Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) was the first human retrovirus to be reported and is associated with neoplastic, neurological, autoimmune, and infectious complications. HTLV-1 is endemic in Peru, with the highest prevalence reported among commercial sex workers. Seroprevalence data collected from Peruvian female sex workers (FSWs) working in Callao over three study periods between 1993 and 2010 were used to examine the secular trend in HTLV-1 prevalence. Between 1993 and 2010, the prevalence of HTLV-1 decreased significantly from 14.5% to 3.1% (P < 0.01). The prevalence of HTLV-1 seropositivity differed significantly by birth cohort (1922–1959, 1960–1969, 1970–1979, and 1980–1992), and for each of the four birth cohorts, the prevalence did not significantly decrease by screening year (P > 0.07). There were no cases of HTLV-1 detected among FSW born after 1979 (N = 224). Participant characteristics associated with HTLV-1 seropositivity were birth in the Andes Mountains region, age, increased time in sex work, younger age of starting sex work, and human immunodeficiency virus (HIV) seropositivity. The secular trend in declining prevalence persisted after adjustment for age, time in sex work, place of birth, and HIV serostatus, with the odds of HTLV-1 infection decreasing approximately 16% per year (adjusted odds ratio = 0.84, 95% confidence interval = 0.78, 0.90). The increasing use of condoms by later birth cohorts noted in our analysis, as well as the increasing availability of free condoms provided by the Peruvian government—which started in the late 1980s before this study— may have been responsible for declining HTLV seroprevalence.
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) is a positive, single-stranded RNA retrovirus that infects approximately 5–10 million people throughout the world.1,2 Although the majority of people infected with HTLV-1 remain asymptomatic, approximately 5% will develop a neoplastic, neurological, or infectious complication, such as adult T-cell lymphoma/leukemia, HTLV-associated myelopathy/tropical spastic paraparesis, Strongyloides stercoralis hyperinfection, or Norwegian scabies.3,4 Autoimmune complications, such as Sjogren's syndrome, arthritis, and uveitis, have also been associated with HTLV-1 infection.5 HTLV-1 infection has also been linked with increased risk of acquiring tuberculosis (TB) and has been associated with decreased survival among people coinfected with either TB or human immunodeficiency virus-1 (HIV-1).6–10 As with TB and HIV-1, socioeconomic factors and risk behaviors may compound the risk of HTLV-1 acquisition and disease progression.11–13
HTLV-1 and HIV share similar modes of transmission, namely sexual intercourse, blood transfusions, injection drug use, and vertical transmission.6,13,14 With HTLV-1 infection, vertical transmission occurs predominantly through breast-feeding with increased prevalence among children who have been breast-fed for longer duration.11,12,15 Factors associated with seropositivity include lower socioeconomic status and less education, older age, a higher number of sexual partners, commercial sex work, and needle sharing.11–14 Condom use has been reported to prevent transmission of HTLV-1, but prior seroprevalence studies have not demonstrated a correlation between reported condom use and HTLV-1 infections.16
HTLV-1 infection is endemic in southern Japan, the Caribbean, equatorial Africa, Australia, and South America.1,11,14 Among some populations, more often indigenous, specific human leukocyte antigen (HLA) classes are more likely to have HTLV-1 infection.17,18 In South America, seroprevalence surveys indicate that the ethnic groups with the highest prevalence of HTLV-1 are the Aymara and Quechua-speaking populations living in the Andean Mountains.19 A 1997 study of a Quechua population living in the Andean region of Peru revealed a 5.1% seroprevalence of HTLV-1.20 A cross-sectional study conducted in 2010 among Shipibo–Konibo women living in the Peruvian Amazon found a 5.9% prevalence of HTLV-1.21–23 In Peru, the prevalence of HTLV-1 has ranged from 0.3% in the general population to 1.3% in blood donors, 1.7% in pregnant women, and 1.8% in men who have sex with men, associated with sexual activity.24–27
Among FSW sampled in Lima, Peru, the overall seroprevalence of HTLV-1 was 7% in 1994 and 3.8% in 1999.13,16 To date, the highest prevalence of HTLV-1 infection in Peru—25%—was reported among female sex workers (FSWs) screened at a public health clinic, Centro de Salud “Alberto Barton” (CSAB) in Callao, Peru, in 1988.28 From 1987 to 1990, a longitudinal study of FSW in Callao found a 1.6% incidence of HTLV-1 infection with 17.6% prevalence on initial screening.29 Commercial sex workers have an increased risk of acquiring sexually transmitted infections (STIs), including HTLV-1, due to occupational risk factors. FSW screened at CSAB between 2008 and 2011 had overall prevalence of 5.1% Chlamydia trachomatis, 0.3% Neisseria gonorrhoeae (NG), 1.7% syphilis, and 1.0% HIV.30
A significant decrease in HTLV-1 prevalence has been reported in Guinea-Bissau (3.5% in 1996 to 2.3% in 2006) and among Haitian women in French Guiana (8.0% in 1991 to 0.3% in 2001).31,32 A secular trend of an endemic area, Nagasaki, Japan, noted a significant annual decline in prevalence of HTLV-1 infections, thought to be due to changing demographics, increased condom use, and decreased breast-feeding among later birth cohorts.33 Trend analysis of first-time blood donors in Japan has revealed a stable prevalence from 1989 to 1996 (0.47–0.13%) and from 2000 to 2006 (1.05–1.41%).34,35 The objective of this analysis was to describe the secular seroprevalence trend and biosociodemographic correlates of HTLV-1 infection in FSW enrolled in a single sexual health clinic in Callao, Peru, during three observational study periods between 1993 and 2010.
