Abstract
Background
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is rare, with better clinical outcomes than other lung cancers. However, reports on advanced LELC characteristics and prognosis are lacking.
Methods
This retrospective study included adults diagnosed with advanced LELC (at least stage IIIA) between January 2003 and December 2015. Clinical characteristics, treatment modalities, and outcomes were recorded.
Results
Study population comprised 23 patients with a mean age of 63.7±10.6 years. The Eastern Cooperative Oncology Group status on diagnosis was 0 in five patients and 1 in the others. Most patients received multimodality treatment and all received cisplatin-based chemotherapy. Median follow-up duration was 28.8 months. The median progression free survival (PFS) was 14.6 months in patients received palliative chemotherapy. There were nine (39.1%) deaths. The median overall survival (OS) was not achieved. Until July 31, 2016, median OS was 54.1 months for stage IIIB and 27.6 months for stage IV. There was no significant difference in OS among all stages. No prognostic factors were found.
Conclusions
Advanced LELC responded well to cisplatin-based chemotherapy and/or radiotherapy. Main tumor resection is probably beneficial for advanced LELC. Long-term survival is possible for advanced LELC after multimodality treatment.
Keywords: Pulmonary lymphoepithelioma-like carcinoma (LELC), overall survival (OS), outcome
Introduction
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a rare lung malignancy, occurring in only 0.92%, that shares a similar morphology with undifferentiated nasopharyngeal carcinoma (1). In Asians, foregut-derived LELC, including primary pulmonary LELC, is highly associated with Epstein-Barr virus (EBV) (2). Higher EBV serology titer in pulmonary LELC is related to larger tumor size and later stage (3). The circulating EBV DNA in serum is also a potential surrogate marker for monitoring disease (4). Primary pulmonary LELC usually occurs in non-smoking patients, non-specific to gender, and patients are usually 10 years younger than those with other types of non-small cell lung cancer (5).
Pulmonary LELC is categorized as a variant of large cell carcinoma, according to the World Health Organization (WHO) classification (6). The prognosis is much better than other large cell carcinomas of the lung (7). Moreover, the outcome is favorable compared to other types of lung cancer (8). In the largest series of LELC among Western patients, the overall median survival was 107 months and that was no difference between early and advanced stages (8). Elderly age and serum albumin levels were the prognostic factors reported to affect overall survival (OS) (1,8).
The majority of pulmonary LELC patients are diagnosed in the early stage (1,8). Surgery is the main treatment strategy for early and operable stage pulmonary LELC (1,5,8). However, there is currently no consensus about the chemotherapy regimen and radiation dose for advanced LELC. As LELC is very rare, information regarding advanced LELC remains limited.
The current study aims to investigate the clinical manifestations, treatment modalities, and prognosis of advanced pulmonary LELC.
Methods
Study design and subjects
This retrospective study included adult patients who were diagnosed with advanced pulmonary LELC between January 2003 and December 2015 at Linkou Medical Center of Chang Gung Memorial Hospital. Pulmonary LELC was diagnosed according to the criteria established by the WHO (9). We excluded patients who had undifferentiated carcinoma without dense lymphoid infiltrates, those with incomplete medical records, and those who were lost to follow-up. Routine nasopharyngeal examination and search for other primary sites were performed in all patients to exclude metastatic nasopharyngeal carcinoma.
Gender, age, smoking history, baseline EBV DNA level, albumin level, tumor size, stage, metastatic site, treatment modalities, and outcomes were collected. Pathologic or clinical staging was performed according to the Seventh Edition of the American Joint Committee on Cancer staging system (10), which was based on tumor size, location, lymph node involvement, and distant metastases. Stage IIIA was included as locally advanced stage. Restaging scans were obtained at 3-month intervals during treatment and were assessed by radiologist, according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Follow-up information was obtained from patient medical records and progression free survival (PFS), OS were assessed.
This study was approved by the institutional review board of Chang Gung Memorial Hospital (No. 201600879B0). As this was a retrospective study and no modification in the management of patients was required, the need for informed consent was waived.
Statistical analysis
Statistical analyses were performed using Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA). Categorical variables were presented as frequencies (percentages), whereas continuous variables were presented as mean ± standard deviation. Kaplan-Meier survival analysis was performed to analyze PFS and OS.
