Abstract
Chlorine-containing bleach can cause acute respiratory distress syndrome (ARDS) and chemical burns. However, simultaneous occurrence of the two conditions caused by this agent is very rare. We describe the case of a 74-year-old female who presented with shortness of breath and hemoptysis following accidental exposure to chlorine-containing bleach. She had second- to third-degree chemical burns on both buttocks and thighs, and received mechanical ventilation because of the development of ARDS. Mechanical ventilation was discontinued on day 6 of hospitalization because of the rapid improvement of hypoxemia, and the patient was transferred to another hospital for further management of the chemical burns on day 18.
Keywords: Chlorine, acute respiratory distress syndrome, chemical burns
Introduction
Chlorine-containing bleach is commonly used as a household cleaning agent. Although chlorine-containing bleach is safe for household use, it can cause damage to the eyes, skin, gastrointestinal tract, and cardiovascular system (1). Respiratory complications that develop after acute exposure to chlorine gas include rhinitis, pneumonitis, pulmonary edema, and acute respiratory distress syndrome (ARDS) (1-3). To date, there has been only one documented case report of a 31-month-old male child who developed simultaneous chemical burns and ARDS following exposure to bleach (4). Here, we present a rare adult case of simultaneous development of chemical burns and ARDS after exposure to chlorine-containing bleach.
Case presentation
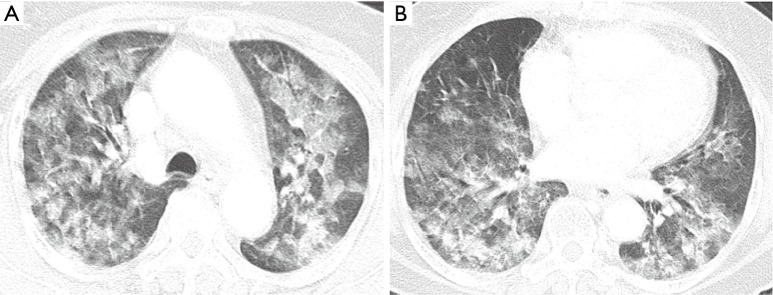
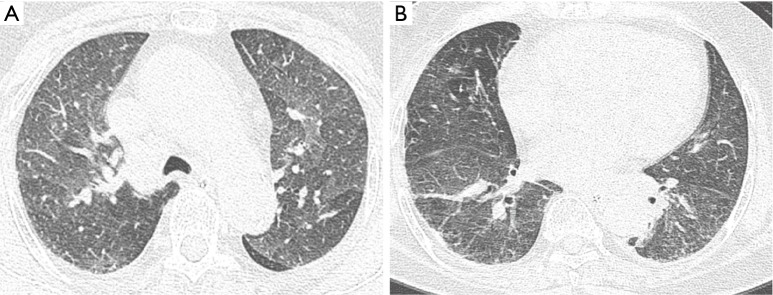
A 74-year-old nonsmoking female presented to the emergency room (ER) with shortness of breath and hemoptysis. About 10 hours before presenting to our hospital, she had slipped and fallen down in her bathroom, and had lost consciousness. At the time of her fall, the patient had been carrying a pouch of chlorine-containing bleach, which tore upon impact, releasing its contents. As a result, she lay in a puddle of the bleach, and she was exposed to chlorine gas for about 1 hour within the narrow enclosed space of the bathroom. The patient had a 1-year history of diabetes mellitus, for which she was taking an oral hypoglycemic agent. On arrival at the ER, her vital signs were normal with the exception of tachypnea (respiratory rate of 32 breaths/min). On physical examination, bilateral wheezes and crackles on auscultation and second- to third-degree chemical burns on both buttocks and thighs were observed. Initial chest radiography showed diffuse bilateral infiltrates (Figure 1). Laboratory data on admission were as follows: arterial blood gas analysis (pH, 7.38; partial pressure of carbon dioxide and oxygen, 32.6 and 42.8 mmHg, respectively); white blood cell count, 21.6×103/mm3 (neutrophils, 92.3%); glucose, 203 mg/dL; pro-brain natriuretic peptide, 282 pg/mL; and albumin, 3.2 g/dL. The patient’s hemoglobin level, platelet count, C-reactive protein level, electrolyte levels, liver function study results, coagulation profile, and lactate level were normal. Chest computed tomography (CT) revealed bilateral diffuse ground-glass opacities with interstitial thickening and patchy consolidation in both lungs (Figure 2). She was admitted to the intensive care unit and administered oxygen (via a high-flow nasal cannula), broad-spectrum antibiotics (piperacillin-tazobactam and levofloxacin), and diuretics. On day 4 following admission, the patient’s hypoxemia worsened despite the use of the high-flow nasal cannula, with fraction of inspired oxygen (FiO2) of 0.9. Because of the development of ARDS, it was decided to intubate the patient and provide mechanical ventilation. The patient’s hypoxemia showed marked improvement following mechanical ventilation, and extubation could be safely performed on day 6. Follow-up chest CT revealed marked improvement in the bilateral diffuse ground-glass opacities and consolidation (Figure 3). The patient’s subjective respiratory symptoms improved, and O2 supplementation was discontinued on day 12. The chemical burns on the patient’s buttocks and thighs were managed with frequent dressings (Figure 4). On day 18, the patient was transferred to a local clinic for further management of the chemical burns.