Methods
Study site and population.
In Peru, an estimated 15,000 FSW work in the Lima-Callao metro area. Sex work is legally permitted for FSW who are registered and are 18 years of age or older. Registration requires monthly health assessments. The CSAB del Callao is one of two designated STI reference centers to provide health care to registered FSW in Lima, Peru. FSW who receive routine medical care at one of these clinics are considered “registered,” whereas sex workers who do not receive regular medical care, typically women who work in bars or on the streets, are considered “unregistered.” Approximately 5,000 women are registered at the two clinics, and have attended one of the clinics at least once during the past 2 years. Approximately 250 women attend CSAB each month. The number of newly registered FSW averages 700 per year at CSAB.
Study enrollment, procedures, and ethics.
FSW 18 years of age or older who presented for medical assessment at CSAB between 1993 and 1997 (Period 1), 2005 and 2006 (Period 2), and 2008 and 2010 (Period 3) were eligible for inclusion. Informed consent was obtained from study participants and the study was approved by the institutional review boards of the University of Washington, Universidad Nacional Mayor de San Marcos, the Directorate of Callao and the U.S. Naval Medical Research Unit No. 6.
For each time period (Period 1: 1993–1997, Period 2: 2005–2006, and Period 3: 2008–2010) each woman completed standardized questionnaires detailing sociodemographic characteristics, sexual practices, and place of work. Participants underwent genital examination with collection of vaginal, endocervical, and blood samples. Genital specimens were tested for NG and Trichomonas vaginalis and blood specimens were tested for syphilis, HIV-1/2, and HTLV-1. Some FSW who enrolled into the study during different time periods underwent repeat serological testing at a subsequent follow-up visit. During Period 2 (2005–2006), Ecuadorian FSW were intentionally oversampled to enable comparisons among FSW characteristics by nationality, but were excluded from this analysis so as to focus analysis on Peruvian FSW.
Screening and confirmatory testing for HTLV-1 infection.
In each study period, sera were screened for HTLV-1 antibody using enzyme immunoassay (EIA). The name and manufacturer of commercial EIA assays used for each study period were as follows: HTLV EIA, Cambridge Bioscience, Worcester, MA (1993–1997); Vironostika HTLV-1/II Microelisa System, Organon Teknika/Biomérieux, Durham, NC (2005–2006); and Bioelisa HTLV I/II 5.0, BioKit, Barcelona, Spain (2008–2011). Women testing positive for HTLV-1 antibodies by EIA underwent confirmatory testing. Sera from women enrolled in Period 1 (1993–1997) were tested using an rp21e-enhanced Western blot assay (Cambridge Bioscience), with infection defined as immunoreactivity to p24, gp46, and p21env(r) bands. If only other viral-specific bands were present, such as p53 or p19, the individual was considered indeterminate. Women enrolled in Period 2 (2005–2006) received confirmatory testing using HTLV I/II Western Blots (Genelabs Diagnostics, Singapore), with positivity defined according to U.S. Public Health Service criteria: immunoreactivity to both the gag gene product p24 and to an env gene product (pg46 and/or gp61/68). Women enrolled during Period 3 (2008–2010) study period with enzyme-linked immunosorbent assay–positive samples underwent confirmatory testing with a line immunoblot assay (HTLV I/II score; Innogenetics, Ghent, Belgium), which defined positivity as the presence of HTLV-1 IgG antibodies in serum, based on two visible antibody bands that included the gp21-I/-II band, or on three or more bands with the sum of the gp46-I and p19-I band intensity greater than the gp46-II band intensity. The presence of the gp21-I/-II band alone, or a combination of any two bands without a detectable gp21-I/-II band, was considered indeterminate. Participants with indeterminate results were excluded from the analysis (N = 20). The sensitivities and specificities of the initial screening tests used in each study and the sensitivities of the confirmatory tests were similar.36–39 The confirmatory test used in Study 2 (by Genelabs Diagnostics) was less specific than the other two (specificity of 50% versus > 92%).40–42 All women testing positive for HTLV-1 received counseling regarding potential long-term manifestations of HTLV-1 infection.
Statistical analysis.
Descriptive statistics were generated using cross tabulations and χ2 tests to determine significant differences by HTLV-1 serostatus. Univariate logistic regression was used to determine whether subject characteristics were associated with the odds of HTLV-1 infection. To investigate trends in HTLV-1 prevalence over time, the prevalence of HTLV-1 was calculated for each year that subjects were screened. Calendar year (1993–2010) was included as the independent linear variable in regression models. Our dependent variable was HTLV-1 serostatus. Multivariate logistic regression was used to model the temporal trend in HTLV-1 prevalence in the total study population with subject age, place of birth, time in sex work, and HIV-1 seropositivity were included as a priori confounders in adjusted models. Other variables considered as potential confounders included birth cohort and self-reported condom use. To investigate the sensitivity of our study results to different analytic approaches, we also conducted the adjustment of logistic regression models without initially including any a priori confounders. We further investigated the potential impact of migration on our study results by conducting the same analyses of the trend in HTLV prevalence stratified by region of birth (coast or Andean Mountains). A significance level of 0.05 was used for all hypothesis testing. All analyses were performed using Stata version 13.1 (StataCorp, College Station, TX).