Results
Data from a total of 23 adult patients who had been diagnosed as advanced LELC were analyzed. Five of our patients had been reported before (11). The group comprised 11 men and 12 women with a mean age of 63.7±10.6 years. Fourteen (60.9%) patients were aged greater than 60 years. Table 1 summarizes the demographics and clinical characteristics of the patients with LELC. Nine (39.1%) were current or ex-smokers. Baseline serum albumin levels were checked in 18 patients and 10 of them had levels greater than 4 g/dL. Six (26.1%) patients have been evaluated for serum EBV DNA levels; all were elevated and ranged from 66 to 146,000 copies/mL. After treatment, three of these patients had non-detectable serum EBV DNA levels and have been disease-free since then.
Table 1. Demographics and analysis of prognostic factors for overall survival.
| Variable | No. of patients (%) | Overall survival (univariate analysis: P value) |
|---|---|---|
| Age, years | 1.0 | |
| Mean | 63.7±10.6 | |
| Range | 48–85 | |
| <60 | 9 (39.1) | |
| ≥60 | 14 (60.9) | |
| Gender | 0.68 | |
| Male | 11 (47.8) | |
| Female | 12 (52.2) | |
| Smoking history | 1.0 | |
| Former or current smoker | 9 (39.1) | |
| Nonsmoker | 14 (60.9) | |
| Albumin level (baseline), g/dL | 18 (78.3) | 0.34 |
| <4 | 8 (34.8) | |
| ≥4 | 10 (43.5) | |
| ECOG | 0.1 | |
| 0 | 5 | |
| 1 | 18 | |
| EBV DNA level (baseline), copies/mL | 6 (26.1) | N/A |
| Median | 18,975 | |
| Range | 66–146,000 | |
| Tumor size, cm | 0.16 | |
| <3 | 6 (26.1) | |
| ≥3 | 17 (73.9) | |
| Stage | 0.38 | |
| IIIA | 7 (30.4) | |
| IIIB | 9 (39.1) | |
| IV | 7 (30.4) | |
| Metastatic site | 7 (30.4) | N/A |
| Lungs | 3 (42.9) | |
| Bone | 2 (28.6) | |
| Adrenals | 2 (28.6) | |
| Others | 2 (28.6) | |
| Treatment | 23 (100.0) | 0.52 |
| Surgery + adjuvant CT ± RT | 3 (13.0) | |
| Neoadjuvant CT ± RT + surgery | 8 (34.8) | |
| Palliative CT ± RT | 12 (52.2) | |
| Surgery | 11 (47.8) | N/A |
| Lobectomy | 9 (39.1) | |
| Wedge resection | 2 (8.7) | |
| First line chemotherapy regimen | 23 (100) | 0.42 |
| Cisplatin + docetaxel | 13 (56.5) | |
| Cisplatin + gemcitabine | 5 (21.7) | |
| Cisplatin + vinorelbine | 3 (13.0) | |
| Cisplatin + 5-FU | 1 (4.3) | |
| ADOC | 1 (4.3) | |
| RT | 15 (65.2) | 0.39 |
| Median, cGy | 5,100 | |
| Range, cGy | 2,500–6,780 | |
| Outcome | N/A | |
| Died | 9 (39.1) | |
| Alive | 14 (60.9) |
ECOG, Eastern Cooperative Oncology Group; EBV, Epstein-Barr virus; CT, chemotherapy; RT, radiotherapy, ADOC, cisplatin + vincristine + doxorubicine + cyclophosphamide.
Seven (30.4%) patients were stage IIIA, nine (39.1%) were stage IIIB, and seven (30.4%) patients were stage IV. The most common site of metastasis was the contralateral lung (42.9%). Brain computed tomography (CT) or magnetic resonance imaging (MRI) performed on 17 patients did not show brain metastasis. The Eastern Cooperative Oncology Group (ECOG) of patients at diagnosis was 0 in five patients and 1 in the remaining patients. Most patients (17 of 23, 73.9%) received multimodality treatment. All were treated with cisplatin-based chemotherapy. Docetaxel (13 of 23, 56.5%) combined with cisplatin was the most frequently used regimen. One patient had received cisplatin and docetaxel as adjuvant therapy after surgery, and twelve patients had received this regimen as first line palliative treatment. The response rate of palliative chemotherapy with cisplatin and docetaxel was 83.3% (10 of 12 patients, Figure 1). Radiation therapy was administered for 15 (65.2%) patients (doses range, 2,500–6,780 cGy). Eleven (42.8%) patients underwent surgical resection; 9 (39.1%) underwent lobectomy and 3 (27%) were down staged from stage IIIB after chemotherapy. Two of 7 patients were diagnosed as stage IV initially received surgery; one had bone metastasis and one had pleural metastasis. After chemotherapy, both patients were negative for distant metastasis on positron emission tomography-CT scan and underwent surgical wedge resection and mediastinum lymph node dissection with negative margins. The patient who had initial bone metastasis remained alive for 35.6 months and has been preparing to receive proton therapy for recurrent mediastinal nodal metastasis. The other patient with pleural metastasis has lived for 57.8 months and has been disease-free since then.
