis and entomological evaluation of malaria vectors, along with cohort studies on coincidence of infection using a more sensitive malaria diagnostic such as polymerase chain reaction and antibody responses to a broader suite of antigens, would help to clarify population heterogeneities.
ACKNOWLEDGMENTS
We thank the Batwa and Bakiga community members who participated in the study as well as Sabastian Twesigomwe and the Batwa Development Programme (BDP) for logistical and research support. Isaac Ssewanyana and Chris Drakeley acknowledge funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases (U19AI089674).
Disclaimer: This study was conducted in collaboration with the Indigenous Health Adaptation to Climate Change (IHACC) project, an international initiative with parallel field sites in the Canadian Arctic and Peruvian Amazon (www.ihacc.ca). IHACC Research Team: James Ford, Alejandro Llanos, Cesar Carcamo, and Victoria Edge.
Footnotes
Authors' addresses: Manisha A. Kulkarni, School of Epidemiology, Public Health and Preventive Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Canada, E-mail: manisha.kulkarni@uottawa.ca. Gala Garrod and Chris Drakeley, Department of Immunology and Infection, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mails: galagarrod@hotmail.co.uk and chris.drakeley@lshtm.ac.uk. Lea Berrang-Ford, Nestor Baraheberwa, and Blanaid Donnelly, Department of Geography, McGill University, Montreal, Canada, E-mails: lea.berrangford@mcgill.ca, nestor.baraheberwa@umontreal.ca, and blanaid.d@gmail.com. Isaac Ssewanyana, Department of Immunology and Infection, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom, and Infectious Disease Research Collaboration (IDRC), Kampala, Uganda, E-mail: sewyisaac@yahoo.co.uk. Sherilee L. Harper and Kaitlin Patterson, Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Canada, E-mails: harpers@uoguelph.ca and kpatte08@uoguelph.ca. Didacus B. Namanya, Ministry of Health, Kampala, Uganda, E-mail: didamanya@yahoo.com. Shuaib Lwasa, Department of Geography, Makerere University, Kampala, Uganda, E-mail: shuaiblwasa@gmail.com.
References
- 1.Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, Otieno S, Carneiro I, Cox J, Msuya E, Kleinschmidt I, Maxwell C, Greenwood B, Riley E, Sauerwein R, Chandramohan D, Gosling R. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201:1764–1774. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni MA, Desrochers RE, Kerr JT. High resolution niche models of malaria vectors in northern Tanzania: a new capacity to predict malaria risk? PLoS One. 2010;5:e9396. doi: 10.1371/journal.pone.0009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:19. doi: 10.1186/1475-2875-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SLR, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WMM, Lemnge MM, Cox J, Reyburn H, Riley EM. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, Cox J, Abeku T, Bousema T, Ghani AC, Drakeley C, Riley E. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195. doi: 10.1186/1475-2875-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helb DA, Tetteh KKA, Felgner PL, Skinner J, Hubbard A, Arinaitwe E, Mayanja-Kizza H, Ssewanyana I, Kamya MR, Beeson JG, Tappero J, Smith DL, Crompton PD, Rosenthal PJ, Dorsey G, Drakeley CJ, Greenhouse B. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci USA. 2015;112:E4438–E4447. doi: 10.1073/pnas.1501705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 8.Lewnard JA, Berrang-Ford L, Lwasa S, Namanya DB, Patterson KA, Donnelly B, Kulkarni MA, Harper SL, Ogden NH, Carcamo CP. Relative undernourishment and food insecurity associations with Plasmodium falciparum among Batwa pygmies in Uganda: evidence from a cross-sectional survey. Am J Trop Med Hyg. 2014;91:39–49. doi: 10.4269/ajtmh.13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis J. The Batwa Pygmies of the Great Lakes Region. London, United Kingdom: Minority Rights Group International; 2000. [Google Scholar]
- 10.Republic of Uganda . The State of the Uganda Population Report: The Role of Culture, Gender and Human Rights in Social Transformation and Sustainable Development. Kampala, Uganda: UNFPA; 2008. [Google Scholar]
- 11.Balenger S, Fried S. Between Forest and Farm: Identifying Appropriate Development Options for the Batwa of Southwestern Uganda. Washington, DC: George Washington University; 2005. [Google Scholar]
- 12.Turyahikayo-Rugyema B. The History of the Bakiga in Southwestern Uganda and Northern Rwanda. Ann Arbor, MI: University of Michigan Press; 1974. pp. 1500–1930. [Google Scholar]
- 13.Clark S, Berrang-Ford L, Lwasa S, Namanya D, Twesigomwe S, Kulkarni M. A longitudinal analysis of mosquito net ownership and use in an Indigenous Batwa population after a targeted distribution. PLoS One. 2016;11:e0154808. doi: 10.1371/journal.pone.0154808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly B, Berrang-Ford L, Labbé J, Twesigomwe S, Lwasa S, Namanya DB, Harper SL, Kulkarni M, Ross NA, Michel P. Plasmodium falciparum malaria parasitaemia among indigenous Batwa and non-indigenous communities of Kanungu district, Uganda. Malar J. 2016;15:254. doi: 10.1186/s12936-016-1299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, Masika P, Mosha J, Bousema T, Shekalaghe S, Cook J, Corran P, Ghani A, Riley EM, Drakeley C. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed GF, Meade BD, Steinhoff MC. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics. 1995;96:600–603. [PubMed] [Google Scholar]
- 17.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 18.Modiano D, Luoni G, Sirima BS, Lanfrancotti A, Petrarca V, Cruciani F, Simporé J, Ciminelli BM, Foglietta E, Grisanti P, Bianco I, Modiano G, Coluzzi M. The lower susceptibility to Plasmodium falciparum malaria of Fulani of Burkina Faso (west Africa) is associated with low frequencies of classic malaria-resistance genes. Trans R Soc Trop Med Hyg. 2001;95:149–152. doi: 10.1016/s0035-9203(01)90141-5. [DOI] [PubMed] [Google Scholar]
- 19.Modiano D, Petrarca V, Sirima BS, Luoni G, Nebie I, Diallo DA, Esposito F, Coluzzi M. Different response to Plasmodium falciparum in west African sympatric ethnic groups: possible implications for malaria control strategies. Parassitologia. 1999;41:193–197. [PubMed] [Google Scholar]