Abstract
The incidence of Japanese encephalitis (JE) decreased sharply after the national vaccination program was implemented in Taiwan in 1968. However, cases of JE still occur. The purpose of this study was to assess the epidemiology and vaccination policy for JE in Taiwan. We analyzed the data on JE cases reported to the Taiwan Centers for Disease Control (Taiwan CDC) between 2000 and 2014. During the 15-year study period, a total of 4,474 cases were reported to the Taiwan CDC. Of these, 379 (8.5%) were classified as confirmed cases, and 4,095 (91.5%) were classified as suspected cases. The incidence of JE ranged from 0.59 to 1.61 per 1,000,000 people and peaked in 2007. Men had a higher incidence of JE than women (1.37 versus 0.84 per 1,000,000; P = 0.03). Patients who were 40–59 years of age had a higher incidence than did patients younger than 20 years (1.82 versus 0.23; P < 0.001). Patients who lived in the eastern region of Taiwan had the highest incidence rate of JE (P < 0.001). Compared with those who were not vaccinated with the JE vaccine, patients who received four doses of JE vaccine had a lower risk of suffering from death and/or hospitalization (adjusted odds ratio: 0.26; 95% confidence interval: 0.08–0.90; P = 0.04). JE is still a public health problem in Taiwan, and monitoring JE via diagnostic testing to determine the best vaccination program along with enforcing JE vaccine boosters for adults is necessary to eliminate JE in Taiwan.
Introduction
Japanese encephalitis (JE) is an important cause of viral encephalitis in most Asian countries, particularly in south Asia, southeast Asia, and east Asia.1 It is estimated that 67,900 cases occur annually, with a fatality rate ranging from 20% to 30%.1,2 The Japanese encephalitis virus (JEV) belongs to the genus Flavivirus, family Flaviviridae, and has a single-stranded, positive-sense RNA genome that is ∼11 kb in length.3 Currently, the most common serological techniques for the diagnosis of JEV infection are IgM and IgG antibody-capture enzyme-linked immunosorbent assays (MAC and GAC ELISAs, respectively). Both of these assays detect antibodies directed toward the membrane (M) and envelope (E) structural proteins of flaviviruses.3 The main transmission vectors of the JEV are Culex mosquitoes, particularly Culex tritaeniorhynchus, and the main vertebrate hosts for amplifying JEV are pigs and ardeid birds.3,4 The illness spectrum of JE for humans ranges from asymptomatic infections to a devastating encephalitis syndrome that is associated with appreciable mortality and frequent central nervous system sequelae in survivors.5 Since childhood JE vaccination programs are not fully implemented across the geographic range of the JEV, in contrast to the pre-vaccination era, the target population for infections has shifted from children to adults in Taiwan.1,2
Taiwan has a population of approximately 23 million people and a population density of 635/km2. The majority (95%) of the population lives in the western region of Taiwan, which is divided into the northern, central, and southern regions. Only 5% of the population lives in eastern Taiwan, where medical care is substandard and the socioeconomic status is classified as low. The Taiwanese government launched a comprehensive vaccination campaign against JE in the 1960s, during which all children younger than 3 years received two doses of the JE vaccine. After 1974, the number of vaccination doses was increased to three, with a booster dose administered 1 year after the two primary doses. After 1976, a fourth booster dose was administered to children during their first year at elementary school. After 1980, the target population for JE vaccinations was mainly children older than 15 months. The vaccination was administered in two doses separated by an interval of 2 weeks, which were administered between March and May, before the epidemic season, and were followed by a booster dose 1 year later, with a final booster dose (the fourth dose) when the child entered the first year of elementary school.6,7 Before 1987, an inactivated vaccine derived from a Nakayama strain JEV-infected mouse brain was the only vaccine used. A version derived from an inactivated freeze-dried Beijing strain was introduced in 1988, but the Nakayama strain of the vaccine has remained the dominant vaccine on the market. Despite the current vaccination program, cases of JE still occur in Taiwan. Although the epidemiology of the disease is changing, the persistent occurrence of JE cases requires careful monitoring. The disease burden caused by JEV needs to be addressed, and effective public health measures should be adopted in Taiwan. The objectives of this study were to explore the epidemiology and vaccination policy for JE in Taiwan based on population surveillance data collected over a 15-year period.
Methods
Data sources.