Figure 1.
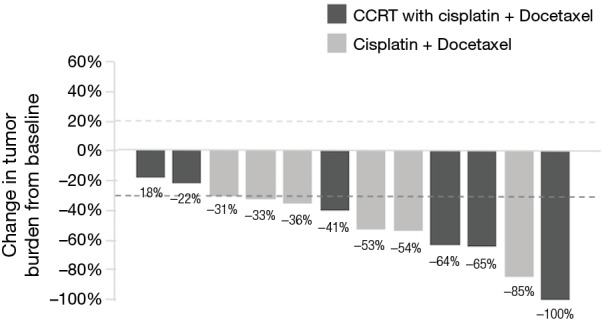
Waterfall plots for change in tumor burden are shown for patients who had received palliative chemotherapy with cisplatin and docetaxel. The response rate was 83.3% (10 of 12 patients). (The dashed line at 20% represents the boundary for determination of progressive disease, and the dashed line at −30% represents the boundary for determination of partial response.)
The median follow-up duration was 28.8 months (range, 3.7–101.5 months). The median PFS was 14.6 months in patients received palliative chemotherapy. The median PFS was 19.4 months in patients received cisplatin and docetaxel, and 10.7 months in patients received cisplatin and non-docetaxel regimen (P=0.29, Figure 2). There were 9 (39.1%) deaths, and the other 14 (60.9%) patients were still alive. One stage IIIB patient died 38 days after surgery because of nosocomial pneumonia. The others died of tumor progression. The median OS for advanced pulmonary LELC was not achieved. Until July 31, 2016, the median OS was 54.1 months in stage IIIB patients and 27.6 months in stage IV patients. The median OS of stage IIIA patients was not achieved and only one patient died (Figure 3). The OS of the study population was not associated with age, gender, smoking status, baseline serum albumin level, baseline serum EBV DNA level, tumor size, and treatment modality. Stage IV LELC patients tended to have the worst survival than those at stages IIIA and IIIB, but there was no significant difference in OS among the different stages (Figure 3, P=0.38).
Figure 2.
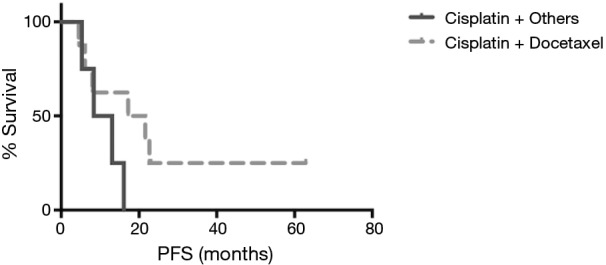
The progression free survival of patients who had received palliative chemotherapy. The median PFS was 19.4 months in patients received cisplatin and docetaxel, and 10.7 months in patients received cisplatin and non-docetaxel regimen (P=0.29). PFS, progression free survival.
Figure 3.
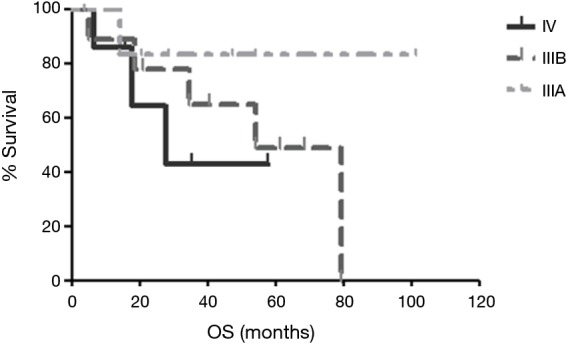
The overall survival of advanced LELC patients in different stages. The median OS of stage IIIA patients was no reached. The OS was 54.1 months in stage IIIB patients and was 27.6 months in stage IV patients (P=0.38). LELC, lymphoepithelioma-like carcinoma; OS, overall survival.
