Abstract
Dengue fever is the most common arbovirus disease, and presents with a large spectrum of clinical manifestations ranging from asymptomatic disease through to the development of dengue hemorrhagic fever. These extreme cases can lead to dengue shock syndrome, and sometimes death. Spinal cord involvement in dengue virus (DENV) infections is rare. Here, we report a case in which the patient developed acute transverse myelitis (TM) without paraparesis following a DENV infection. This case highlights the importance of physicians' awareness of the possible link between DENV and TM in endemic areas.
Background
Dengue fever (DF), with its high morbidity and mortality rates, is the most common disease caused by an arbovirus globally, and is a major public health issue in many tropical and subtropical countries. DF was reported in at least 128 countries, and it is estimated that around 3.97 billion people are at risk.1 The World Health Organization estimates that around 50–100 million cases occur annually.2 The etiological agent for DF is dengue virus (DENV), which is classified into a group of four distinct but genetically related serotypes (DENV-1 to DENV-4). These viruses belong to the genus Flavivirus, family Flaviviridae, and are small, spherical, and lipid enveloped, with a genome composed of a single-stranded positive-sense RNA molecule.3
DF presents with a large spectrum of clinical manifestations ranging from asymptomatic to dengue hemorrhagic fever (DHF). DHF can progress to dengue shock syndrome (DSS), which is characterized by vascular leakage and multiorgan failure that can cause death.4 A diverse range of neurological complications of DENV infections involving the central and peripheral nervous systems have been reported. These include encephalopathy, encephalitis, febrile seizures, aseptic meningitis, intracranial hemorrhages, intracranial thrombosis, subdural effusions, mononeuropathies, polyneuropathies, and Guillain–Barré syndrome.5,6 However, spinal cord involvement following DENV infections is rare, and presents as postinfectious myelopathy, acute disseminated encephalomyelitis, or transverse myelitis (TM).7 TM can be para- or postinfectious. It has been suggested that parainfectious TM is more frequently associated with flaccid paraparesis, whereas postinfectious TM is typically associated with spastic paraparesis.8–10
Other arboviruses can infect the nervous system, causing TM. The emergence or re-emergence of new arboviruses in the Americas as Zika, chikungunya, Mayaro, West Nile, and others,11,12 highlights the need of the physicians pay more attention to the diagnosis of TM.
We report a case in which the patient developed acute TM without paraparesis following a DENV infection.
Case Report
A 21-year-old male patient with complaints of motor dysfunction of the bladder and lower limbs was admitted to the Hospital de Base, in São José do Rio Preto, São Paulo, Brazil. He was admitted on April 2015, 10 days after of the onset of suggestive symptoms of dengue without any warning signs. He had complaints of fever associated with leukopenia and thrombocytopenia (70,000 platelets/mm3; normal values for complete blood count [CBC]: leukocytes, 4,000–11,000/mm3; platelets, 1,50,000–3,00,000/mm3).
On examination, the patient was afebrile for 4 days and his blood count was within normal limits. The patient presented with a positive Babinski sign, bilateral clonus of the lower limbs, absence of cutaneous and abdominal reflexes bilaterally, and tactile and pain sensitivity in the T4–T6 regions of the dermatome.
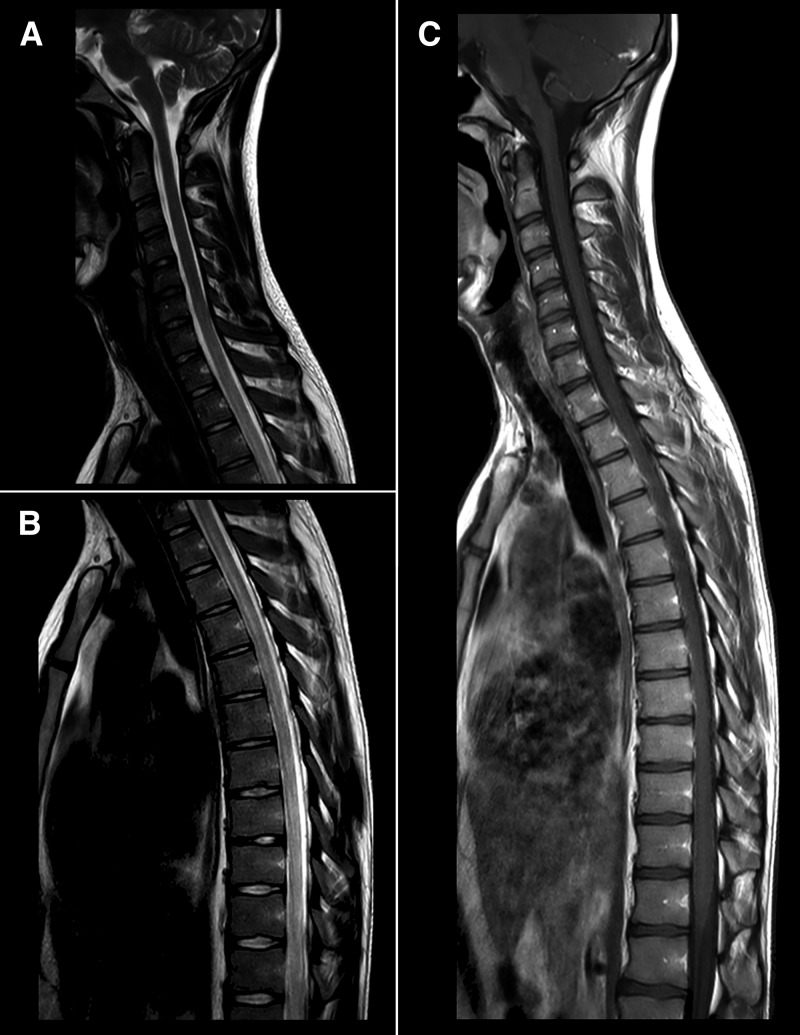