Since 1955, the National Notifiable Diseases Surveillance System (NNDSS) has reported JE cases to the Centers for Disease Control of Taiwan (Taiwan CDC).6,8 Physicians are required to report all cases that meet the case definition of JE, collect samples, and send them to the Taiwan CDC within 1 week of the case being reported for examination.6 After the reports are received, an epidemiological team (field epidemiologist, entomologist, public health nurse) is assigned to perform a patient follow-up, verify the diagnosis, acquire complete patient information, and ensure vector control.
We collected all JE-related data reported to the NNDSS at the Taiwan CDC from January 2000 to December 2014. The reported information included patient age, sex, area of residence, geographic location of exposure, personal contact, travel history, vaccination status, and date of JE onset. If the patient had been vaccinated, the vaccination date and number of doses received were also reported. In addition, clinical details were reported, including signs/symptoms and disease outcome (either complications or death). Serum and/or cerebrospinal fluid (CSF) samples were collected from the patients for a serological confirmation of the diagnosis. Serological testing was performed by the virus laboratory of the Taiwan CDC.
Institutional review board approval for this study was obtained from the National Cheng Kung University Hospital.
Case definitions.
A clinical case was defined as a person of any age with an acute onset of fever and a change in mental status and/or a new onset of seizures (excluding simple febrile seizures) at any time of the year.6,9
A suspected case was defined as a case that met the clinical case definition in which diagnostic testing was performed but the etiological agent of JE was not found or the test results were indeterminate.6,9
A confirmed case was defined as a clinical case with a positive laboratory test (presence of IgM antibodies specific to the JE virus in a single sample of CSF or serum; and/or a 4-fold increase in IgG antibodies; and/or the detection of JE virus antigens in tissue via immunohistochemistry or the detection of the JE virus genome in serum, plasma, blood, CSF, or tissue or that met the clinical case definition and was epidemiologically linked to a confirmed case).6,9
Laboratory tests.
In 1998, an E/M-specific capture IgM and IgG enzyme-linked immunosorbent assay (E/M-specific IgM/IgG ELISA) for JE and dengue fever (DF) was developed by the Taiwan CDC.7,10 Antibodies against both JE and DF are screened using hemagglutination inhibition (HI)11,12 or an E/M-specific IgM/ IgG ELISA.13 A differential testing algorithm was used as described in previous study,13 in which samples that tested positive in the JE ELISA were subsequently tested using the DF ELISA, and the results of both tests were considered in the final interpretation of the data. Positive results in the JE ELISA and negative results in the DF ELISA indicated the presence of JEV antibodies only, whereas positive results in both tests indicated a false-positive JE result. A long-term evaluation revealed that the E/M-specific IgM/IgG ELISA is highly sensitive and specific and that it can effectively differentiate JEV infections from dengue virus infections.13 Since 2001, acute-phase serum obtained within 7 days and CSF from individuals with JE have been used for diagnoses via real-time polymerase chain reaction (PCR) analyses14; however, the E/M-specific IgM/IgG ELISA remains the primary method of diagnosis. The JE laboratory confirmed that clinical cases that meet one of the following specific laboratory criteria should be defined as confirmed cases of JE: 1) an HI titer of the convalescent serum of 1:160 and at least a 4-fold increase between the HI titers of convalescent and acute sera11,12; 2) an HI titer from a single serum sample of 1:32011,12; 3) an IgM-positive serum sample as determined based on an ELISA test, or the IgG of paired serum samples exhibiting a 4-fold increase12; 4) a sample that exhibits a positive real-time PCR reaction12; or 5) a sample positive for indirect immuno-fluorescence antibody staining after isolating the virus from CSF.12,15 Cell culture techniques using a mosquito C6/36 cell line or plaque assays using the BHK-21 cell line were used for virus isolation.16 Viral RNA was exacted from the JEV-infected culture medium using the QIAamp Viral RNA Mini Kit (Qiagen, Germantown, MD).16
Statistical analysis.
The annual incidence rate of JE was calculated by dividing the number of confirmed JE cases by the number of individuals in the total population of the same age as reported in Taiwan's census between 2000 and 2014 and is expressed as the number of JE cases per 1,000,000 people. Age-specific incidence rates were estimated for the following age groups: ≤ 9 years, 10–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, and ≥ 60 years. All statistical analyses were performed using SAS V.9.2 (SAS Institute Inc., Cary, NC). We used χ2 tests with Yates' correction for categorical data and Student's t tests for continuous data. The accepted level of significance for all analyses was P < 0.05.