Discussion
Given the rare incidence of pulmonary LELC, only a few cases have been reported in the literature. To the best of our knowledge, this was the first study that discussed the clinical manifestations and outcomes of advanced primary pulmonary LELC. In addition to cisplatin-based chemotherapy and radiotherapy, we demonstrated that surgery is also probably beneficial to advanced LELC.
The gender predilection of LELC varies, women predominance has been reported before (5,12), while others (13) showed preponderance of men. The men/women ratio was 0.92:1 (11:12) in our study. Only nine of our cases were former or current smokers. This finding was compatible with that of previous studies (1,3,5), suggesting that smoking is not probably an etiologic role of LELC. Among Asians, the median age of LELC patients has been reported to range from 47–57 years (3,5,7,11,14), which is around 10 years younger than other patients with non-small cell lung cancer (5). In our advanced pulmonary LELC patients, the median age of 61 years (mean, 63.7±10.6 years) was much closer to the reported age range of Western patients with LELC (8). Although our patients were relatively older, their PS was good, despite being in the advanced stage.
The majority of foregut-derived LELC cases, which were highly associated with EBV, were reported in the Asian population (2,3,8,13). Most of those LELC cases were positive for EBV-encoded small non-polyadenylated RNA (EBER) on in situ hybridization (13). Chang et al. found that in 15 pulmonary LELC patients with available serum samples, a higher EBV serology titer represented higher tumor stage and larger tumor size (3). Moreover, in the prospective study presented by Ngan et al. (4), the level of circulating EBV DNA have been demonstrated to have a close relationship with clinical treatment response and tumor recurrence. They also found that patients with pre-therapy serum EBV DNA >10,000 copies/mL had poor outcome; whereas 5 of 19 cases without detectable serum EBV DNA did not have evidence of tumor after treatment (15). In the current study, only six patients have been evaluated for serum EBV DNA level: one patient died from nosocomial pneumonia after surgery and the others remained alive. Therefore, we could not analyze the association between EBV DNA titer and outcome. Nevertheless, three of the six patients who had no detectable serum EBV DNA after therapy remained alive and disease-free. This finding was similar to the one reported by Ngan et al. (15), suggesting that EBV DNA may be an adequate marker to monitor LELC status.
There were only two studies that discussed the outcome of advanced LELC. In one of the largest studies among Asians, Liang et al. (1) reported that the median OS in non-resectable patients was 39.1 months. In another study in Taiwan, the median OS of patients with stage III and stage IV was 3.4 years (40.8 months). In our review, because of only nine deaths, the median OS of stage IIIA patients and all advanced LELC cases were not achieved. However, we found the median OS to be 54.1 months in stage IIIB patients and 27.6 months in stage IV patients. While surgery is the main treatment strategy for early stage pulmonary LELC (5,8), multimodality treatment including cisplatin-based chemotherapy and radiation may play important roles in patients with advanced stage (3,8). However, there is currently no consensus on the regimen of chemotherapy (11). Ho et al. (16,17) suggested that combined 5-FU and cisplatin chemotherapy provided a favorable response and capecitabine was useful as a salvage agent. Liang et al. (1) demonstrated that pulmonary LELC was sensitive to paclitaxel-based or docetaxel-based regimen. As the PS was good in our cases, all of them received systemic cisplatin-based chemotherapy. More than half of them (56.5%) were treated with cisplatin and docetaxel, followed by gemcitabine and vinorelbine (Table 1). Only one patient with stage IV LELC received 5-FU and cisplatin combined chemotherapy, followed by sequential radiotherapy; this patient died 27.6 months later. After curative surgery with adjuvant chemotherapy and/or radiotherapy, stage IIIA patients had good prognosis. The longest survival after surgery in patients with stage IIIA LELC was 101.5 months through July 31, 2016. Three cases with initial stage IIIB status have been down staged to operable status after responding well to chemotherapy. The patient with the longest survival has been alive for 79.4 months. Moreover, there were two stage IV patients who nearly had remission after chemotherapy. They had received surgical intervention and both were alive well now. Based on our experience, we verified that LELC was a chemosensitive and radiosensitive type of lung cancer. There were no brain metastases in our 17 cases of advanced LELC, as evaluated by brain imaging. Moreover, our results indicated that LELC patients with advanced stage had relative favorable outcomes if their PS were good enough to allow them to receive multimodality treatment.