Figure 1.
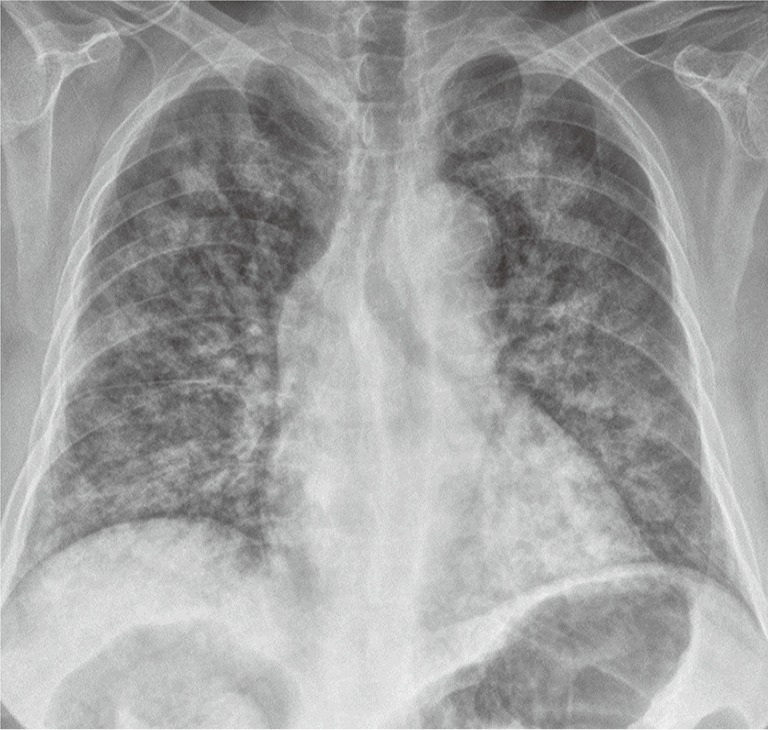
Chest plain radiograph acquired at presentation reveals diffuse bilateral infiltrates.
Figure 2.
Chest CT image acquired at presentation shows bilateral diffuse ground-glass opacities with interstitial thickening and patchy consolidation in the upper (A) and lower lobes (B).
Figure 3.
Chest CT image acquired on day 9 of hospitalization shows marked improvement in the bilateral diffuse ground-glass opacities in the upper (A) and lower lobes (B).
Figure 4.