Results
Over the three study periods, 1,938 female commercial sex workers were screened for HTLV-1 infection, with 184 (9.6%) testing positive for HTLV-1; 20 women had indeterminate Western blot results and were excluded from further analysis. An indeterminate result may have represented participants in the process of seroconversion; however, follow-up data were not available. Overall, HTLV-1 serostatus differed significantly by place of birth, age of subject, time in sex work, age of starting sex work, HIV seropositivity, and screening year (Table 1; all P values ≤ 0.02).
Table 1.
Characteristics of 1,918 female sex workers by HTLV-1 serostatus in Callao, Peru, 1993–2010
| Total (N = 1,918) | HTLV-1 positive (N = 184) n (%) | P value | |
|---|---|---|---|
| Age (years) | < 0.001 | ||
| 18–24 | 499 | 21 (11.4) | |
| 25–29 | 507 | 27 (14.7) | |
| 30–34 | 383 | 44 (23.9) | |
| 35+ | 527 | 92 (50.0) | |
| Period | < 0.001 | ||
| Period 1: 1993–1997 | 1,477 | 171 (92.9) | Ref |
| Period 2: 2005–2006 | 62 | 1 (1.0) | 0.04 |
| Period 3: 2008–2010 | 379 | 12 (6.5) | < 0.001 |
| Place of birth | < 0.001 | ||
| Coast | 1,193 | 89 (48.4) | Ref |
| Andes | 574 | 88 (47.8) | < 0.001 |
| Amazon | 151 | 7 (3.8) | 0.21 |
| Condom use | 0.07 | ||
| Always/almost always | 1311 | 130 (9.9) | Ref |
| Frequently/sometimes/rarely | 422 | 47 (11.1) | 0.47 |
| Never/almost never | 128 | 4 (3.1) | 0.02 |
| Mean years in sex work (±SD) | 2.7 (5.1) | 2.6 (4.8) | 0.02 |
| Mean age in years at starting sex work (±SD) | 27.9 (7.8) | 32.2 (9.5) | < 0.001 |
| HIV positive | 10 | 4 (2.7) | < 0.001 |
HIV = human immunodeficiency virus; HTLV-1 = human T-cell lymphotropic virus type 1; SD = standard deviation.
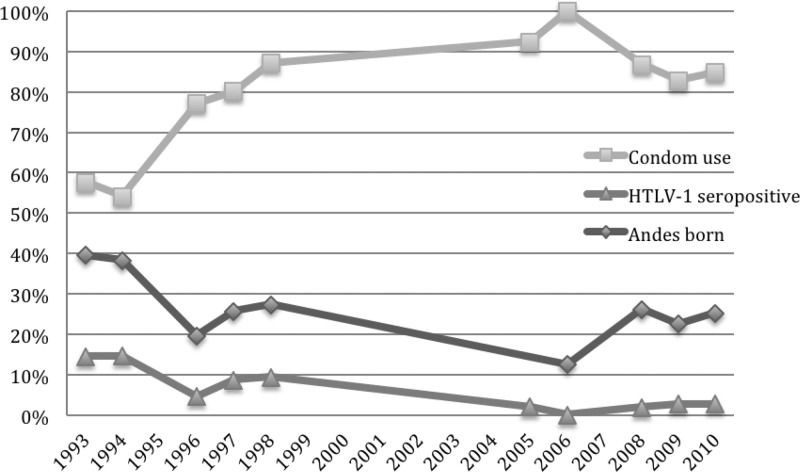
Condom use increased significantly over time (Figure 1 ) with a reported prevalence of always using a condom of 240/416 (57.7%) in 1993 compared with 106/125 (84.8%) in 2010 (P < 0.01). Although the increase in condom use has been correlated with the decline of HTLV seroprevalence, we found reported condom use within the last week (always/less than always) was more frequent among participants with HTLV-1 infection (70.2% versus 67.3%), although the association was not significant (P = 0.43).
Figure 1.
Annual trends of prevalence of human T-cell lymphotropic virus type 1 infection, Andean birthplace, and reported condom use of “always” or “almost always,” 1993–2010.
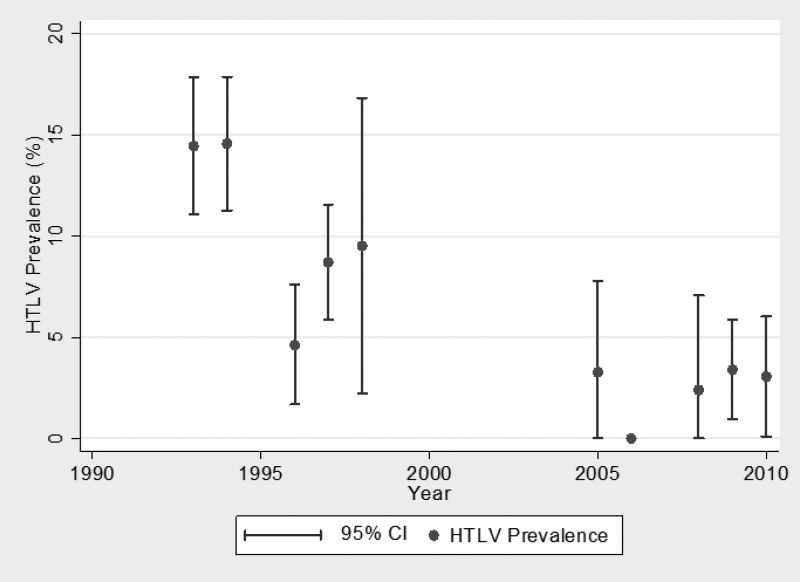
The prevalence of HTLV-1 decreased significantly from 14.5% in 1993 to 3.1% in 2010 (P < 0.01) (Figure 2 ). This trend remained significant even after adjustment for age, time in sex work, birthplace in the Andes, and HIV status, with the odds of HTLV-1 infection decreasing approximately 16% per year (adjusted odds ratio [aOR] = 0.84, 95% confidence interval [CI] = 0.78, 0.90). Adjustment of multivariate regression models without the inclusion a priori confounders yielded similar results.
