Abstract
Mass drug administration (MDA) targeting school-age children is recommended by the World Health Organization for the global control of soil-transmitted helminth (STH) infections. Although considered safe and cost-effective to deliver, benzimidazole anthelminthics are variably effective against the three most common STHs, and widespread use has raised concern about the potential for emerging resistance. To identify factors mediating response to albendazole, we conducted a cross-sectional study of hookworm infection in the Kintampo North Municipality of Ghana in 2011. Among 140 school-age children residing in five contiguous communities, the hookworm prevalence was 59% (82/140). The overall cure rate following administration of single-dose albendazole (400 mg) was 35% (27/76), with a community-wide fecal egg reduction rate (ERR) of 61% (95% confidence interval: 51.8–71.1). Significant disparities were observed in albendazole effectiveness by community, with a cure rate as low as 0% (N = 24) in Jato Akuraa and ERRs ranging from 53% to 95% across the five study sites. Individual host factors associated with response to deworming treatment included time since last meal, pretreatment blood hemoglobin level, and mid-upper arm circumference. These data demonstrate significant community-level variation in the effectiveness of albendazole, even among populations living in close proximity. Identification of host factors that influence response to albendazole, most notably the timing of drug administration and nutritional factors, creates an opportunity to enhance the effectiveness of deworming through targeted interventions. These findings also demonstrate the importance of measuring anthelminthic response as part of the monitoring and evaluation of community-based deworming programs.
Introduction
Soil-transmitted helminth (STH) infections, which include hookworms (Necator americanus and Ancylostoma spp.), Ascaris lumbricoides, and Trichuris trichiura, represent a leading global cause of anemia, malnutrition, and growth delay, especially among children and women of childbearing age.1–4 Together, these three intestinal nematodes infect more than 1 billion people worldwide, with twice that number currently living in endemic areas. Among the STHs, hookworms are associated with a significant burden of disease, which is primarily attributable to gastrointestinal blood loss caused by adult worms that attach to the intestinal mucosa and feed on blood from lacerated capillaries. Recent estimates suggest that there are more than 400 million people infected with hookworm, nearly all of whom live in rural poverty.5
The World Health Organization (WHO) currently recommends periodic (annual or semiannual) mass drug administration (MDA) of anthelminthics to school-age children (SAC) to reduce morbidity associated with moderate-to-high intensity infection.6,7 The costs associated with the distribution of single-dose treatment with benzimidazole anthelminthics (albendazole or mebendazole) have been significantly reduced by large-scale donations from pharmaceutical manufacturers.8–10 With rare exceptions, the long-standing experience with benzimidazoles suggests that they are safe, even in young children and pregnant women, making them the preferred agents for MDA programs in most endemic areas.11,12 Modeling studies suggest that MDA has a favorable cost-benefit ratio and that scaling up of drug distribution could reduce costs further through economies of scale.13–15 However, despite the favorable financial profile of MDA, evidence in support of sustainable health and educational benefits from deworming is lacking, as results from carefully controlled trials vary widely.16–20
Beyond the individual health impact, questions have also been raised about the degree to which current deworming strategies are likely to achieve widespread disease control or elimination in endemic areas. For example, MDA programs that only treat children enrolled in formal education will not reach those who do not regularly attend, nor will it address the potentially significant adult reservoirs of infection within targeted communities.21–24 Second, the widespread distribution of benzimidazole agents may lead to resistance among human STHs, the impact of which on global control efforts has not been carefully assessed.25–27 In fact, reduced efficacy in communities subjected to long-standing MDA suggests that resistance may already have emerged, a phenomenon that is well described in veterinary nematodes.27–33
Previous studies in Kintampo North Municipality (KNM), Ghana, have shown that malnutrition (as measured by body mass index [BMI]) is a risk factor for hookworm infection in adults,34 whereas infection in SAC was associated with reduced dietary intake of animal-based food.35 To further characterize the association between host nutritional factors on hookworm infection status and deworming response in SAC, we conducted a follow-up cross-sectional study of SAC in KNM. The data show that albendazole response varies by community and baseline nutritional status, and that potentially modifiable factors like timing of drug administration significantly improve deworming effectiveness. These results confirm the previously identified link between malnutrition and hookworm infection, while also suggesting a potentially important role for nutritional status in mediating anthelminthic activity in humans.
Methods
Ethical review and enrollment.
Prior to recruitment, ethical approval was obtained from the Yale University Human Investigation Committee (HIC no. 07050022669) and the Institutional Review Boards of the Ghana Health Service (GHS), the Noguchi Memorial Institute for Medical Research, and the Kintampo Health Research Center (KHRC). Information meetings were organized at local schools and parents of eligible children were invited to attend. School children were selected from five communities previously identified as having a high prevalence of hookworm infection.34,35 Children between the ages of 6 and 13 were eligible if they were enrolled in primary school, resided within the study area, and were willing and able to give informed consent. Only one child per household was enrolled in the study. Sample size was calculated to provide power to test differences between dietary recall data and child dietary diversity, as the primary objective of the study was to identify nutritional factors associated with hookworm infection and response to treatment. Fecal samples and household surveys were collected from 140 study subjects who were randomly selected from the pool of 254 eligible children (Figure 1 ).
Figure 1.
Study sample flow diagram.
Household survey.
Two teams of researchers and translators conducted in home interviews during June and July 2011. A pretested standardized questionnaire was used to gather socioeconomic data, including types of household construction, sources of water and sanitation, level of parental education and occupation, and ownership of select household assets (e.g., consumer goods, land, and livestock).35 Standard measures of food security,36 dietary diversity,37 and details of recent medical history were collected as part of the individual survey. All survey questions were conducted in the local language, Twi.
Anthropometry.
Weight was measured to the nearest 0.1 kg using an electronic balance and height was measured to the nearest 0.1 cm using a portable fixed stadiometer. BMI for age Z scores were calculated for all participants.38 Mid-upper arm circumference (MUAC) was measured for all participants to the nearest 0.1 cm at the midpoint of the left arm using a standardized tape ribbon.39,40
Hookworm diagnosis and treatment.