Results
Surveillance.
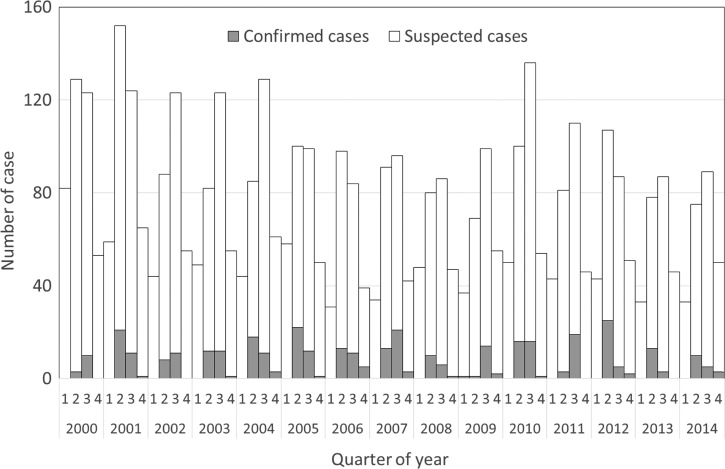
Figure 1 shows the number of cases (suspected and confirmed cases) of JE reported to the Taiwan CDC between January 2000 and December 2014. The annual number of reported cases varied yearly (range: 244–400 cases per year). The highest number of cases occurred in 2001 (400 reported cases), and the lowest occurred in 2013 (244 reported cases). For confirmed JE cases, seasonal peaks occurred every year during the summer and autumn during the 15-year study period. The mean number of confirmed JE cases was 25, and the number varied annually (range: 13–37 cases per year). The highest number of confirmed cases occurred in 2007 (37 cases), and the lowest occurred in 2000 (13 cases). The JE-positive rate among reported cases was 3.4% in 2000, 8.3% in 2001, 6.1% in 2002, 8.1% in 2003, 10.0% in 2004, 11.4% in 2005, 11.5% in 2006, 14.1% in 2007, 6.5% in 2008, 6.9% in 2009, 9.7% in 2010, 7.9% in 2011, 11.1% in 2012, 6.6% in 2013, and 7.3% in 2014; the mean annual JE-positive rate among reported cases was 8.5%.
Figure 1.
Number of suspected and confirmed cases of Japanese encephalitis in Taiwan by quarter, 2000–2014.
Morbidity.
During the study period, a total of 4,474 JE cases were reported to the Taiwan CDC and underwent a laboratory evaluation to confirm JE. Of these, 379 (8.5%) were classified as confirmed cases, and 4,095 (91.5%) were classified as suspected cases. The demographic characteristics of the confirmed JE cases are presented in Table 1. The male-to-female ratio was 1.7:1, and the median age was 40 years (range: < 1 to 80 years). The highest number of cases was in the 40–49 years age group (24.5%) and lowest was in the ≤ 9 years age group (1.8%). The number of patients was different for the different regions of Taiwan, with the highest number in the southern region (42.7%) and lowest in the eastern region (11.1%). Of the patients, 171 (45.1%) had received four doses of vaccine, and 47 (12.4%) were unvaccinated. The number of hospitalized cases was 331 (87%), and there were 27 deaths; a fatality rate of 7.1% (Table 1).
Table 1.
Demographic characteristics of JE in Taiwan, 2000–2014
| Variables | N | % |
|---|---|---|
| Sex | ||
| Male | 237 | 62.5 |
| Female | 142 | 37.5 |
| Age group (years) | ||
| ≤ 9 | 7 | 1.8 |
| 10–19 | 13 | 3.4 |
| 20–29 | 46 | 12.1 |
| 30–39 | 87 | 23.0 |
| 40–49 | 93 | 24.5 |
| 50–59 | 85 | 22.4 |
| ≥ 60 | 48 | 12.7 |
| Imported cases | ||
| Yes | 6 | 1.6 |
| No | 373 | 98.4 |
| Region of residence | ||
| Northern | 85 | 22.4 |
| Central | 90 | 23.7 |
| Southern | 162 | 42.7 |
| Eastern | 42 | 11.1 |
| JE vaccination status | ||
| 4 doses | 171 | 45.1 |
| 3 doses | 79 | 20.8 |
| 2 doses | 82 | 21.6 |
| Not vaccinated | 47 | 12.4 |
| Hospitalization | ||
| Yes | 331 | 87.3 |
| No | 48 | 12.7 |
| Death | ||
| Yes | 27 | 7.1 |
| No | 352 | 92.9 |
JE = Japanese encephalitis.