Figure 2.
Annual human T-cell lymphotropic virus type 1 (HTLV-1) prevalences among 1,918 female sex workers in Callao, Peru, 1993–2010. CI = confidence interval.
We also examined differences in subject characteristics by study period to investigate whether differences in study populations contributed to changes in HTLV-1 prevalence over time. The percentage of FSW born in the Andean region decreased over time (Figure 1); in 1993, 230/420 (39.5%) of FSW were born in this region compared with 33/146 (22.6%) in 2010. Birthplace in the Andes Mountains was associated with HTLV-1 seropositivity (OR = 2.35, 95% CI = 1.73, 3.20). The association of Andes birthplace with HTLV-1 seropositivity remained significant after adjusting for screening year (OR = 2.07, 95% CI = 1.52, 2.83) (P < 0.01). There was no significant difference in the odds of seropositivity for women born in the Amazonian region compared with women born in the coastal region. To further investigate the potential effect of Andean migration on our overall prevalence trend, we analyzed the trends in HTLV prevalence separately among participants born on the coast and those born in the Andes. In both regions, the prevalence of HTLV-1 decreased significantly in unadjusted models (coastal OR = 0.91, 95% CI = 0.87, 0.96; Andes OR = 0.90, 95% CI = 0.85, 0.96). The same approaches to adjustment were used for these regional models as for the overall model; there was no evidence of confounding by any variable; however, in some models, the trend became nonsignificant after adjustment for birth cohort.
Women screened during Period 1 (1993–1997) had spent significantly less time in sex work as compared with women screened in Periods 2 (2005–2006) or 3 (2008–2010) (P < 0.001). Although the odds of HTLV-1 infection decreased since 1993 among all age groups, the youngest age group screened (18- to 25-year-old women) had the greatest decrease in HTLV-1 seropositivity (aOR = 0.49, 95% CI = 0.34, 0.71). The decreasing trend in HTLV-1 prevalence among subjects aged 46 and older did not reach significance (P = 0.08).
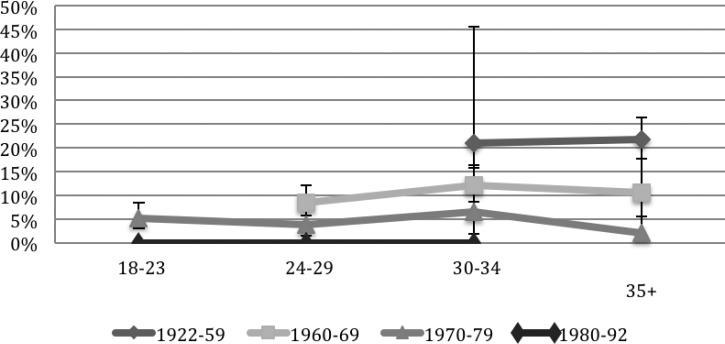
Across all three periods, increasing age was significantly associated with HTLV-1 prevalence (P < 0.001 in all three periods). The association between age and HTLV-1 was no longer significant when adjusted for birth cohort (P = 0.29). The prevalence of HTLV-1 seropositivity differed significantly, by birth cohort (1922–1959, 1960–1969, 1970–1979, and 1980–1992) even when adjusted for age (Figure 3 ). For each of the four birth cohorts, the prevalence did not significantly change by screening year (P > 0.07). There were no cases of HTLV-1 detected among FSW born after 1979 (N = 224).
Figure 3.
Human T-cell lymphotropic virus type 1 seroprevalence (%) by age and birth cohorts for all screening years (95% confidence interval).
Discussion
Our analysis demonstrates a dramatic decline in HTLV-1 seroprevalence—from 14.5% to 3.1%—among Peruvian FSWs between 1993 and 2010. This decline is even more impressive considering the HTLV-1 seroprevalence in FSW at the same clinic in Callao was 25% in 1988 and 17.6% in 1990.28 Individual level data on FSW tested for HTLV-1 in Callao from 1988 to 1993 were not available for analysis. The decreasing trend in HTLV-1 seroprevalence detected in our population of FSW remained significant even after controlling for factors such as age, region of birth, years in sex work, and HIV status. Several sociodemographic, biological, and behavioral characteristics were not measured across the entire study period or were not included in the analysis due to substantial missing data and may have confounded the trend in HTLV-1 seroprevalence we observed; these include migration within Peru and a number of high-risk behaviors.
Breast-feeding and birth cohort.