Children were asked to provide a fresh, morning fecal sample in a previously provided clean plastic cup. Individual samples were analyzed using the Kato–Katz fecal thick smear technique for estimation of eggs per gram (EPG) of feces.34,35,41 All samples were processed according to WHO-recommended laboratory methods,42 and microscopy readings were taken within 30–60 minutes of Kato–Katz slide preparation. Duplicate counts were analyzed from each sample and the mean value recorded. Children infected with hookworm were referred to GHS officials for treatment with a single oral dose of albendazole (Zentel 400 mg; Glaxo Smith Kline, Bangalore, India).34,35 All study medications were administered under direct observation by GHS Pharmacists based at the Kintampo Health Center on one of two treatment days (June 24 or June 30, 2011), and the anthelminthic activity of the albendazole preparation used in the study was confirmed in the field using an in vitro assay.43 Stool specimens were collected from treated individuals 10–14 days later and evaluated by fecal microscopy using the Kato-Katz method as described above. This time point was chosen to measure the effect of treatment on fecal egg excretion before reinfection.44 Subjects who remained infected were referred for a second treatment with albendazole.
Blood and serum analysis.
Approximately 1 mL of blood was obtained by venipuncture from study participants for automated complete blood count analysis conducted at the KHRC. Whole blood was analyzed using a malaria rapid diagnostic test kit (First Response Malaria Ag HRP-2; Premier Medical Corporation Ltd., Kachigam Daman, India) for the presence of circulating Plasmodium falciparum antigens.35 Thick and thin blood smears were prepared from positive samples for further confirmation using light microscopy. All study subjects appeared well and without symptoms of malaria at the time of screening.
Statistical analysis.
The response to treatment of STH infections is traditionally assessed using two indicators: the fecal egg reduction rate (ERR) and the cure rate (CR), both of which are useful for monitoring effectiveness in field settings.25,30,34,35 The individual ERR was calculated according to the following formula:
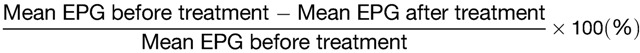 |
Community ERR values were calculated by averaging the individual values for study subjects living within each community. In subjects with higher EPG values observed posttreatment, which would result in a negative value for ERR, we defined the value as zero for the purposes of community averages.
The CR was calculated as the percentage of treated children in whom the findings of posttreatment fecal microscopy exam were negative:
 |
Data were entered into Microsoft Excel (Redmond, WA) and analyzed using SPSS (version 19; IBM, Armonk, NY) and Stata (Intercooled 12.0; Stata Corp, College Station, TX) software packages.
To define socioeconomic status, we used principal components analysis and extracted the first component, as an index of wealth, from a consumer goods index (improved cooking fuel, improved toilet, improved drinking water, electricity, radio, television, telephone, refrigerator, bicycle, bank account, improved floor, and improved roof) and an agricultural goods index (land, horse or donkey, goat or sheep, poultry, and pigs).45 Univariate analysis was performed for descriptive purposes of baseline and posttreatment populations. χ2 tests, Kruskal–Wallis, and analysis of variance were used to test for differences between populations and groups. A zero-or-one inflated beta regression model46 was used to identify predictors of ERR, allowing separate analysis of children with 100% reduction, 0% reduction, and variable reduction from 1% to 99%. Logistic regression was used to identify predictors of cure, that is, 100% ERR.
Results
Study population characteristics.
Of the 254 potentially eligible SAC residing in five villages in the Kintampo North District in the Brong Ahafo region in central Ghana, 140 subjects (range 6–13 years of age) were randomly selected and enrolled after providing informed consent (Figure 1). Significant differences across the five communities were identified in measures of anthropometry, nutritional status, wealth index, and hookworm infection status (Table 1). Specific anthropometric and nutritional factors that varied significantly between communities included age (P = 0.001) and MUAC (P = 0.01). Although average household wealth and consumer/financial wealth were comparable across the five study communities, we did find a significant difference in agricultural wealth index (P = 0.003). Other socioeconomic measures that varied between communities included access to improved drinking water (P = 0.03) and pig ownership (P = 0.01).
Table 1.
Study population and community variation
