Introduction
The presence of a cavitary lesion is a radiographic hallmark of pulmonary tuberculosis (TB). The sizes of the cavitary lesion and its proximity to the bronchial tree have been associated with mycobacterial burden (1). However, the task of evaluating the size of a cavitary lesion is problematic due to differences in reporting methods (e.g., uni-dimensional maximum in the axial plane vs. bi-dimensional) and inter-observer variance (2,3). The evaluation of a cavitary lesion is often complicated by their irregular shape, thus the radiographic reporting of the lesion (e.g., spherical volume) might underestimate or overestimate its actual size. The problem with having several methods of reporting is that global communication becomes impaired, and results from different groups more difficult to compare. A computer-aided algorithm would help to diminish the inter-rater differences in reporting, and provide a more precise evaluation of the size of a lesion and its distance from the bronchial tree.
The objective of this correspondence is to report an improved computer-aided algorithm to automatically (A) detect a cavitary lesion, (B) report its size, and (C) find the closest distance from the bronchial tree in patients with pulmonary TB.
Methods
Our previously reported algorithm evaluated cavitary lesions in rabbits (4). This algorithm uses a machine learning method which calculates the volume based on the voxel size. To assess cavitary lesions in humans, we improved the lung segmentation technique to cover smaller cavities (5). The improved algorithm automatically detects the presence of a cavitary lesion, reports its size, and calculates the minimum distance between the lesion and the bronchial tree from a person’s computerized tomography (CT) scan. The minimum distance was calculated based on Euclidean distance transformation methods (6). To test this improvement, we evaluated human CT scans available from patients with pulmonary TB who were enrolled in a TB cough frequency study in Peru (7).
In brief, this study evaluated adults with suspected pulmonary TB that was confirmed with auramine smear and by the microscopic observation drug susceptibility (MODS) broth culture assay (8-10). Characteristics of the patients’ cough were obtained with the ambulatory Cayetano Cough Monitor (CayeCoM) device (7), which measures and groups consecutive coughs that are within 2 seconds from each other to be part of the same episode. CT scans were performed with a 1.25-mm slice thickness.
Results
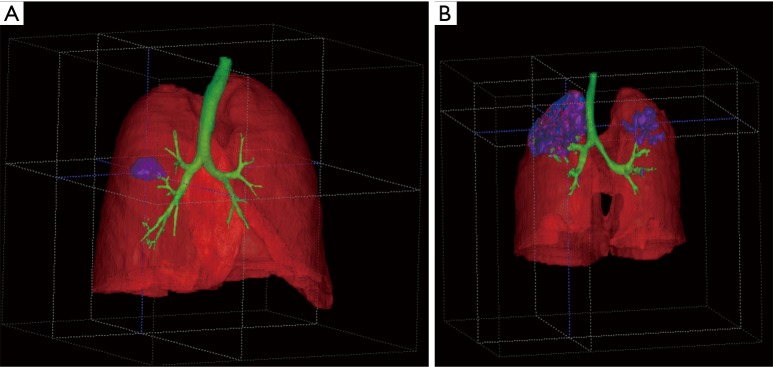
Two CT scans were evaluated. The first one from a 49-year-old HIV-negative male, whose initial sputum sample was smear positive (++) and culture positive according to MODS culture. This patient was started on standard quadruple TB treatment (first line). Baseline time to positivity of the MODS culture was 5 days, and the isolate was susceptible to Rifampicin and Isoniazid. The CayeCoM device recorded this patient for 20 continuous hours on the day of treatment initiation (day 0), and showed a cough rate of 1.7 cough episodes per hour. The CT scan with 273 slices was obtained 4 days after treatment started. The algorithm 3D-rendering results are shown in Figure 1A. The lung volume was 6,402.2 mL, and a single 20.1 mL cavity was observed in the right lung, yielding a cavity-to-volume percentage of 0.3%. The shortest distance between the bronchial tree and the cavitary lesion was 5.2 mm in this case.
Figure 1.
3-D rendering of a cavitary lesions in TB drug-susceptible HIV-negative patients. (A) Shows a lung volume of 6,402.2 mL with a single 20.1 mL cavity in the patient’s right lung; (B) shows a lung volume of 2,646.3 mL, with 12 cavities in both lungs (133.6, 9.9, 1.6, 1.4, 1.2, 1.1, 0.7, 0.6, 0.2, 0.2, 0.2, 0.1 mL).
The second CT scan was obtained from a 48-year-old HIV-negative female, whose initial sputum sample was smear positive (+++) and MODS culture positive for TB, with a baseline time to positivity on MODS of 6 days and a fully sensitive isolate. This patient was also started on standard quadruple TB treatment. The CayeCoM device recorded this patient for 24 continuous hours on day 1 of treatment, and showed a cough rate of 0.8 cough episodes per hour. The CT scan with 217 slices was obtained 26 days after treatment started. The algorithm 3D-rendering results are shown in Figure 1B. The analysis found that lung volumes were of 2,646.3 mL, with 12 cavities in both lungs. The volumes of the cavities were as follows: 133.6, 9.9, 1.6, 1.4, 1.2, 1.1, 0.7, 0.6, 0.2, 0.2, 0.2, 0.1 mL, and the cumulative cavity-to-volume percentage were 5.7%. The closest distance between the bronchial tree and a cavitary lesion was of 0.7 mm.