Breast-feeding exposure and duration have been associated with increased prevalence of HTLV-1 infections, but breast-feeding history was not collected in these data.11,12,15 A possible marker for changes in breast-feeding practice is analysis of HTLV-1 prevalence by birth cohorts. Earlier birth cohorts, especially those born before 1970, revealed much higher prevalence of HTLV-1 compared with later birth cohorts. The strong association with birth cohort may be linked to exposures in life before sex work. A prior longitudinal study in Callao has, however, shown acquisition of HTLV-1 during time as a commercial sex worker suggesting that exposure in adulthood played a role in the early 1990s.29 The shorter duration of sex work, coupled with more frequent use of condoms reported in our study may have reduced sexual transmission of HTLV-1 in this population.
Birthplace and migration.
In 1993, over a third (39.5%) of FSW receiving health services at the CSAB reported a birthplace in the Andes Mountains of Peru, compared with 54.8% from coastal areas and 5.7% from the Amazonian region. Over the screening years, the percentage of FSW born in the Andean region decreased to 25.2% (2010). Birthplace in the Andean region was significantly associated with increased risk of HTLV-1 infection; however, the declining trend in HTLV-1 prevalence over time remained significant even after adjustment for region of birth and among participants stratified by region of birth, which indicates that migration from the Andean region to the coast does not entirely explain the trend.7,19,43 Although the exact reason for higher prevalence of HTLV-1 infection among Andean populations is unknown, some researchers have posited that geographic, cultural factors, or HLA type may be responsible.44,45
Condom use.
Self-reported condom use among Peruvian FSW working in Callao has continued to steadily increase since 1986. Surveys conducted in Callao in 1986 and 1988 found that 2/135 (1.5%) and 196/636 (30.8%) FSW reported always using a condom, respectively.46,47 In our data, there was a significant increase in reported “always using a condom” from 1993 (58%) to 2010 (85%), but this behavior was not associated with HTLV-1 infection. Self-report of always using a condom increased for later birth cohorts (P < 0.01). Compared with participants born between 1922 and 1959, participants born between 1980 and 1992 were more than three times as likely to report always using a condom (OR = 3.26, P < 0.01).
Given the striking increase in self-reported condom use reported by FSW receiving care at the same clinic, it is plausible that increasing condom use among FSW over the study periods played a role in the decline in HTLV-1 prevalence. Government-purchased condoms were first distributed in the late 1980s, suggesting the decline in HTLV-1 seroprevalence among Peruvian FSW could be a direct result of the Peruvian STI control programs.47,48 We believe that the lack of association between condom use and HTLV-1 risk that we observed in this study is related to the difficulty in evaluating condom use via self-report, which has been documented among other FSWs in Latin America.49 This unexpected finding highlights the need for higher validity measures of condom use in this and other FSW populations.
Needle sharing.
Although injection drug use was not reported by any FSW and is generally very rare in Peru, a common behavior among FSW working in Callao during the 1980s and 1990s was use of parenteral injection of antibiotics, vitamins, or steroids purchased outside the medical clinic. Needles used for these injections were sometimes shared needles—which may have contributed to higher HTLV-1 seroprevalence.46 Although over half of FSW reported receiving such injections in 1986, data regarding the use of injectable antibiotics and frequency of sharing needles to inject penicillin were not collected in later studies or these data. It is plausible that higher HTLV-1 seroprevalence was perpetuated through reuse of nonsterile needles during the 1980s and early 1990s.
Diagnostic techniques and technology.
The diagnostic techniques and criteria have changed over the last three decades and have been cited as a possible cause of higher rates of HTLV-1 prevalence using earlier criteria and technology.14 Evidence from validation studies indicates that the sensitivities and specificities of our screening tests were comparable except for the confirmatory test used in Study 2, which was less sensitive as compared with the confirmatory tests of Studies 1 and 3. Because a single HTLV-1 seropositive case was detected during Study 2, false positives resulting from the use of a less specific test did not substantially bias our results.
Conclusions
The prevalence of HTLV-1 infections among Peruvian FSW working in Callao, Peru–previously one of the most endemic populations in the world—has declined significantly over the last two decades. Increasing age, earlier birth cohort, birth place in the Andes, time of sex work, age at time of starting sex work, and HIV seropositivity were positively associated with HTLV-1 infection in this population. The decrease in HTLV-1 infection over time persisted even after adjustment for these factors. Although similar percentages of FSW with and without HTLV-1 infection reported less than 100% condom usage, the increasing availability of free condoms in the late 1980s makes it plausible that increasing condom use by all women may have been an important factor associated with declining HTLV seroprevalence. Unfortunately, we were not able to examine other specific behavioral factors potentially associated with trends, such as HLA type, exposure, and duration of breast-feeding, or higher risk sexual behavior, such as anal receptive intercourse. The intense internal migration from the rural Andes region to the coastal cities during the 1980s appeared to partially explain the decreasing trend; our data also suggest that changes in early life exposures associated with birth cohort contributed to decreasing HTLV prevalence. We believe that the results of our study would be generalizable to populations in other large cities on the Peruvian coast because they also experienced major immigration from Andean Peruvians during the 1980s. With a growing urban population, it is likely that the decrease in HTLV-1 prevalence we observed in this study would impact national trends. A declining prevalence in the Andean region, as suggested by our study, would compound this. More research is needed to confirm the national representativeness of our results.
ACKNOWLEDGMENTS
We thank Silvia Montano for her support and guidance. We also thank the staff at Centro de Salud Alberto Barton, the female sex workers who have volunteered to participate in years on epidemiologic studies at CSAB, Evelyn Hseih, and data management teams at NAMRU-6 and the University of Washington.