| Village (North > South) | Atta Akuraa (N = 40) | Cheranda (N = 26) | Jato Akuraa (N = 34) | Mahama Akuraa (N = 18) | Tahiru Akuraa (N = 22) | P value |
|---|---|---|---|---|---|---|
| Anthropometry/nutrition | ||||||
| Age (years)* | 10.1 (9.8, 10.5) | 10.2 (9.3, 11.1) | 9.1 (8.6, 9.7) | 10.6 (9.8, 11.4) | 9.0 (8.6, 9.4) | 0.001 |
| Female | 21 (53%) | 17 (65%) | 20 (59%) | 7 (39%) | 8 (36%) | 0.2 |
| Body mass index | 15.3 (15.1, 15.6) | 15.6 (15.2, 16.0) | 15.2 (14.8, 15.6) | 16.0 (15.4, 16.6) | 15.2 (14.7, 15.6) | 0.07 |
| Mid-upper arm circumference (cm) | 18.9 (18.5, 19.3) | 19.3 (18.5, 20.0) | 18.3 (17.7, 18.8) | 19.2 (18.6, 19.7) | 18.0 (17.6, 18.4) | 0.01 |
| Hemoglobin (g/dL) | 12.0 (11.2, 12.7) | 11.9 (11.1, 12.6) | 11.2 (10.8, 11.6) | 11.6 (11.1, 12.0) | 11.9 (11.5, 12.2) | 0.3 |
| WBC (× 103) | 7.44 (6.7, 8.2) | 6.72 (6.0, 7.4) | 7.88 (7.0, 8.8) | 6.67 (5.7, 7.6) | 7.22 (6.3, 8.1) | 0.3 |
| Food insecure households | 33 (83%) | 21 (81%) | 29 (85%) | 16 (89%) | 16 (72%) | 0.7 |
| Measures of Wealth | ||||||
| Average wealth index | −0.45 (−0.81, −0.08) | −0.01 (−0.65, 0.62) | 0.35 (−0.19, 0.88) | 0.20 (−0.6, 0.99) | 0.16 (−0.44, 0.77) | 0.2 |
| Consumer and financial wealth index | 0.09 (−0.36, 0.55) | 0.32 (−0.24, 0.88) | −0.07 (−0.57, 0.42) | −0.25 (−0.90, 0.39) | −0.22 (−0.65, 0.21) | 0.6 |
| Agricultural wealth index |
−0.52 (−0.82, −0.23) | −0.10 (−0.55, 0.35) | 0.41 (−0.00, 0.81) | 0.31 (−0.21, 0.84) | 0.21 (−0.21, 0.63) | 0.003 |
| Improved toilet | 12 (30%) | 18 (69%) | 0 (0%) | 0 (0%) | 15 (68%) | < 0.001 |
| Improved drinking water | 7 (18%) | 5 (19%) | 10 (29%) | 8 (44%) | 11 (50%) | 0.03 |
| Visible trash | 12 (30%) | 8 (32%) | 13 (39%) | 5 (28%) | 7 (32%) | 0.9 |
| Pig ownership | 3 (8%) | 6 (23%) | 14 (41%) | 5 (28%) | 9 (41%) | 0.01 |
| Baseline hookworm infection status | ||||||
| Positive (N = 82) | 23 (58%) | 11 (42%) | 24 (71%) | 15 (83%) | 9 (41%) | 0.02 |
| 95% CI | 40–72 | 27–57 | 53–85 | 59–96 | 21–64 | |
| Negative (N = 58) | 17 (43%) | 15 (58%) | 10 (29%) | 3 (17%) | 13 (59%) | |
| Baseline EPG (arithmetic mean) | 322 (109) | 175 (81) | 298 (84) | 324 (157) | 161 (72) | 0.1 |
| Baseline EPG (geometric mean) | 230 (125) | 200 (85) | 181 (97) | 126 (56) | 181 (51) | 0.07 |
| Malaria intensity | ||||||
| Negative | 13 (33%) | 9 (38%) | 8 (24%) | 2 (11%) | 6 (29%) | 0.1 |
| 1–999 parasites/μL | 21 (54%) | 11 (46%) | 12 (35%) | 11 (61%) | 9 (43%) | |
| ≥1,000 parasites/μL | 5 (13%) | 4 (17%) | 14 (41%) | 5 (28%) | 6 (29%) | |
| Posttreatment hookworm infection status | ||||||
| Positive (N = 49) | 6 (29%) | 2 (18%) | 24 (100%) | 12 (86%) | 7 (88%) | < 0.001 |
| Negative (N = 27) | 15 (71%) | 9 (82%) | 0 | 2 (14%) | 1 (13%) | |
CI = confidence interval; EPG = eggs per gram; WBC = white blood cell.
Numbers in parentheses represent standard deviation from mean. Arithmetic means analyzed using Kruskal–Wallis nonparametric test; geometric means analyzed by analysis of variance.
Community-level variation in the prevalence of hookworm and response to deworming treatment.
The baseline prevalence of hookworm infection among all study participants within the surveyed communities was 59% (82/140) (Figure 1). Among the 82 subjects infected at baseline, 79 (96%) were classified as light intensity (1–1,999 EPG), whereas three (4%) were in the moderate (2,000–4,999 EPG) category. As shown in Table 1, there was significant variation in hookworm prevalence among the five study communities: Mahama Akuraa (83%), Jato Akuraa (71%), Atta Akuraa (58%), Cheranda (42%), and Tahiru Akuraa (41%) (P = 0.02). Despite the variation in hookworm prevalence, there was no difference in intensity of infection, as measured by mean hookworm EPG excreted in feces. There was also no statistically significant difference in the prevalence of P. falciparum malaria infection, which ranged from 62% in Cheranda to 89% in Mahama.
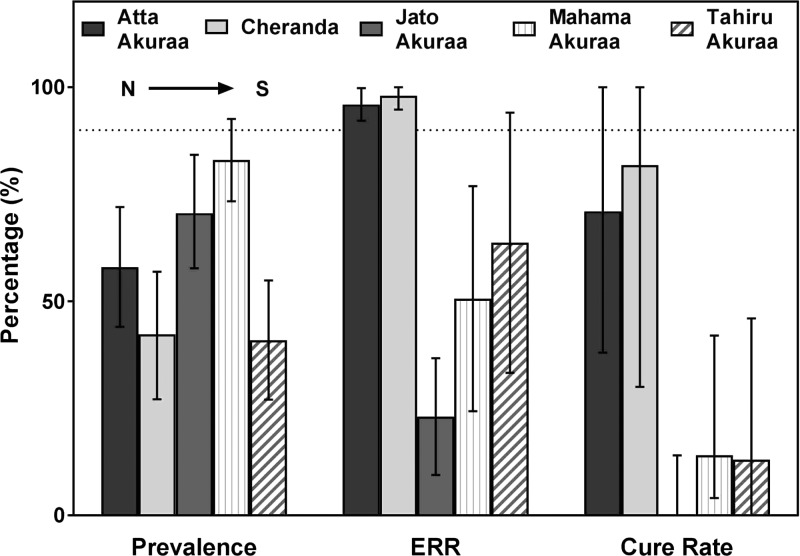
The overall CR following single-dose albendazole across the five communities was 36% (27/76) (Figure 1), which is comparable to prior studies of hookworm in Kintampo.34,35 However, there was a statistically significant difference in CR following albendazole treatment between communities (Table 1; P < 0.001) (Figure 2 ). The lowest CR recorded among the five study communities was 0% (N = 24) in Jato Akuraa, whereas Cheranda had the highest CR (82%; N = 11). The difference in effectiveness did not correlate with pretreatment community prevalence or intensity of infection. For example, the community with the lowest prevalence, Tahiru Akuraa (41%), showed a CR of only 22% (N = 8).
Figure 2.
Community variation in hookworm prevalence and response to albendazole, as measured by egg reduction rate (ERR) and cure rate. Error bars represent 95% confidence intervals. Horizontal dotted line represents the World Health Organization's 2013 ERR standard (90%) for deworming effectiveness.