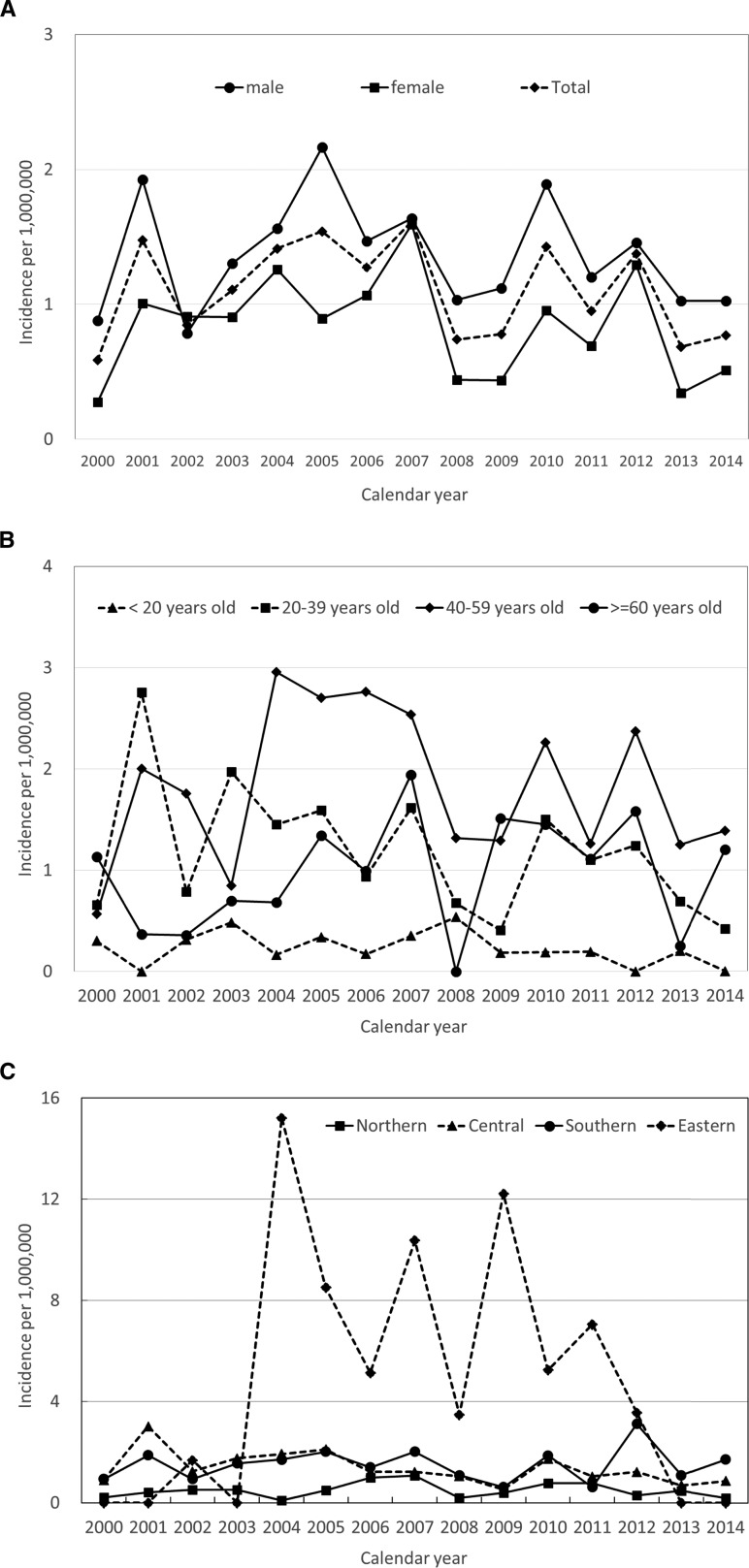
The incidence of confirmed JE cases is shown in Figure 2A . Overall, the average annual incidence rate was 1.10 per 1,000,000 from 2000 through 2014 in Taiwan; the highest annual incidence rate was 1.61 per 1,000,000 in 2007, and lowest was 0.59 per 1,000,000 in 2000 (χ2 test for a linear trend = 0.05; P = 0.82).
Figure 2.