Footnotes
Financial support: This work was partially funded by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, and National Institutes of Health Office of Women's Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Authors' addresses: Jenell Stewart, Department of Internal Medicine, University of Washington, Seattle, WA, E-mail: jenells@uw.edu. Kristen Heitzinger, Department of Epidemiology, University of Washington, Seattle, WA, E-mail: heitzk@uw.edu. Simon Pollett, Marie Bashir Institute for Infectious Diseases and Biosecurity, University of Sydney, Sydney, Australia, E-mail: spollett@med.usyd.edu.au. Martha Calderón, Clínica de Salud Pública, “Alberto Barton” del Callao, Callao, Peru, E-mail: marthacalderonsilva@yahoo.com. Jorge Alarcón, Instituto de Medicina Tropical, Universidad Nacional Mayor de San Marcos, Lima, Peru, E-mail: joav06@gmail.com. Thanh G. N. Ton and Joseph R. Zunt, Department of Global Health and Neurology, University of Washington, Seattle, WA, E-mails: thanhton@gmail.com and jzunt@u.washington.edu.
References
- 1.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Thé G, Kazanji M. An HTLV-I/II vaccine: from animal models to clinical trials? J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13((Suppl 1)):S191–S198. doi: 10.1097/00042560-199600001-00029. [DOI] [PubMed] [Google Scholar]
- 3.Takatsuki K. Discovery of adult T-cell leukemia. Retrovirology. 2005;2:16. doi: 10.1186/1742-4690-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 5.Quaresma JA, Yoshikawa GT, Koyama RV, Dias GA, Fujihara S, Fuzii HT. HTLV-1, immune response and autoimmunity. Viruses. 2015;8:pii. doi: 10.3390/v8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedral-Sampaio DB, Martins Netto E, Pedrosa C, Brites C, Duarte M, Harrington W., Jr Co-Infection of tuberculosis and HIV/HTLV retroviruses: frequency and prognosis among patients admitted in a Brazilian Hospital. Braz J Infect Dis. 1997;1:31–35. [PubMed] [Google Scholar]
- 7.Verdonck K, Gonzalez E, Schrooten W, Vanham G, Gotuzzo E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. Epidemiol Infect. 2008;136:1076–1083. doi: 10.1017/S0950268807009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastos M de L, Santos SB, Souza A, Finkmoore B, Bispo O, Barreto T, Cardoso I, Bispo I, Bastos F, Pereira D, Riley L, Carvalho EM. Influence of HTLV-1 on the clinical, microbiologic and immunologic presentation of tuberculosis. BMC Infect Dis. 2012;12:199. doi: 10.1186/1471-2334-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marinho J, Galvao-Castro B, Rodrigues LC, Barreto ML. Increased risk of tuberculosis with human T-lymphotropic virus-1 infection: a case-control study. J Acquir Immune Defic Syndr. 2005;40:625–628. doi: 10.1097/01.qai.0000174252.73516.7a. [DOI] [PubMed] [Google Scholar]
- 10.Beilke MA. Retroviral coinfections: HIV and HTLV: taking stock of more than a quarter century of research. AIDS Res Hum Retroviruses. 2012;28:139–147. doi: 10.1089/aid.2011.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Palacios C, Gotuzzo E, Vandamme AM, Maldonado Y. Seroprevalence and risk factors for human T-cell lymphotropic virus (HTLV-I) infection among ethnically and geographically diverse Peruvian women. Int J Infect Dis. 2003;7:132–137. doi: 10.1016/s1201-9712(03)90009-9. [DOI] [PubMed] [Google Scholar]
- 13.Gotuzzo E, Sanchez J, Escamilla J, Carrillo C, Phillips IA, Moreyra L, Stamm W, Ashley R, Roggen EL, Kreiss J. Human T cell lymphotropic virus type I infection among female sex workers in Peru. J Infect Dis. 1994;169:754–759. doi: 10.1093/infdis/169.4.754. [DOI] [PubMed] [Google Scholar]
- 14.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 15.Gotuzzo E, Moody J, Verdonck K, Cabada MM, González E, Van Dooren S, Vandamme AM, Terashima A, Vermund SH. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev Panam Salud Publica. 2007;22:223–230. doi: 10.1590/s1020-49892007000900001. [DOI] [PubMed] [Google Scholar]
- 16.Trujillo L, Munoz D, Gotuzzo E, Yi A, Watts DM. Sexual practices and prevalence of HIV, HTLV-I/II, and Treponema pallidum among clandestine female sex workers in Lima, Peru. Sex Transm Dis. 1999;26:115–118. doi: 10.1097/00007435-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Biggar RJ, Ng J, Kim N, Hisada M, Li HC, Cranston B, Hanchard B, Maloney EM. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J Infect Dis. 2006;193:277–282. doi: 10.1086/498910. [DOI] [PubMed] [Google Scholar]