The ERR measured among all study subjects was lower than expected at 61% (95% confidence interval [CI]: 51.8–71.1), with significant variation noted between communities (Figure 2). The overall ERR, as well as individual community values from Jato (53%), Mahama Akuraa (69%), and Tahiru Akuraa (84%) each fell short of the 90% target defined in 2013 by the WHO for effective response to single-dose albendazole.30,42 By contrast, Atta Akuraa (95%) and Cheranda (96%) met the expected standard.
Individual host factors associated with hookworm infection status and response to treatment.
Among all study subjects (N = 140), those infected with hookworm at baseline were older in age (10.1 ± 4 versus 9.3 ± 4 years; P = 0.005), and the prevalence was higher in males (46/67; 69%) than females (36/73; 49%) (P = 0.02). In addition, hookworm-infected children were less likely to reside in households using an improved toilet (26% versus 41%; P = 0.049), and more likely to live in households with visible trash (40% versus 23%; P = 0.04).
Regarding response to treatment (Table 2), children who were cured of hookworm following albendazole treatment had a higher mean MUAC (19.5 versus 18.7 cm; P = 0.02) and a higher mean blood hemoglobin level (12.8 versus 11.4 g/dL; P < 0.001). Cure was also significantly more likely in children living in households with access to improved toilets (41% versus 18%; P = 0.03). The timing of food intake also impacted response to albendazole. Among infected children who had not eaten at least 6 hours before treatment, the CR was 90%, whereas in those who had eaten more recently, the CR was significantly lower at 59% (P = 0.02).
Table 2.
Individual host factors associated with albendazole response
| Hookworm status posttreatment | |||
|---|---|---|---|
| Positive (N = 51) | Negative (N = 27) | P value | |
| Age (years)* (N = 74)† | 9.9 (9.4, 10.3) | 10.5 (9.9, 11.1) | 0.1 |
| Female (%) | 20 (39.2%) | 14 (51.9%) | 0.3 |
| Hemoglobin (g/dL)* | 11.43 (11.2, 11.7) | 12.8 (11.9, 13.8) | < 0.001 |
| Mid-upper arm circumference (cm)* | 18.7 (18.3, 19.1) | 19.5 (18.9, 20.1) | 0.02 |
| Body mass index* | 15.6 (15.2, 15.9) | 15.7 (15.4, 16.1) | 0.5 |
| Improved toilet | 9 (17.7%) | 11 (40.7%) | 0.03 |
| Visible trash (N = 77) | 21 (42.0%) | 11 (40.7%) | 0.9 |
| Last meal less than 6 hours before treatment (N = 77) | 45 (90.0%) | 16 (59.3%) | 0.002 |
| Number of bowel movements (24 hours)* | 1.1 (0.9, 1.3) | 1.5 (1.0, 1.9) | 0.1 |
Numbers in parentheses represent standard deviation from mean.
N = 78 unless otherwise indicated.
Using multivariate analysis, fasting at least 6 hours before albendazole treatment remained a strong predictor of hookworm cure. The unadjusted odds ratio (OR) predicting the likelihood of cure was 9.52 (95% CI: 2.6–34.9; P < 0.01) in subjects who had not eaten in 6 hours or more before treatment. Blood hemoglobin level and MUAC also correlated with albendazole effectiveness (Table 3). For every increase of 1 g/dL in blood hemoglobin level, the OR for being cured of hookworm was 1.75 (95% CI: 1.2–2.6; P < 0.01). Likewise, for every increase of 1 cm in MUAC, the OR for hookworm cure was 1.47 (95% CI: 1.03–2.1; P < 0.05). When controlling for age and sex of study subjects, the adjusted odds ratio (AOR) for albendazole cure remained statistically significant for time (> 6 hours) since last meal (AOR: 8.59; 95% CI: 2.3–32.3; P < 0.001) and blood hemoglobin level (AOR: 1.77; 95% CI: 1.17–2.67; P < 0.01) (Table 3). However, the AOR (1.36) for MUAC was no longer statistically significant (CI: 0.91–2.03; P = 0.1) when controlling for age and sex.
Table 3.
Host factors associated with complete response to albendazole treatment†
| Crude OR | Adjusted OR† | |
|---|---|---|
| Last meal more than 6 hours before treatment (N = 73) | 9.52 (2.6, 34.9)** | 8.59 (2.3, 32.3)** |
| Hemoglobin (g/dL)‡ (N = 74) | 1.75 (1.2, 2.6)** | 1.77 (1.2, 2.7)** |
| MUAC (cm)§ (N = 74) | 1.47 (1.03, 2.1)* | 1.36 (0.91, 2.03) |
MUAC = mid-upper arm circumference; OR = odds ratio.
P < 0.05;
P < 0.01.
Adjusted for child age and sex.
For every increase of 1 g/dL in blood hemoglobin, there is a corresponding 75% (crude OR) increase in the probability of being negative following treatment. After adjusting for child age and sex, for every 1 g/dL increase in hemoglobin there is a 77% increase in the probability of being negative following drug treatment.
For every 1 cm increase in MUAC, there is a corresponding 47% increase in the probability of being negative following albendazole treatment (unadjusted). After adjusting for child age and sex, there is a 36% increase in the probability of being negative following treatment of every 1-cm increase in MUAC.
Discussion
A central finding of this study is the identification of modifiable host factors associated with improved treatment response following single-dose albendazole (400 mg). We and others have previously observed that host nutritional factors, most notably BMI, anemia, and dietary diversity, were predictors of hookworm infection among people living in endemic areas.35,47–49 To our knowledge, however, this is the first report of an association between specific host nutritional factors and the effectiveness of anthelminthic chemotherapy, specifically the commonly used drug albendazole. Initial multivariate analysis revealed that increasing blood hemoglobin level (OR: 1.75; P < 0.01) and increasing MUAC (OR: 1.47; P < 0.05) reduced the probability of remaining infected after treatment (Table 3). We also observed that children who had fasted 6 hours before treatment were significantly more likely to be cured of hookworm (OR: 9.52; P < 0.01). When further adjusted for potential confounding variables, for example, age and sex, these factors remained strongly associated with enhanced treatment response.