Tests for autoantibodies and serological assessments for human immunodeficiency virus, Venereal Disease Research Laboratory test, hepatitis B and C were all nonreactive. Nuclear magnetic resonance of the brain and spinal cord showed a small hyperintense image on T2 in the frontal white matter of the right cerebral hemisphere. The spinal cord showed enhancement from T1 through T10, compatible with inflammation, and hyperintense lesions in T2 in the thoracic segment of the spinal cord, compatible with TM (Figure 1 ). The cerebrospinal fluid (CSF) showed lymphocytic pleocytosis and normal glucose and protein levels, without signs of intracranial hypertension (normal values for CSF: opening pressure, 5–20 cmH2O; aspect, clear; cell count, until 4 mm3; lymphocytes, 50–70%; monocytes, 30–50%; proteins, until 40 mg/dL; glucose, 2/3 of the glycemia).
Figure 1.
T2-weighted magnetic resonance imaging images of the spinal cord. (A and B) Hyperintense lesion of the thoracic segment on T2-weighted images, extending from T2 through T10. No evidence of medullar expansion or significant impregnation by paramagnetic contrast, consistent with transverse myelitis. (C) T1-weighted image showing no correspondence.
Rapid tests for viral antigen detection (NS1), and for IgG and IgM detection, were performed using patient serum and CSF samples. The tests were positive for IgG and IgM in serum and for IgG in CSF.
The patient was diagnosed with DENV-associated TM, and then treated with intravenous methylprednisolone pulse therapy for 5 days. At the end of treatment, the patient was able to walk with assistance and to urinate with Crede maneuver, and then was discharged. After 10 months of follow-up, the patient still had urinary control difficulties.
Discussion
Despite the increasing number of dengue cases, the pathophysiology of neurological complications remains elusive. Viral antigens can be isolated from brain, brainstem, and spinal cord in humans, showing the virus can infect neural tissues. So at the beginning of the infection, the virus may have a direct pathogenic effect. However, in postinfectious TM, the role of the immune system in pathogenesis appears to be greater, although the presence of the virus at these sites cannot be proven. In animal models, DENV-2 can replicate within the nervous system, spreading to the spinal cord via the CSF.13
The actual prevalence of dengue-associated TM, especially postinfectious myelitis, may be underestimated. There are some reports of TM arising weeks after dengue symptoms onset,9,10,14,15 and the patients may not be followed up for so long. The reports given very different figures for the prevalence of this disease. For example, in one study involving 116 dengue patients in India, despite 79% of the patients presents some neurological manifestation, only 1% had TM.16 On the other hand, in a retrospective study conducted in Rondonia State (north region of Brazil), involving 59 dengue patients with neurological manifestations, 26 had TM.17
There are no reports of differences in the clinical presentation of TM with or without DENV infection. Therefore, our patient management followed the usual treatment of TM, which consists of pulse therapy with methylprednisolone for 3–7 days.18 The emergence of new arboviruses, such as Zika virus and chikungunya virus, poses new challenges to the public health system, especially in DENV-endemic regions. Careful evaluation and follow-up of patients, with special attention to neurological complications, may allow better understanding of the role of DENV and other arboviruses in the pathogenesis of TM.
Footnotes
Authors' addresses: Mânlio Tasso de Oliveira Mota, Cássia Fernanda Estofolete, Nathalia Zini, Ana Carolina Bernardes Terzian, Delzi Vinha Nunes Gongora, Irineu Luiz Maia, and Maurício Lacerda Nogueira, Laboratory of Virology, Faculty of Medicine of São José do Rio Preto, São Paulo, Brazil, E-mails: manliotasso@gmail.com, cassiafestofolete@gmail.com, nathalia_zini@hotmail.com, anacarolinaterzian@gmail.com, delzi@famerp.br, maia@famerp.com.br, and mnogueira@famerp.br.