- 18.Fujiyoshi T, Yashiki S, Fujiyama C, Kuwayama M, Miyashita H, Ohnishi H, Blank M, Zaninovic V, Blank A, Cartier L, Byrnes J, Harrington WJ, Miura T, Hayami M, Tajima K, Sonoda S. Ethnic segregation of HTLV-I and HTLV-II carriers among South American native Indians. Int J Cancer. 1995;63:510–515. doi: 10.1002/ijc.2910630409. [DOI] [PubMed] [Google Scholar]
- 19.Fujiyoshi T, Li HC, Lou H, Yashiki S, Karino S, Zaninovic V, Oneegllo SG, Camacho M, Andrade R, Hurtado LV, Gomez LH, Damiani E, Cartier L, Dipierri JE, Hayami M, Sonoda S, Tajima K. Characteristic distribution of HTLV type I and HTLV type II carriers among native ethnic groups in South America. AIDS Res Hum Retroviruses. 1999;15:1235–1239. doi: 10.1089/088922299310124. [DOI] [PubMed] [Google Scholar]
- 20.Zurita S, Costa C, Watts D, Indacochea S, Campos P, Sanchez J, Gotuzzo E. Prevalence of human retroviral infection in Quillabamba and Cuzco, Peru: a new endemic area for human T cell lymphotropic virus type 1. Am J Trop Med Hyg. 1997;56:561–565. doi: 10.4269/ajtmh.1997.56.561. [DOI] [PubMed] [Google Scholar]
- 21.Blas MM, Alva IE, Garcia PJ, Carcamo C, Montano SM, Muñante R, Zunt JR. Association between human papillomavirus and human T-lymphotropic virus in indigenous women from the Peruvian Amazon. PLoS One. 2012;7:e44240. doi: 10.1371/journal.pone.0044240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alva IE, Orellana ER, Blas MM, Bernabe-Ortiz A, Cotrina A, Chiappe M, Kochel TJ, Carcamo CP, García PJ, Zunt JR, Buffardi AL, Montano SM. HTLV-1 and -2 infections among 10 indigenous groups in the Peruvian Amazon. Am J Trop Med Hyg. 2012;87:954–956. doi: 10.4269/ajtmh.2012.12-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blas MM, Alva IE, Garcia PJ, Cárcamo C, Montano SM, Mori N, Muñante R, Zunt JR. High prevalence of human T-lymphotropic virus infection in indigenous women from the Peruvian Amazon. PLoS One. 2013;8:e73978. doi: 10.1371/journal.pone.0073978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carcamo CP, Campos PE, Garcia PJ, Hughes JP, Garnett GP, Holmes KK. Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: a national population-based survey. Lancet Infect Dis. 2012;12:765–773. doi: 10.1016/S1473-3099(12)70144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotuzzo E, Arango C, de Queiroz-Campos A, Isturiz RE. Human T-cell lymphotropic virus-I in Latin America. Infect Dis Clin North Am. 2000;14:211–239. doi: 10.1016/s0891-5520(05)70225-7. x-xi. [DOI] [PubMed] [Google Scholar]
- 26.Alarcon JO, Friedman HB, Montano SM, Zunt JR, Holmes KK, Quinnan GV., Jr High endemicity of human T-cell lymphotropic virus type 1 among pregnant women in Peru. J Acquir Immune Defic Syndr. 2006;42:604–609. doi: 10.1097/01.qai.0000221680.52563.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Rosa AM, Zunt JR, Peinado J, Lama JR, Ton TG, Suarez L, Pun M, Cabezas C, Sanchez J, Peruvian HIV Sentinel Surveillance Working Group Retroviral infection in Peruvian men who have sex with men. Nephrol Dial Transplant. 2009;49:112–117. doi: 10.1086/599609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wignall FS, Hyams KC, Phillips IA, Escamilla J, Tejada A, Li O, Lopez F, Chauca G, Sanchez S, Roberts CR. Sexual transmission of human T-lymphotropic virus type I in Peruvian prostitutes. J Med Virol. 1992;38:44–48. doi: 10.1002/jmv.1890380110. [DOI] [PubMed] [Google Scholar]
- 29.Hyams KC, Phillips IA, Tejada A, Wignall FS, Roberts CR, Escamilla J. Three-year incidence study of retroviral and viral hepatitis transmission in a Peruvian prostitute population. J Acquir Immune Defic Syndr. 1993;6:1353–1357. [PubMed] [Google Scholar]
- 30.Pollett S, Calderon M, Heitzinger K, Solari V, Montano SM, Zunt J. Prevalence and predictors of cervicitis in female sex workers in Peru: an observational study. BMC Infect Dis. 2013;13:195. doi: 10.1186/1471-2334-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva ZJ, Nielsen J, Andersen A, Oliveira I, Dias F, Rodrigues A, Holmgren B, Andersson S, Aaby P. Decline in human T-cell lymphotropic virus-1 prevalence in urban areas of Bissau, Guinea-Bissau: exploring the association with HIV infections. AIDS. 2009;23:637–639. doi: 10.1097/QAD.0b013e32832403e8. [DOI] [PubMed] [Google Scholar]
- 32.Tortevoye P, Tuppin P, Carles G, Peneau C, Gessain A. Comparative trends of seroprevalence and seroincidence rates of human T cell lymphotropic virus type I and human immunodeficiency virus 1 in pregnant women of various ethnic groups sharing the same environment in French Guiana. Am J Trop Med Hyg. 2005;73:560–565. [PubMed] [Google Scholar]