Hookworms infect more than 400 million people worldwide, and are responsible for two-thirds of the nearly 5 million years lived with disability that have been attributed to STH infections.5 These blood-feeding intestinal parasites have long been associated with malnutrition, the putative mechanisms of which include an effect on appetite, absorption of macronutrients, the loss of iron and serum proteins through gastrointestinal hemorrhage, chronic intestinal inflammation, and malabsorption.47,49–54 Data from animal models have also shown that poor host nutritional status is also associated with an increased susceptibility to helminth infections, and that malnutrition represents an independent risk factor for infection.53,55–57
The global strategy for control of hookworm and other STH infections, as outlined by the WHO, involves preventive chemotherapy using one of four anthelminthics, administered 1–2 times per year depending on estimates of community prevalence. Currently, MDA is broadly recommended for SAC, in whom the morbidity from hookworm is thought to be highest. Recent targets established by the WHO propose scaling-up MDA to reach 75% of the population at risk by 2020,6 with much of the cost defrayed through donations of anthelminthics by the pharmaceutical industry.
However, recent controversy affirms that there is no consensus view on the long-term benefit of MDA as a sustainable approach to helminth control.16,58 First, although deworming confers short-term nutritional benefit to individual children, randomized trials have not shown consistent long-term benefits in terms of physical development, cognitive function, school performance, or birth outcomes.16 Second, because infected adults serve as a substantial reservoir of STHs across endemic communities, targeting SAC alone may not substantially reduce overall prevalence or risk of reinfection, both of which are necessary for ultimate elimination.9,21 Third, widespread exposure to benzimidazoles, for example, albendazole and mebendazole, has the potential to accelerate the emergence of genetically mediated resistance in human nematode parasites.25,27,59
Although it has been observed in the veterinary context, there is no evidence to date that this genetically mediated resistance has reduced the effectiveness of benzimidazoles for STH infections in human populations.33,60–63 However, it may be possible to extrapolate from ongoing lymphatic filariasis control programs in Ghana, which advocate treatment of all community members, not just SAC. In these populations, there have been reports of reduced effectiveness of ivermectin against Onchocerca volvulus,64 whereas Wuchereria bancrofti worms collected in communities exposed to combination treatment (albendazole and ivermectin) are more likely to harbor genetic mutations associated with benzimidazole resistance.65 These observations highlight the need for regular monitoring of treatment response in endemic communities, as well as periodic assessment of resistance using genetic analysis of parasite DNA.
Studies published over the past 15 years have defined the epidemiology of hookworm infection in Ghana, both in the centrally located Kintampo and more northern regions of the country.34,35,43,66–69 Our work has characterized risk factors for infection and the response to deworming treatment in communities located along approximately 100 km of paved road in KNM. These communities exhibit a moderate prevalence of hookworm in the absence of other STHs, as well as a high prevalence of asymptomatic P. falciparum malaria. The response to treatment with single-dose albendazole (400 mg) has been highly variable, as measured by CR and ERR.34,35 The cross-sectional study reported here was designed to identify nutritional factors associated with deworming response, and revealed significant variability at both the community and individual level. First, among the five villages surveyed along the main Kintampo road, we identified significant community-level variation in hookworm prevalence, which ranged from 41% to 83% (Figure 2; Table 1). We also observed a substantial disparity in the effectiveness of albendazole at the community level, as measured by CR and ERR. For example, Atta Akuraa (71%) and Cheranda (82%) showed very high CRs compared with the southern communities Mahama Akuraa (14%) and Tahiru Akuraa (13%). Most striking, and of greatest concern, is the CR of 0% (N = 24) among treated children in Jato Akuraa, despite an identical treatment protocol using the same source of drug. Of note, the ERR data roughly mirrored the CR data, with the highest reductions in intensity of infection found in the villages of Atta Akuraa (95%) and Cheranda (96%) (Figure 2) and more modest therapeutic effect in Jato Akuraa (53%), Mahama Akuraa (67%), and Tahiru Akuraa (84%).
Although the cause of these widely variable responses to albendazole across the Kintampo communities is likely multifactorial, WHO guidelines recommend that an ERR of less than 90% for hookworm following albendazole treatment should elicit concern about potential parasite resistance.42 These concerns have been raised even though at present there is no evidence that genetically mediated resistance plays a role in reducing the effectiveness of albendazole against human hookworms. In fact, studies published have failed to demonstrate significant frequencies of resistance associated gene mutations circulating in N. americanus hookworm samples collected from individuals living in endemic areas.31,70 Although the low CRs and ERRs reported here, especially in Jato Akuraa (0% CR), are worrisome, genetically mediated benzimidazole resistance is only one of a number of potential explanations.
The observation that fasting enhances albendazole activity against hookworm is supported by animal studies on drug bioavailability and pharmacokinetics.71,72 In fact, Lange and others previously suggested that treatment of intraluminal infections, such as hookworm, might be enhanced if the drugs were administered on an empty stomach, since absorption of albendazole is thought to be increased when taken with meals.71 The data reported here supports the idea that conditions associated with reduced absorption of albendazole may improve its effectiveness against hookworm and potentially other intestinal nematodes. Therefore, given our observation that food intake influences treatment response in children, attention to this modifiable factor represents an intervention that could increase CRs and enhance the positive impact of deworming programs at no additional cost. We therefore recommend that specific guidelines be developed to address the timing of albendazole when administered under school-based deworming programs.
Because malnutrition also appears to be an independent risk factor for hookworm infection,34,35 improving the nutritional status of children living in hookworm-endemic areas could potentially produce a double benefit, that is, by both enhancing the response to deworming and reducing susceptibility to infection. For example, defining the prevalence of anemia and nutritional status (via MUAC) within schools or communities could identify children in whom albendazole effectiveness might be less than adequate. Conversely, careful monitoring of treatment response could identify individuals in whom deworming effectiveness might be improved through nutritional support. Of course, results from this relatively small, cross-sectional study will need to be validated using a prospective study design before any changes in current recommendations or policies regarding the implementation of MDA programs can be considered.