Incidence of confirmed Japanese encephalitis among patients in Taiwan according to (A) sex, (B) age, and (C) region by year from 2000 to 2014.
Men had a higher average annual incidence rate than women between 2000 and 2014 (1.37 versus 0.84 per 1,000,000; P = 0.03). For men, the lowest annual incidence rate was 0.79 in 2002, and the highest was 2.16 in 2005 (χ2 test for a linear trend = 0.03; P = 0.86). For women, the lowest annual incidence rate was 0.28 in 2000, and the highest was 1.59 in 2007 (χ2 test for a linear trend = 0.28; P = 0.60). There was no significant change in the annual incidence rates of JE in men and women during the 15-year study period (P > 0.05 for both) (Figure 2A).
The annual incidence rates of JE cases among various age groups are shown in Figure 2B. We divided the age groups into four categories (< 20 years, 20–39 years, 40–59 years, and ≥ 60 years). During the study period, the incidence of JE was higher among people 20 years or older than it was among those under 20 years. Annual incidence rates increased as age increased, demonstrating a peak incidence rate in the age group of 40–59 years and lowest in the age group of less than 20 years (1.82 versus 0.23 per 1,000,000; P < 0.001). There was no significant change in the annual incidence rates of JE in all age groups (P > 0.005 for all).
Figure 2C shows the incidence rates for the four regions of Taiwan. The eastern region had the highest overall incidence rate (4.83 per 1,000,000); however, for the years 2000–2001, 2003, and 2013–2014, it was on par with the rates in other regions. The lowest incidence rate (0.49 per 1,000,000) was observed in the central region.
Risk factors for mortality and/or hospitalization.
Table 2 presents the results of a multiple logistic regression analysis of risk factors associated with mortality and/or hospitalization among patients with JE infections. Compared with those who were not vaccinated with the JE vaccine, patients who received four doses of JE vaccine had a lower risk of suffering from death and/or hospitalization [adjusted odds ratio: 0.26; 95% confidence interval [CI]: 0.08–0.90; P = 0.04]. Other risk factors, including sex, age groups, and resident region, were not significantly associated with death among patients with JE infections.
Table 2.
Multiple logistic regression analysis of factors associated with death among patients with JE in Taiwan, 2000–2014
| Variables | AOR | 95% CI | P value |
|---|---|---|---|
| JE vaccination status | |||
| 4 doses | 0.26 | 0.08–0.90 | 0.04 |
| 3 doses | 1.01 | 0.23–4.43 | 0.71 |
| 2 doses | 1.05 | 0.24–4.61 | 0.75 |
| 0 dose | 1.00 | ||
| Sex | |||
| Female | 1.37 | 0.62–3.01 | 0.72 |
| Male | 1.00 | ||
| Age groups (years) | |||
| ≥ 30 | 5.89 | 0.78–44.19 | 0.62 |
| < 30 | 1.00 | ||
| Region of residence | |||
| Central | 0.34 | 0.10–1.22 | 0.31 |
| Southern | 0.88 | 0.33–2.33 | 0.24 |
| Eastern | 1.02 | 0.28–3.72 | 0.39 |
| Northern | 1.00 | ||
AOR = adjusted odds ratio; CI = confidence interval; JE = Japanese encephalitis.
Discussion
Using the national surveillance database, this study provides an estimate of JE morbidity and mortality in Taiwan from 2000 to 2014. The results detail the overall incidence rates for JE as well as age-specific rates and the vaccination status of patients. The epidemiological evidence supports the conclusion that JE is still a public health concern in Taiwan.
The study of Hsu and others7 shows that the highest incidence rates of JE were in the people aged 0–29 years from 1966 to 1970; the incidence rates were similar for age groups (< 30 and ≥ 30 years) between 1992 and 2000; however, after 2001, the highest incidence rates shift to ages ≥ 30 years. The age of JE occurrence shifted from a younger age group to an older age group. Similar to the results of a previous study,7 this study found that the number of JE cases reported between 2000 and 2014 in Taiwan was far below that of the pre-vaccination era, when at least 5–15 times more cases were reported annually (the average number of JE cases reported in 1968 was 200).6 The incidence of JE was higher among people 20 years or older than it was among those under 20 years. We did an advanced analysis and found the incidence rates of JE increased as age increased, with a peak incidence rate in the age group of 40–59 years. The previous study7 shows that the positive rates of JE neutralizing antibodies in Taiwanese people were 74%, 63%, 55%, 54%, 68%, and 86% among 1981–1986, 1976–1980, 1970–1975, 1963–1969, and 1912–1952 cohorts, respectively. The countries neighboring Taiwan have confronted similar problems, with the majority of JE cases shifting from children to adults. Seventy-eight percent of confirmed JE cases in Japan occurred in people 40 years or older.17 In Korea, 86.7% of the confirmed cases were in adults older than 40 years.18 However, we performed a more detailed analysis here on the incidence rates of JE in the four regions of Taiwan and found that incidence rates were variable in the different regions (Figure 2C), which resulted in providing us more information about the risk of JE infections.