- 33.Koga Y, Iwanaga M, Soda M, Inokuchi N, Sasaki D, Hasegawa H, Yanagihara K, Yamaguchi K, Kamihira S, Yamada Y. Trends in HTLV-1 prevalence and incidence of adult T-cell leukemia/lymphoma in Nagasaki, Japan. J Med Virol. 2010;82:668–674. doi: 10.1002/jmv.21738. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi K. Declining trends in HTLV-I prevalence among blood donors in Japan. Intern Med. 2001;40:1–2. doi: 10.2169/internalmedicine.40.1. [DOI] [PubMed] [Google Scholar]
- 35.Iwanaga M, Chiyoda S, Kusaba E, Kamihira S. Trends in the seroprevalence of HTLV-1 in Japanese blood donors in Nagasaki Prefecture, 2000–2006. Int J Hematol. 2009;90:186–190. doi: 10.1007/s12185-009-0366-6. [DOI] [PubMed] [Google Scholar]
- 36.Kline RL, Brothers T, Halsey N, Boulos R, Lairmore MD, Quinn TC. Evaluation of enzyme immunoassays for antibody to human T-lymphotropic viruses type I/II. Lancet. 1991;337:30–33. doi: 10.1016/0140-6736(91)93343-8. [DOI] [PubMed] [Google Scholar]
- 37.Cossen C, Hagens S, Fukuchi R, Forghani B, Gallo D, Ascher M. Comparison of six commercial human T-cell lymphotropic virus type I (HTLV-I) enzyme immunoassay kits for detection of antibody to HTLV-I and -II. J Clin Microbiol. 1992;30:724–725. doi: 10.1128/jcm.30.3.724-725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karopoulos A, Silvester C, Dax EM. A comparison of the performance of nine commercially available anti-HTLV-I screening assays. J Virol Methods. 1993;45:83–91. doi: 10.1016/0166-0934(93)90142-e. [DOI] [PubMed] [Google Scholar]
- 39.Andersson S, Thorstensson R, Ramirez KG, Krook A, von Sydow M, Dias F, Biberfeld G. Comparative evaluation of 14 immunoassays for detection of antibodies to the human T-lymphotropic virus types I and II using panels of sera from Sweden and west Africa. Transfusion. 1999;39:845–851. doi: 10.1046/j.1537-2995.1999.39080845.x. [DOI] [PubMed] [Google Scholar]
- 40.Lal RB, Brodine S, Kazura J, Mbidde-Katonga E, Yanagihara R, Roberts C. Sensitivity and specificity of a recombinant transmembrane glycoprotein (rgp21)-spiked western immunoblot for serological confirmation of human T-cell lymphotropic virus type I and type II infections. J Clin Microbiol. 1992;30:296–299. doi: 10.1128/jcm.30.2.296-299.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinman SH, Kaplan JE, Khabbaz RF, Calabro MA, Thomson R, Busch M. Evaluation of a p21e-spiked western blot (immunoblot) in confirming human T-cell lymphotropic virus type I or II infection in volunteer blood donors. The Retrovirus Epidemiology Donor Study Group. J Clin Microbiol. 1994;32:603–607. doi: 10.1128/jcm.32.3.603-607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorstensson R, Albert J, Andersson S. Strategies for diagnosis of HTLV-I and -II. Transfusion. 2002;42:780–791. doi: 10.1046/j.1537-2995.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- 43.Gastaldello R, Hall WW, Gallego S. Seroepidemiology of HTLV-I/II in Argentina: an overview. J Acquir Immune Defic Syndr. 2004;35:301–308. doi: 10.1097/00126334-200403010-00012. [DOI] [PubMed] [Google Scholar]
- 44.Gotuzzo E, Yamamoto V, Kanna M, Chauca G, Watts D. Human T-cell lymphotropic virus type I infection among Japanese immigrants in Peru. Int J Infect Dis. 1996;1:75–77. [Google Scholar]
- 45.Biglione MM, Pizarro M, Puca A, Salomon HE, Berria MI. A cluster of human T-cell lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis in Jujuy, Argentina. J Acquir Immune Defic Syndr. 2003;32:441–445. doi: 10.1097/00126334-200304010-00015. [DOI] [PubMed] [Google Scholar]
- 46.Golenbock DT, Guerra J, Pfister J, Golubjatnikov R, Tejada A, Abugattas J, Kemper R, Maki DG. Absence of infection with human immunodeficiency virus in Peruvian prostitutes. AIDS Res Hum Retroviruses. 1988;4:493–499. doi: 10.1089/aid.1988.4.493. [DOI] [PubMed] [Google Scholar]
- 47.Alarcón J, Palacios OA, Tejada AV, Foreit J, Piscoya J, Wignal S, Phillips I. Investigacion Operacional de Prevencion del SIDA en Prostitutas del Callao, Lima-Peru, 1988–1989. Rev Peru Epidemiol. 1991;4:16–26. [Google Scholar]
- 48.Sanchez J, Campos PE, Courtois B, Gutierrez L, Carrillo C, Alarcon J, Gotuzzo E, Hughes J, Watts D, Hillier SL, Buchanan K, Holmes KK. Prevention of sexually transmitted diseases (STDs) in female sex workers: prospective evaluation of condom promotion and strengthened STD services. Sex Transm Dis. 2003;30:273–279. doi: 10.1097/00007435-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Weir SS, Fox LJ, DeMoya A, Gomez B, Guerrero E, Hassig SE. Measuring condom use among sex workers in the Dominican Republic. Int J STD AIDS. 1998;9:223–226. doi: 10.1258/0956462981922089. [DOI] [PubMed] [Google Scholar]