In summary, these data demonstrate the potential value of monitoring the response to drug treatment at both the community and individual level, so that significant variations in outcome can be factored into predictive models of prevalence, intensity, and emerging resistance. More importantly, the identification of specific host nutritional factors that significantly impact drug effectiveness highlights the critical importance of providing nutritional support as a means of reducing the global health impact of hookworm and other globally important STN infections.
ACKNOWLEDGMENTS
We would like to thank the staff of the Kintampo Health Research Center, as well as Martin Keil, Rebecca Treger, and Jon Vermeire for technical assistance during the course of the field study.
Footnotes
Financial support: This work was supported by National Institutes of Health grant 1R01AI099623, a Wilbur Downs International Health Research Fellowship, and the Yale College Dean's Research Fellowship.
Authors' addresses: Debbie Humphries and Sara Nguyen, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, Yale University, New Haven, CT, E-mails: debbie.humphries@yale.edu and sara.ann.nguyen@gmail.com. Sunny Kumar, Lisa M. Harrison, and Michael Cappello, Department of Pediatrics, Yale School of Medicine, New Haven, CT, E-mails: sunnykumar.yale@gmail.com, lisa.harrison@yale.edu, and michael.cappello@yale.edu. Josephine E. Quagraine, Joseph Otchere, and Michael Wilson, Department of Parasitology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana, E-mails: jquagraine2@gmail.com, jotchere@noguchi.ug.edu.gh, and mwilson@noguchi.ug.edu.gh.
References
- 1.Pullan RL, Gitonga C, Mwandawiro C, Snow RW, Brooker SJ. Estimating the relative contribution of parasitic infections and nutrition for anaemia among school-aged children in Kenya: a subnational geostatistical analysis. BMJ Open. 2013;3:e001936. doi: 10.1136/bmjopen-2012-001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooker SJ, Pullan RL. Ascaris lumbricoides and ascariasis: estimating numbers infected and burden of disease. In: Holland C, editor. Ascaris: The Neglected Parasite. Oxford, United Kingdom: Elsevier; 2013. pp. 343–362. [Google Scholar]
- 3.Gyorkos TW, Gilbert NL. Blood drain: soil-transmitted helminths and anemia in pregnant women. PLoS Negl Trop Dis. 2014;8:e2912. doi: 10.1371/journal.pntd.0002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Pediatrics Collaboration Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. 2016;170:267–287. doi: 10.1001/jamapediatrics.2015.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . Soil-Transmitted Helminthiases: Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 7.WHO . Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 8.Uniting to Combat NTDs London declaration on neglected tropical diseases. 2012. http://unitingtocombatntds.org/downloads/press/ntd_event_london_declaration_on_ntds.pdf Available at. Accessed October 1, 2016.
- 9.Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis. 2013;7:e2027. doi: 10.1371/journal.pntd.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry MA, Simon GG, Mistry N, Hotez PJ. Global trends in neglected tropical disease control and elimination: impact on child health. Arch Dis Child. 2013;98:635–641. doi: 10.1136/archdischild-2012-302338. [DOI] [PubMed] [Google Scholar]
- 11.Gyorkos TW, Larocque R, Casapia M, Gotuzzo E. Lack of risk of adverse birth outcomes after deworming in pregnant women. Pediatr Infect Dis J. 2006;25:791–794. doi: 10.1097/01.inf.0000234068.25760.97. [DOI] [PubMed] [Google Scholar]
- 12.Montresor A, Stoltzfus RJ, Albonico M, Tielsch JM, Rice AL, Chwaya HM, Savioli L. Is the exclusion of children under 24 months from anthelmintic treatment justifiable? Trans R Soc Trop Med Hyg. 2002;96:197–199. doi: 10.1016/s0035-9203(02)90303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner HC, Truscott JE, Fleming FM, Hollingsworth TD, Brooker SJ, Anderson RM. Cost-effectiveness of scaling up mass drug administration for the control of soil-transmitted helminths: a comparison of cost function and constant costs analyses. Lancet Infect Dis. 2016;16:838–846. doi: 10.1016/S1473-3099(15)00268-6. [DOI] [PubMed] [Google Scholar]
- 14.Turner HC, Truscott JE, Hollingsworth TD, Bettis AA, Brooker SJ, Anderson RM. Cost and cost-effectiveness of soil-transmitted helminth treatment programmes: systematic review and research needs. Parasit Vectors. 2015;8:355. doi: 10.1186/s13071-015-0885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo NC, Bogoch II, Blackburn BG, Raso G, N'Goran EK, Coulibaly JT, Becker SL, Abrams HB, Utzinger J, Andrews JR. Comparison of community-wide, integrated mass drug administration strategies for schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Glob Health. 2015;3:e629–e638. doi: 10.1016/S2214-109X(15)00047-9. [DOI] [PubMed] [Google Scholar]
- 16.Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin, and school performance. Cochrane Database Syst Rev. 2015;7:CD000371. doi: 10.1002/14651858.CD000371.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davey C, Aiken AM, Hayes RJ, Hargreaves JR. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: a statistical replication of a cluster quasi-randomized stepped-wedge trial. Int J Epidemiol. 2015;44:1581–1592. doi: 10.1093/ije/dyv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aiken AM, Davey C, Hargreaves JR, Hayes RJ. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: a pure replication. Int J Epidemiol. 2015;44:1572–1580. doi: 10.1093/ije/dyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Njenga S, Mutungi F, Wamae C, Mwanje M, Njiru K, Bockarie M. Once a year school-based deworming with praziquantel and albendazole combination may not be adequate for control of urogenital schistosomiasis and hookworm infection in Matuga District, Kwale County, Kenya. Parasit Vectors. 2014;7:74. doi: 10.1186/1756-3305-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries D, Nguyen S, Boakye D, Wilson M, Cappello M. The promise and pitfalls of mass drug administration to control intestinal helminth infections. Curr Opin Infect Dis. 2012;25:584–589. doi: 10.1097/QCO.0b013e328357e4cf. [DOI] [PubMed] [Google Scholar]