JE has historically been a childhood disease1,8; so, why did people 20 years or older have a higher incidence of JE over the period examined in this study? A possible explanation is that vaccine-induced immunity wanes over time, regardless of the number of doses received. The JE vaccination campaign in Taiwan has been running for more than 40 years, and the continual changes to the immunization policy ensures that various birth cohorts have received different doses of the JE vaccine. The results reported here might have been caused by the loss of antibodies because individuals received their last dose long ago or received fewer than four doses of the vaccine. Because the immunization program is national and compulsory, two- and three-dose schedules have achieved a coverage rate greater than 80%, and the coverage of the four-dose schedule has exceeded 95%.6,7 Our study found that the patients who received four doses of the JE vaccine had a lower risk of mortality and/or hospitalization compared with those who were not vaccinated with JE vaccine. A previous study showed that in people aged ≤ 51 years who were vaccinated, the positive antibody rates decreased as people aged7,19; for those who received four doses of the vaccine, the positive antibody rate was 63% for the 16–20 years since the last booster dose7,19; for people who received two or three doses, the positive antibody rate was 54% after more than 20 years since the last booster dose.7,19
Another possible reason is that there has been a dramatic shift in the dominant genotype of the JEV from GIII to GI circulating in Taiwan over the past two decades.15,20,21 It has been found that antibodies elicited by JE-VAX (Nakayama strain) among Taiwanese children show reduced neutralizing potency against the genotype of the emerging GI JEV strain.22
Taiwan has been using an inactivated mouse brain Nakayama-NIH vaccine strain since 1968, except for the brief use of an inactivated freeze-dried Beijing vaccine strain in 1988. It has been reported that the JE-VAX had several disadvantages, including vaccine-induced adverse events and the need for two or three primary doses plus boosters.23,24 Several new cell-culture-derived JE vaccines have been produced since 1989,25,26 and determining which vaccine provides the best way to reduce the occurrence of adult cases of JE is critical to the control and prevention of JE in Taiwan in the future.
In our study, male patients had a higher annual incidence rate of JE than female patients (1.37 versus 0.84 per 1,000,000). The study by Hsu and others shows the odds of presenting JE neutralizing antibodies are higher in male than in female (ratio: 1.25, 95% CI: 1.12–1.40) in Taiwanese people.7 However, no sex differences were reported in the incidence of JE cases in India.9 We are unable to explain this epidemiological finding in Taiwan but postulate that contributing factors may include different behavioral patterns or different reporting patterns.
In our study, a high percentage (42.7%) of JE cases occurred in the southern region of Taiwan. There are several possible reasons for this, which are discussed as follows: 1) In Taiwan, most confirmed cases of JE involved patients who lived near paddy fields or pig farms,15 and southern Taiwan is an agricultural region. Compared with other region, there are more pig farms near rice paddy fields and wetland habits for water birds in southern Taiwan. These provide suitable environments for maintaining the JEV infection cycle.16,27 2) In Taiwan, almost all DF cases (approximately > 95%) over the last decade occurred in the southern region of Taiwan.6,7 This fact and the high proportion of JE cases suggest that mosquitoes and the JE virus are well adapted to this region. 3) Finally, the higher proportion of cases in the southern region of Taiwan is also a reflection of the population numbers.
In contrast, this study found that the eastern rural areas had the highest overall incidence rate except in 2000–2001, 2003, and 2013–2014 (Figure 2C). The explanation for this interesting trend is unclear. However, we have not yet further investigated the possible epidemiological or public health factors that underlie these dramatic differences. Furthermore, the lower overall number of cases in this region could be a result of the low population density (104/km2) and may indicate that the extent of natural infection is substantially affected by the degree of urbanization and that it is the result of accumulated exposure.