The present study had some limitations. First, this was a retrospective study and some of the data were incomplete. Second, the number of cases was small. However, by far, this has been the largest study to discuss advanced LELC. Third, because the chemotherapy regimens and radiation doses were heterogeneous, the optimal treatment for this rare disease remains to be elucidated. Nevertheless, chemotherapy and radiation were obviously effective for advanced LELC. Surgery may also have a role in treating advanced LELC.
In conclusion, patients with advanced LELC generally had good PS and responded well to cisplatin-based chemotherapy and radiation therapy. If possible, main tumor resection is probably beneficial for advanced LELC. Patients with advanced LELC could have good prognosis and possibly, long-term survival after multimodality treatment. Further large-scale, prospective studies are needed to determine the optimal treatment.
Acknowledgements
We thank all the investigators and members of the Department of Thoracic Care Medicine and the Department of Internal Medicine at Chang Gung Memorial Hospital for their effort.
Ethical Statement: The study was approved by the institutional review board of Chang Gung Memorial Hospital (No. 201600879B0). As this was a retrospective study and no modification in the management of patients was required, the need for informed consent was waived.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T, Haba R, Tanizawa J, et al. Cytopathologic features and differential diagnostic considerations of primary lymphoepithelioma-like carcinoma of the lung. Diagn Cytopathol 2012;40:820-5. [DOI] [PubMed] [Google Scholar]
- 3.Chang YL, Wu CT, Shih JY, et al. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol 2002;26:715-23. [DOI] [PubMed] [Google Scholar]
- 4.Ngan RK, Yip TT, Cheng WW, et al. Circulating Epstein-Barr virus DNA in serum of patients with lymphoepithelioma-like carcinoma of the lung: a potential surrogate marker for monitoring disease. Clin Cancer Res 2002;8:986-94. [PubMed] [Google Scholar]
- 5.Lin Z, Situ D, Chang X, et al. Surgical treatment for primary pulmonary lymphoepithelioma-like carcinoma. Interact Cardiovasc Thorac Surg 2016;23:41-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimosato Y. Histological Typing of Lung and Pleural Tumors (3rd edition, 1999): Malignant epithelial tumors. Nihon Rinsho 2002;60 Suppl 5:123-31. [PubMed] [Google Scholar]
- 7.Sun YH, Lin SW, Hsieh CC, et al. Treatment outcomes of patients with different subtypes of large cell carcinoma of the lung. Ann Thorac Surg 2014;98:1013-9. [DOI] [PubMed] [Google Scholar]
- 8.He J, Shen J, Pan H, et al. Pulmonary lymphoepithelioma-like carcinoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Dis 2015;7:2330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. [DOI] [PubMed] [Google Scholar]
- 10.Goldstraw P. The 7th Edition of TNM in Lung Cancer: what now? J Thorac Oncol 2009;4:671-3. [DOI] [PubMed] [Google Scholar]
- 11.Huang CJ, Feng AC, Fang YF, et al. Multimodality treatment and long-term follow-up of the primary pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer 2012;13:359-62. [DOI] [PubMed] [Google Scholar]
- 12.Chang YL, Wu CT, Shih JY, et al. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282-7. [DOI] [PubMed] [Google Scholar]
- 13.Han AJ, Xiong M, Zong YS. Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol 2000;114:220-6. [DOI] [PubMed] [Google Scholar]
- 14.Printz C. New AJCC cancer staging manual reflects changes in cancer knowledge. Cancer 2010;116:2-3. [DOI] [PubMed] [Google Scholar]
- 15.Ngan RK, Yip TT, Cheng WW, et al. Clinical role of circulating Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci 2004;1022:263-70. [DOI] [PubMed] [Google Scholar]
- 16.Ho JC, Lam WK, Wong MP, et al. Lymphoepithelioma-like carcinoma of the lung: experience with ten cases. Int J Tuberc Lung Dis 2004;8:890-5. [PubMed] [Google Scholar]
- 17.Ho JC, Lam DC, Wong MK, et al. Capecitabine as salvage treatment for lymphoepithelioma-like carcinoma of lung. J Thorac Oncol 2009;4:1174-7. [DOI] [PubMed] [Google Scholar]