- 21.Truscott J, Turner H, Anderson R. What impact will the achievement of the current World Health Organisation targets for anthelmintic treatment coverage in children have on the intensity of soil transmitted helminth infections? Parasit Vectors. 2015;8:1–12. doi: 10.1186/s13071-015-1135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson RM, Turner HC, Truscott JE, Hollingsworth TD, Brooker SJ. Should the goal for the treatment of soil transmitted helminth (STH) infections be changed from morbidity control in children to community-wide transmission elimination? PLoS Negl Trop Dis. 2015;9:e0003897. doi: 10.1371/journal.pntd.0003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truscott JE, Hollingsworth TD, Brooker SJ, Anderson RM. Can chemotherapy alone eliminate the transmission of soil transmitted helminths? Parasit Vectors. 2014;7:266. doi: 10.1186/1756-3305-7-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafiz I, Berhan M, Keller A, Haq R, Chesnaye N, Koporc K, Rahman M, Rahman S, Mathieu E. School-based mass distributions of mebendazole to control soil-transmitted helminthiasis in the Munshiganj and Lakshmipur districts of Bangladesh: an evaluation of the treatment monitoring process and knowledge, attitudes, and practices of the population. Acta Trop. 2015;141:385–390. doi: 10.1016/j.actatropica.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Albonico M, Levecke B, LoVerde PT, Montresor A, Prichard R, Vercruysse J, Webster JP. Monitoring the efficacy of drugs for neglected tropical diseases controlled by preventive chemotherapy. J Glob Antimicrob Resist. 2015;3:229–236. doi: 10.1016/j.jgar.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5:e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, Montresor A, Levecke B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist. 2011;1:14–27. doi: 10.1016/j.ijpddr.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ. 2003;81:343–352. [PMC free article] [PubMed] [Google Scholar]
- 29.Albonico M. Methods to sustain drug efficacy in helminth control programmes. Acta Trop. 2003;86:233–242. doi: 10.1016/s0001-706x(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 30.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, Engels D, Guillard B, Nguyen TV, Kang G, Kattula D, Kotze AC, McCarthy JS, Mekonnen Z, Montresor A, Periago MV, Sumo L, Tchuente LA, Dang TC, Zeynudin A, Levecke B. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5:e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwenkenbecher JM, Albonico M, Bickle Q, Kaplan RM. Characterization of beta-tubulin genes in hookworms and investigation of resistance-associated mutations using real-time PCR. Mol Biochem Parasitol. 2007;156:167–174. doi: 10.1016/j.molbiopara.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Diawara A, Halpenny CM, Churcher TS, Mwandawiro C, Kihara J, Kaplan RM, Streit TG, Idaghdour Y, Scott ME, Basanez MG, Prichard RK. Association between response to albendazole treatment and beta-tubulin genotype frequencies in soil-transmitted helminths. PLoS Negl Trop Dis. 2013;7:e2247. doi: 10.1371/journal.pntd.0002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews JB. Anthelmintic resistance in equine nematodes. Int J Parasitol Drugs Drug Resist. 2014;4:310–315. doi: 10.1016/j.ijpddr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphries D, Mosites E, Otchere J, Twum WA, Woo L, Jones-Sanpei H, Harrison LM, Bungiro RD, Benham-Pyle B, Bimi L, Edoh D, Bosompem K, Wilson M, Cappello M. Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: patterns of malaria coinfection, anemia, and albendazole treatment failure. Am J Trop Med Hyg. 2011;84:792–800. doi: 10.4269/ajtmh.2011.11-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphries D, Simms BT, Davey D, Otchere J, Quagraine J, Terryah S, Newton S, Berg E, Harrison LM, Boakye D, Wilson M, Cappello M. Hookworm infection among school age children in Kintampo north municipality, Ghana: nutritional risk factors and response to albendazole treatment. Am J Trop Med Hyg. 2013;89:540–548. doi: 10.4269/ajtmh.12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coates J, Swindale A, Bilinsky P. In: Household Food Insecurity and Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (version 3) Project FaNTA, editor. Washington, DC: Academy for Educational Development; 2007. [Google Scholar]
- 37.Arimond M, Ruel MT. Dietary diversity is associated with child nutritional status: evidence from 11 demographic and health surveys. J Nutr. 2004;134:2579–2585. doi: 10.1093/jn/134.10.2579. [DOI] [PubMed] [Google Scholar]
- 38.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguayo VM, Aneja S, Badgaiyan N, Singh K. Mid upper-arm circumference is an effective tool to identify infants and young children with severe acute malnutrition in India. Public Health Nutr. 2015;18:3244–3248. doi: 10.1017/S1368980015000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO/UNICEF . WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland: World Health Organization/United Nations Children's Fund; 2009. [PubMed] [Google Scholar]
- 41.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 42.WHO . Assessing the Efficacy of Anthelminthic Drugs against Schistosomiasis and Soil-transmitted Helminthiases. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 43.Treger RS, Otchere J, Keil MF, Quagraine JE, Rai G, Mott BT, Humphries DL, Wilson M, Cappello M, Vermeire JJ. In vitro screening of compounds against laboratory and field isolates of human hookworm reveals quantitative differences in anthelmintic susceptibility. Am J Trop Med Hyg. 2014;90:71–74. doi: 10.4269/ajtmh.12-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherrer AU, Sjoberg MK, Allangba A, Traore M, Lohourignon LK, Tschannen AB, N'Goran EK, Utzinger J. Sequential analysis of helminth egg output in human stool samples following albendazole and praziquantel administration. Acta Trop. 2009;109:226–231. doi: 10.1016/j.actatropica.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 46.Ospina R, Ferrari SLP. A general class of zero-or-one inflated beta regression models. Comput Stat Data Anal. 2012;56:1609–1623. [Google Scholar]