Notably, only 27 deaths, with a fatality rate of 7.1%, were attributable to JE over the 15 years of surveillance in Taiwan. Compared with results of previous studies,28 the fatality rate of JE in Taiwan was very low (20–40% versus 7.1%). The lower fatality rate in Taiwan may be due to the national health insurance system, through which people have greater access to hospital services.
Our study has several limitations. First, the data were obtained via a surveillance-based method, where the completeness of reporting is important in determining the impact of a disease. Second, most cases were diagnosed by physicians according to clinical presentations and laboratory tests. Misdiagnoses and the underestimation of cases likely occurred because adequate specimens were not always collected. Nonetheless, this information is a reasonable substitute for population-level data.
In conclusion, most cases of clinically diagnosed JE in Taiwan do not have a JEV infection, and there appears to be waning protection if people are not revaccinated. Although the incidence rates of JE have decreased considerably since 1971, this disease remains a public health challenge in Taiwan. JE cases have shifted from children to adults. Monitoring JE via diagnostic testing and determining the best new vaccination program to reduce the occurrence of adult cases of JE is critical to the future control and prevention of JE in Taiwan.
ACKNOWLEDGMENTS
We thank the staff of the Taiwan Centers for Diseases Control for their help in collecting the information for all study patients.
Footnotes
Financial support: The study was supported by a grant (MOST-104-2314-B-217-001) from the Ministry of Science and Technology, Taiwan.
Authors' addresses: Yu-Kang Chang, Department of Radiology, Liouying Campus, Chi-Mei Medical Center, Tainan, Taiwan, E-mail: cangtd@ms29.hinet.net. Hsiao-Ling Chang, Division of Infection Control and Biosafety, Centers for Disease Control, Ministry of Health and Welfare, Taipei, Taiwan, E-mail: hlchang@cdc.gov.tw. Ho-Sheng Wu, Hsinchu Blood Center, Taiwan Blood Services Foundation, Hsinchu, Taiwan, E-mail: wuhs.sc@blood.org.tw. Kow-Tong Chen, Department of Occupational Medicine, Tainan Municipal Hospital, Tainan, Taiwan, E-mail: ktchen@mail.ncku.edu.tw.
References
- 1.Campbell G, Hill S, Fischer M, Jacobson J, Hoke C, Hombach J, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS. Estimated global incidence of Japanese encephalitis: a systemic review. Bull World Health Organ. 2011;89:766–774. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Japanese encephalitis surveillance and immunization—Asia and the Western Pacific, 2012. MMWR. 2013;62:658–662. [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenbach B, Rice C. Flaviviridae: the viruses and their replication. In: Fields BN, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Stratus S, Knipe D, editors. Fields Virology. 4th edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 991–1041. [Google Scholar]
- 4.Vaughn DW, Hoke CJ. The epidemiology of Japanese encephalitis prospects for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh D, Basu A. Japanese encephalitis—a pathological and clinical perspective. PLoS Negl Trop Dis. 2009;3:e437. doi: 10.1371/journal.pntd.0000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control, Taiwan Notifiable Infectious Disease Statistical System. 2016. http://nidss.cdc.gov.tw Available at. Accessed February 15, 2016.
- 7.Hsu LC, Chen YJ, Hsu FK, Huang JH, Chang CM, Chou P, Lin IF, Chang FY. The incidence of Japanese encephalitis in Taiwan—a population-based study. PLoS Negl Trop Dis. 2014;8:e3030. doi: 10.1371/journal.pntd.0003030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YC, Huang YS, Chien LJ, Lin TL, Yueh YY, Tseng WL, Chang KJ, Wang GR. The epidemiology of Japanese encephalitis on Taiwan during 1966–1997. Am J Trop Med Hyg. 1999;61:78–84. doi: 10.4269/ajtmh.1999.61.78. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization A Cohort Study to Assess the New WHO Japanese Encephalitis Surveillance Standards. 2007. http://www.who.int/bulletin/Volumes/86/3/07-04330/en/ Available at. Accessed February 21, 2016. [DOI] [PMC free article] [PubMed]