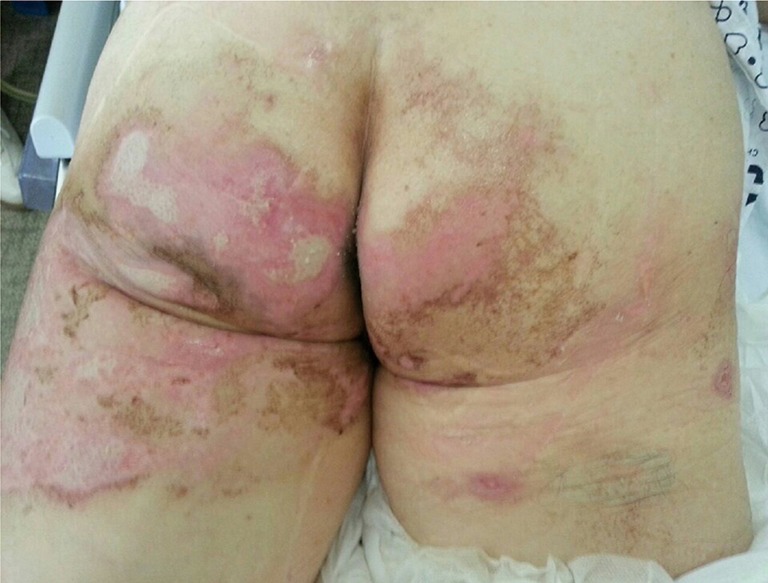
Photograph of the buttocks and thighs on day 9 of hospitalization. Second- to third-degree chemical burns are observed on both buttocks and thighs.
Discussion
Chlorine has various industrial and domestic uses (5). However, chlorine inhalation at toxic levels can cause ARDS, and skin exposure to chlorine can cause chemical burns even when used for domestic purposes. In this report, we document the successful management of a rare case of simultaneous development of chemical burns on the skin and ARDS caused by accidental exposure to chlorine-containing bleach.
Chlorine gas can damage large and small airways and the lung parenchyma, because it is a highly irritant gas with intermediate water solubility (5,6). The duration and concentration of exposure seem to determine the toxicity of chlorine gas (7). Recent studies have shown that exposure to low-concentration chlorine gas is associated with nasal irritation in patients with seasonal allergic rhinitis, while exposure to high-concentration chlorine gas can be fatal (2,8). In the present case, we could not measure the concentration of chlorine gas. However, the patient was directly exposed to undiluted chlorine-containing bleach. Furthermore, she inhaled the chlorine gas in a narrow enclosed space for about 1 hour. Therefore, she might have inhaled highly concentrated chlorine gas.
Chlorine gas exposure has been reported in transportation accidents (9) industrial use (10), or for intentional purposes such as during the First World War and the Iraq war (11). The development of ARDS following acute exposure to chlorine gas is very rare, but it can be fatal (1-3). ARDS caused by chlorine gas exposure is generally managed with supportive care, i.e., oxygen supplementation and administration of inhaled bronchodilators (6). The use of corticosteroids is controversial in patients with ARDS caused by chlorine gas exposure because of inconsistent survival benefits and concerns regarding superimposed infection (1,12,13). Therefore, we did not use corticosteroids in the present case. In animal experimental studies, systemic or inhaled corticosteroids showed benefits in pulmonary function recovery immediately after chlorine exposure (13-16). In a previous case report, the clinical manifestations demonstrated improvement within 5 days with corticosteroid administration (17). In our index case, hypoxemia developed following exposure to chlorine gas. The patient was managed with supportive care without corticosteroid administration; however, her lung injury worsened, and she eventually received mechanical ventilation. It is unclear whether corticosteroids can reduce the mortality rate (13). Furthermore, the risk of superimposed infection is high within 5 to 7 days after the initial inhalation injury (1,12). A previous study using an animal model showed that treatment with inhaled corticosteroids within 30 min after chlorine gas exposure resulted in a greater improvement in the symptoms and signs of lung injury than did delayed treatment with inhaled corticosteroids after 60 min (13). In our case, the patient arrived at our hospital about 10 hours after exposure to chlorine gas. Although she received mechanical ventilation without corticosteroid administration, she eventually recovered with near-complete resolution of the lung parenchymal injury.
Chlorine has the potential to induce deep-tissue damage through saponification of proteins and fats and liquefactive necrosis (18). The management of chemical burns is similar to that used for thermal burn injuries, i.e., copious irrigation, pH neutralization, pain management, and antimicrobial therapy (19). When dermal hypersensitivity reactions develop after chlorine exposure, systemic or topical corticosteroids or antihistamines may be necessary (18). In our case, the patient sustained chemical burns on both buttocks and thighs. We cleaned the burn wounds with normal saline and applied wet dressings. Although the patient was transferred to another hospital, her wounds had improved before she left our hospital.
Conclusions
The simultaneous occurrence of ARDS and chemical burns caused by chlorine-containing bleach is very rare. The treatment for ARDS caused by chlorine inhalation is supportive, and the clinical use of corticosteroids is controversial. In the present case, we were able to manage ARDS without corticosteroids. The chemical burns were managed according to standard thermal burn injury protocols.