- 47.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121((Suppl)):S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 48.Hadju V, Abadi K, Stephenson LS, Noor NN, Mohammed HO, Bowman DD. Intestinal helminthiasis, nutritional status, and their relationship; a cross-sectional study in urban slum school children in Indonesia. Southeast Asian J Trop Med Public Health. 1995;26:719–729. [PubMed] [Google Scholar]
- 49.Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Weight gain of Kenyan school children infected with hookworm, Trichuris trichiura and Ascaris lumbricoides is improved following once- or twice-yearly treatment with albendazole. J Nutr. 1993;123:656–665. doi: 10.1093/jn/123.4.656. [DOI] [PubMed] [Google Scholar]
- 50.Crompton DW. The public health importance of hookworm disease. Parasitology. 2000;121((Suppl)):S39–S50. doi: 10.1017/s0031182000006454. [DOI] [PubMed] [Google Scholar]
- 51.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 52.Chu D, Bungiro RD, Ibanez M, Harrison LM, Campodonico E, Jones BF, Mieszczanek J, Kuzmic P, Cappello M. Molecular characterization of Ancylostoma ceylanicum Kunitz-type serine protease inhibitor: evidence for a role in hookworm-associated growth delay. Infect Immun. 2004;72:2214–2221. doi: 10.1128/IAI.72.4.2214-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koski KG, Scott ME. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu Rev Nutr. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- 54.Zaralis K, Tolkamp BJ, Houdijk JG, Wylie AR, Kyriazakis I. Consequences of protein supplementation for anorexia, expression of immunity and plasma leptin concentrations in parasitized ewes of two breeds. Br J Nutr. 2009;101:499–509. doi: 10.1017/S000711450802401X. [DOI] [PubMed] [Google Scholar]
- 55.Hoste H, Torres-Acosta JF, Aguilar-Caballero AJ. Nutrition-parasite interactions in goats: is immunoregulation involved in the control of gastrointestinal nematodes? Parasite Immunol. 2008;30:79–88. doi: 10.1111/j.1365-3024.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 56.Coop RL, Kyriazakis I. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 2001;17:325–330. doi: 10.1016/s1471-4922(01)01900-6. [DOI] [PubMed] [Google Scholar]
- 57.Coop RL, Holmes PH. Nutrition and parasite interaction. Int J Parasitol. 1996;26:951–962. doi: 10.1016/s0020-7519(96)80070-1. [DOI] [PubMed] [Google Scholar]
- 58.Montresor A, Addiss D, Albonico M, Ali SM, Ault SK, Gabrielli A-F, Garba A, Gasimov E, Gyorkos T, Jamsheed MA, Levecke B, Mbabazi P, Mupfasoni D, Savioli L, Vercruysse J, Yajima A. Methodological bias can lead the cochrane collaboration to irrelevance in public health decision-making. PLoS Negl Trop Dis. 2015;9:e0004165. doi: 10.1371/journal.pntd.0004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prichard RK, Basanez MG, Boatin BA, McCarthy JS, Garcia HH, Yang GJ, Sripa B, Lustigman S. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis. 2012;6:e1549. doi: 10.1371/journal.pntd.0001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolstenholme AJ, Martin RJ. Int J Parasitol Drugs Drug Resist. 2014;4:218–219. doi: 10.1016/j.ijpddr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutherland IA, Leathwick DM. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Barrere V, Alvarez L, Suarez G, Ceballos L, Moreno L, Lanusse C, Prichard RK. Relationship between increased albendazole systemic exposure and changes in single nucleotide polymorphisms on the beta-tubulin isotype 1 encoding gene in Haemonchus contortus. Vet Parasitol. 2012;186:344–349. doi: 10.1016/j.vetpar.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 63.Diawara A, Schwenkenbecher JM, Kaplan RM, Prichard RK. Molecular and biological diagnostic tests for monitoring benzimidazole resistance in human soil-transmitted helminths. Am J Trop Med Hyg. 2013;88:1052–1061. doi: 10.4269/ajtmh.12-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Awadzi K, Boakye DA, Edwards G, Opoku NO, Attah SK, Osei-Atweneboana MY, Lazdins-Helds JK, Ardrey AE, Addy ET, Quartey BT, Ahmed K, Boatin BA, Soumbey-Alley EW. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann Trop Med Parasitol. 2004;98:231–249. doi: 10.1179/000349804225003253. [DOI] [PubMed] [Google Scholar]
- 65.Schwab AE, Boakye DA, Kyelem D, Prichard RK. Detection of benzimidazole resistance-associated mutations in the filarial nematode Wuchereria bancrofti and evidence for selection by albendazole and ivermectin combination treatment. Am J Trop Med Hyg. 2005;73:234–238. [PubMed] [Google Scholar]
- 66.Kotze AC, Dobson RJ, Humphries D, Wilson M, Cappello M. Application of a Poisson distribution quality control measure to the analysis of two human hookworm drug treatment studies in Ghana. Int J Parasitol Drugs Drug Resist. 2014;4:64–70. doi: 10.1016/j.ijpddr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziem JB, Kettenis IM, Bayita A, Brienen EA, Dittoh S, Horton J, Olsen A, Magnussen P, Polderman AM. The short-term impact of albendazole treatment on Oesophagostomum bifurcum and hookworm infections in northern Ghana. Ann Trop Med Parasitol. 2004;98:385–390. doi: 10.1179/000349804225003370. [DOI] [PubMed] [Google Scholar]
- 68.Ziem JB, Magnussen P, Olsen A, Horton J, Asigri VL, Polderman AM. Impact of repeated mass treatment on human Oesophagostomum and hookworm infections in northern Ghana. Trop Med Int Health. 2006;11:1764–1772. doi: 10.1111/j.1365-3156.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 69.Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, Van Lieshout L. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg. 2007;77:685–690. [PubMed] [Google Scholar]
- 70.Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DA, Scott ME, Halpenny C, Stothard JR, Prichard RK. Assays to detect beta-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLoS Negl Trop Dis. 2009;3:e397. doi: 10.1371/journal.pntd.0000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange H, Eggers R, Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol. 1988;34:315–317. doi: 10.1007/BF00540964. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez S, Alvarez L, Pis A, Quiroga M, Lanusse C. Differences in plasma and abomasal kinetics of albendazole and its metabolites in calves grazed on pasture or fed a grain-based diet. Res Vet Sci. 1999;66:223–230. doi: 10.1053/rvsc.1998.0264. [DOI] [PubMed] [Google Scholar]