Discussion
Our results are promising, we were able to (I) detect cavitary lesions, (II) assess the size of each cavity, and (III) to calculate the closest distance of the lesions from the bronchial tree, in patients with pulmonary TB. With use of this algorithm we hope to standardize the reporting of cavitary lesion findings, which can in turn help researchers and clinicians communicate better regardless of radiologist background and clinical setting. We anticipate that results from this algorithm will not only help us to speak a common language in the clinical management of patients, but will also provide a significant contribution to TB research by providing important information into the role a cavitary lesion plays in TB pathophysiology and transmission.
In a separate study, we will use the algorithm results to test the previously stated association between cavity size and distance from the airways, and mycobacterial burden. We hope to make this algorithm easier to implement in clinical settings ideally providing clinicians a fast, reproducible, and precise method of evaluating cavitary lesions in patients with pulmonary TB. Its usage may additionally help clinicians and researchers improve TB diagnostics, methods of monitoring treatment response, and assessment of transmission risk.
Finally, this algorithm has the potential of evaluating cavitary lesions from not only pulmonary TB, but also from other etiologies such as lung cancer or Staphylococcus aureus (11).
Acknowledgements
Other members of the Tuberculosis Working Group in Peru include: Lilia Cabrera and Marco Varela (Asociación Benéfica PRISMA, Lima, Peru); Nancy M Vu (Cleveland Clinic, Cleveland, Ohio, USA); María Prado and Richard Rodríguez (Hospital María Auxiliadora, Lima, Peru); Aldo Vivar (Hospital Nacional Arzobispo Loayza, Lima, Peru); Jesus Chacaltana and José L Cabrera (Hospital Nacional Daniel Alcides Carrion, Lima, Peru); Antonio Salas, Eduardo Ticona, Felix Llanos and Marco Ñavincopa (Hospital Nacional Dos De Mayo, Lima, Peru); Eduardo Sanchez (Hospital Nacional Hipólito Unanue, Lima, Peru); Carlton A Evans, Daniela E Kirwan, Jon S Friedland, Louis Grandjean, Maria-Cristina I Loader, Roderick Escombe and Sumona Datta (Imperial College London, London, UK); José W López (Instituto Nacional de Salud del Niño San Borja, Lima, Perú); David A Moore (London School of Hygiene and Tropical Medicine, London, UK); Marjory A Bravard (Massachusetts General Hospital, Boston, Massachusetts, USA); José Gómez-Márquez (Massachusetts Institute of Technology, Cambridge, Massachusetts, USA); Michelle A Beam (Oregon Health & Science University, Portland, Oregon, USA); Brian H Tracey (Tufts University, Medford, Massachusetts, USA); Germán Comina, Gwenyth O Lee, Gustavo Hernández-Córdova, Kelly Jensen and Richard Oberhelman (Tulane University, New Orleans, Louisiana, USA); Jorge Coronel, Mirko Zimic, Patricia Fuentes and Patricia Sheen (Universidad Peruano Cayetano Heredia, Lima, Peru); David Bui (University of Arizona, Tucson, Arizona, USA); Nehal Naik (Virginia Commonwealth University, Richmond, Virginia, USA); and nurses from the Peruvian National TB Program.
Funding: This research is supported by CIDI, the intramural research program of the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Biomedical Imaging and Bioengineering (NIBIB). It also received support from the National Institutes of Health (5D43TW006581 “Infectious Diseases Training Program in Peru”, 5R21AI094143-02 “Cough–a rapid indicator of response to therapy in pulmonary TB”, and 5D43TW009349-03 “Inter-American Training for Innovations in Emerging Infectious Diseases”).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ors F, Deniz O, Bozlar U, et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging 2007;22:154-9. 10.1097/01.rti.0000213590.29472.ce [DOI] [PubMed] [Google Scholar]
- 2.Sakurada S, Hang NT, Ishizuka N, et al. Inter-rater agreement in the assessment of abnormal chest X-ray findings for tuberculosis between two Asian countries. BMC Infect Dis 2012;12:31. 10.1186/1471-2334-12-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics 2008;28:329-44. 10.1148/rg.282075068 [DOI] [PubMed] [Google Scholar]
- 4.Xu Z, Bagci U, Kubler A, et al. Computer-aided detection and quantification of cavitary tuberculosis from CT scans. Med Phys 2013;40:113701. 10.1118/1.4824979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansoor A, Bagci U, Xu Z, et al. A generic approach to pathological lung segmentation. IEEE Trans Med Imaging 2014;33:2293-310. 10.1109/TMI.2014.2337057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer CR, Qi R, Raghavan V. A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Transactions on Pattern Analysis and Machine Intelligence 2003;25:265-70. 10.1109/TPAMI.2003.1177156 [DOI] [Google Scholar]
- 7.Proaño A, Bravard MA, Tracey BH, et al. Protocol for studying cough frequency in people with pulmonary tuberculosis. BMJ Open 2016;6:e010365. 10.1136/bmjopen-2015-010365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore DA, Mendoza D, Gilman RH, et al. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol 2004;42:4432-7. 10.1128/JCM.42.10.4432-4437.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 2006;355:1539-50. 10.1056/NEJMoa055524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caviedes L, Lee TS, Gilman RH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru. J Clin Microbiol 2000;38:1203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev 2008;21:305-33. 10.1128/CMR.00060-07 [DOI] [PMC free article] [PubMed] [Google Scholar]