Acknowledgements
Funding: This study was supported by a grant (CRI16005-1) from Chonnam National University Hospital.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Babu RV, Cardenas V, Sharma G. Acute respiratory distress syndrome from chlorine inhalation during a swimming pool accident: a case report and review of the literature. J Intensive Care Med 2008;23:275-80. 10.1177/0885066608318471 [DOI] [PubMed] [Google Scholar]
- 2.Parimon T, Kanne JP, Pierson DJ. Acute inhalation injury with evidence of diffuse bronchiolitis following chlorine gas exposure at a swimming pool. Respir Care 2004;49:291-4. [PubMed] [Google Scholar]
- 3.Rabinowitz PM, Siegel MD. Acute inhalation injury. Clin Chest Med 2002;23:707-15. 10.1016/S0272-5231(02)00025-4 [DOI] [PubMed] [Google Scholar]
- 4.Mangat HS, Stewart TL, Dibden L, et al. Complications of chlorine inhalation in a pediatric chemical burn patient: a case report. J Burn Care Res 2012;33:e216-21. 10.1097/BCR.0b013e318254d1c8 [DOI] [PubMed] [Google Scholar]
- 5.Evans RB. Chlorine: state of the art. Lung 2005;183:151-67. 10.1007/s00408-004-2530-3 [DOI] [PubMed] [Google Scholar]
- 6.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 2010;7:257-63. 10.1513/pats.201001-008SM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sexton JD, Pronchik DJ. Chlorine inhalation: the big picture. J Toxicol Clin Toxicol 1998;36:87-93. 10.3109/15563659809162593 [DOI] [PubMed] [Google Scholar]
- 8.Shusterman DJ, Murphy MA, Balmes JR. Subjects with seasonal allergic rhinitis and nonrhinitic subjects react differentially to nasal provocation with chlorine gas. J Allergy Clin Immunol 1998;101:732-40. 10.1016/S0091-6749(98)70302-1 [DOI] [PubMed] [Google Scholar]
- 9.Van Sickle D, Wenck MA, Belflower A, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 2009;27:1-7. 10.1016/j.ajem.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leikauf GD, Pope-Varsalona H, Concel VJ, et al. Functional genomics of chlorine-induced acute lung injury in mice. Proc Am Thorac Soc 2010;7:294-6. 10.1513/pats.201001-005SM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garner JP. Some recollections of Porton in World War 1. Commentary. J R Army Med Corps 2003;149:138-41. 10.1136/jramc-149-02-11 [DOI] [PubMed] [Google Scholar]
- 12.Sheridan RL. Airway management and respiratory care of the burn patient. Int Anesthesiol Clin 2000;38:129-45. 10.1097/00004311-200007000-00009 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med 2002;28:352-7. 10.1007/s00134-001-1175-4 [DOI] [PubMed] [Google Scholar]
- 14.Gunnarsson M, Walther SM, Seidal T, et al. Effects of inhalation of corticosteroids immediately after experimental chlorine gas lung injury. J Trauma 2000;48:101-7. 10.1097/00005373-200001000-00017 [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. J Trauma 2004;56:850-62. 10.1097/01.TA.0000078689.45384.8B [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Winskog C, Edston E, et al. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand 2005;49:183-90. 10.1111/j.1399-6576.2004.00563.x [DOI] [PubMed] [Google Scholar]
- 17.Mapp CE, Pozzato V, Pavoni V, et al. Severe asthma and ARDS triggered by acute short-term exposure to commonly used cleaning detergents. Eur Respir J 2000;16:570-2. 10.1034/j.1399-3003.2000.016003570.x [DOI] [PubMed] [Google Scholar]
- 18.Calcium Hypochlorite (CaCl2O2)/Sodium Hypochlorite (NaOCl) CAS 7778-54-3/7681-52-9; UN 1748/1791. Available online: https://www.atsdr.cdc.gov/MHMI/mmg184.pdf
- 19.Smith ML. Pediatric burns: management of thermal, electrical, and chemical burns and burn-like dermatologic conditions. Pediatr Ann 2000;29:367-78. 10.3928/0090-4481-20000601-10 [DOI] [PubMed] [Google Scholar]