References
- 1.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilder-Smith A, Byass P. The elusive global burden of dengue. Lancet Infect Dis. 2016;16:629–631. doi: 10.1016/S1473-3099(16)00076-1. [DOI] [PubMed] [Google Scholar]
- 3.Achee NL, Gould F, Perkins TA, Reiner RC, Jr, Morrison AC, Ritchie SA, Gubler DJ, Teyssou R, Scott TW. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9:e0003655. doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla P, Yadav A, Chawla V. Clinical implications and treatment of dengue. Asian Pac J Trop Med. 2014;7:169–178. doi: 10.1016/S1995-7645(14)60016-X. [DOI] [PubMed] [Google Scholar]
- 5.Chanthamat N, Sathirapanya P. Acute transverse myelitis associated with dengue viral infection. J Spinal Cord Med. 2010;33:425–427. doi: 10.1080/10790268.2010.11689722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thisyakorn U, Thisyakorn C. Dengue with central nervous system involvement. Southeast Asian J Trop Med Public Health. 2015;46((Suppl 1)):118–122. [PubMed] [Google Scholar]
- 7.Verma R, Sahu R, Holla V. Neurological manifestations of dengue infection: a review. J Neurol Sci. 2014;346:26–34. doi: 10.1016/j.jns.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Kunishige M, Mitsui T, Tan BH, Leong HN, Takasaki T, Kurane I, Mihara A, Matsumoto T. Preferential gray matter involvement in dengue myelitis. Neurology. 2004;63:1980–1981. doi: 10.1212/01.wnl.0000144194.29643.d0. [DOI] [PubMed] [Google Scholar]
- 9.Leao RN, Oikawa T, Rosa ES, Yamaki JT, Rodrigues SG, Vasconcelos HB, Sousa MR, Tsukimata JK, Azevedo RS, Vasconcelos PF. Isolation of dengue 2 virus from a patient with central nervous system involvement (transverse myelitis) Rev Soc Bras Med Trop. 2002;35:401–404. doi: 10.1590/s0037-86822002000400018. [DOI] [PubMed] [Google Scholar]
- 10.Seet RC, Lim EC, Wilder-Smith EP. Acute transverse myelitis following dengue virus infection. J Clin Virol. 2006;35:310–312. doi: 10.1016/j.jcv.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo LT. Emergent arboviruses in Brazil. Rev Soc Bras Med Trop. 2007;40:224–229. doi: 10.1590/s0037-86822007000200016. [DOI] [PubMed] [Google Scholar]
- 12.Sampathkumar P, Sanchez JL. Zika virus in the Americas: a review for clinicians. Mayo Clin Proc. 2016;91:514–521. doi: 10.1016/j.mayocp.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 13.An J, Zhou DS, Kawasaki K, Yasui K. The pathogenesis of spinal cord involvement in dengue virus infection. Virchows Arch. 2003;442:472–481. doi: 10.1007/s00428-003-0785-3. [DOI] [PubMed] [Google Scholar]
- 14.Weeratunga PN, Caldera MC, Gooneratne IK, Gamage R, Perera P. Neurological manifestations of dengue: a cross sectional study. Travel Med Infect Dis. 2014;12:189–193. doi: 10.1016/j.tmaid.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Miranda de Sousa A, Puccioni-Sohler M, Dias Borges A, Fernandes Adorno L, Papais Alvarenga M, Papais Alvarenga RM. Post-dengue neuromyelitis optica: case report of a Japanese-descendent Brazilian child. J Infect Chemother. 2006;12:396–398. doi: 10.1007/s10156-006-0475-6. [DOI] [PubMed] [Google Scholar]
- 16.Misra UK, Kalita J, Mani VE, Chauhan PS, Kumar P. Central nervous system and muscle involvement in dengue patients: a study from a tertiary care center. J Clin Virol. 2015;72:146–151. doi: 10.1016/j.jcv.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 17.de Sousa AM, Alvarenga MP, Alvarenga RM. A cluster of transverse myelitis following dengue virus infection in the Brazilian Amazon region. Trop Med Health. 2014;42:115–120. doi: 10.2149/tmh.2014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott TF, Frohman EM, De Seze J, Gronseth GS, Weinshenker BG, Therapeutics and Technology Assessment Subcommittee of American Academy of Neurology Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;77:2128–2134. doi: 10.1212/WNL.0b013e31823dc535. [DOI] [PubMed] [Google Scholar]