- 10.Ravi V, Robinson JS, Russell BJ, Desai A, Ramamurty N, Featherstone D, Johnson BW. Evaluation of IgM antibody capture enzyme-linked immunosorbent assay kits for detection of IgM against Japanese encephalitis virus in cerebrospinal fluid samples. Am J Trop Med Hyg. 2009;81:1144–1150. doi: 10.4269/ajtmh.2009.09-0144. [DOI] [PubMed] [Google Scholar]
- 11.Clark CH, Casals J. Techniques for hemagglutination inhibition with arthropod viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Manual for the Laboratory Diagnosis of Japanese Encephalitis Virus Infection. Geneva, Switzerland: WHO; 2007. pp. 1–52. [Google Scholar]
- 13.Johnson BW, Goodman CH, Jee Y, Featherstone DA. Differential diagnosis of Japanese encephalitis virus infections with the Inbios JE Detect™ and DEN Detect™ MAC-ELISA Kits. Am J Trop Med Hyg. 2016;94:820–828. doi: 10.4269/ajtmh.15-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sapkal GN, Wairagkar NS, Ayachit VM, Bondre VP, Gore MM. Detection and isolation of Japanese encephalitis virus from blood clots collected during the acute phase of infection. Am J Trop Med Hyg. 2007;77:1139–1145. [PubMed] [Google Scholar]
- 15.Mathur A, Kumar R, Sharma S, Kulshreshtha R, Kumar A, Chaturvedi UC. Rapid diagnosis of Japanese encephalitis by immunofluorescent examination of cerebrospinal fluid. Indian J Med Res. 1990;91:1–4. [PubMed] [Google Scholar]
- 16.Su CL, Yang CF, Teng HJ, Lu LC, Lin C, Tsai KH, Chen YY, Chen LY, Chang SF, Shu PY. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS Negl Trop Dis. 2014;8:e3122. doi: 10.1371/journal.pntd.0003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai S, Matsunaga Y, Takasaki T, Tanaka-Taya K, Taniguchi K, Okabe N, Kurane I, Vaccine Preventable Diseases Surveillance Program of Japan Japanese encephalitis: surveillance and elimination effort in Japan from 1982 to 2004. Jpn J Infect Dis. 2008;61:333–338. [PubMed] [Google Scholar]
- 18.Lee DW, Choe YJ, Kim JH, Song KM, Cho H, Bae GR, Lee JK. Epidemiology of Japanese encephalitis in South Korea, 2007–2010. Int J Infect Dis. 2012;16:e448–e452. doi: 10.1016/j.ijid.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tseng HF, Tan HF, Chang CK, Huang WL, Ho WC. Seroepidemiology study of Japanese encephalitis neutralizing antibodies in southern Taiwan: a comparative study between urban city and country townships. Am J Infect Control. 2003;31:435–440. doi: 10.1067/mic.2003.73. [DOI] [PubMed] [Google Scholar]
- 20.Konishi E, Kitai Y, Tabei Y, Nishimura K, Harada S. Natural Japanese encephalitis virus infection among humans in west and east Japan shows the need to continue a vaccination program. Vaccine. 2010;28:2664–2670. doi: 10.1016/j.vaccine.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Su CL, Yang CF, Chang SF, Su PY. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan, 2013–1014 [in Chinese] Epidemiol Bull. 2016;32:148–157. [Google Scholar]
- 22.Fan YC, Chen JM, Chiu HC, Chen YY, Lin JW, Shih CC, Chen CM, Chang CC, Chang GJJ, Chiou SS. Partially neutralizing potency against emerging genotype I virus among children received formalin-inactivated Japanese encephalitis virus vaccine. PLoS Negl Trop Dis. 2012;6:e1834. doi: 10.1371/journal.pntd.0001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon T. Control of Japanese encephalitis—within our grasp? N Engl J Med. 2006;355:869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 24.Fischer M, Lindsey N, Staples JE, Hills S, Centers for Disease Control and Prevention (CDC) Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 25.Yun SI, Lee YM. Japanese encephalitis: the virus and vaccines. Hum Vaccin Immunother. 2014;10:263–270. doi: 10.4161/hv.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halsted SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10:355–364. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka A, Mulyatno KC, Susilowati H, Hendrianto E, Utsumi T, Amin M, Lusida MI, Soegijanto S, Konishi E. Prevalence of antibodies to Japanese encephalitis virus among pigs in Bali and East Java, Indonesia, 2008. Jpn J Infect Dis. 2010;63:58–60. [PubMed] [Google Scholar]
- 28.Kari K, Liu W, Gautama K, Mamman MP, Clemens JD, Nisalak A, Subrata K, Kim HK, Xu ZY. A hospital-based surveillance for Japanese encephalitis in Bali, Indonesia. BMC Med. 2006;4:8. doi: 10.1186/1741-7015-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]