Abstract
Background
The combined small cell lung cancer (c-SCLC) was rare and its clinicopathological characteristics had not been thoroughly described. The aim of this study was to determine prognostic factors and survival in c-SCLC patients.
Methods
Clinical records of patients with c-SCLC who underwent surgery between January 2009 and December 2013 in two institutions were retrospectively reviewed.
Results
Ninety-seven patients were identified. The most common pathology was combined SCLC and large cell neuroendocrine carcinoma (LCNEC, N=46), followed by combined SCLC and squamous cell carcinoma (SCC) (N=32), combined SCLC and adenocarcinoma (AC) (N=12), and combined SCLC and adenosquamous carcinoma (ASC) (N=7). The overall survival (OS) rates of the entire cohort were 42.4% and 35.2% at 3 and 5 years, respectively. Multivariate analysis identified sex [female vs. male, hazards ratio (HR) =0.38; 95% confidence interval (CI): 0.19–0.79; P=0.010], age (<53 vs. >53 years, HR =0.28; 95% CI: 0.09–0.81; P=0.019), performance status (<2 vs. >2, HR =0.08; 95% CI: 0.02–0.32; P<0.001), combined non-small cell lung cancer (NSCLC) components (LCNEC vs. non-LCNEC, HR =3.00; 95% CI: 1.03–8.76; P=0.045), adjuvant therapy (yes vs. no, HR =0.33; 95% CI: 0.17–0.67; P=0.002) as significantly prognostic factors of OS in patients with complete resection and lymphadenectomy.
Conclusions
The mixed NSCLC components within c-SCLCs had a significant influence on the survival. Compared with surgery alone, adjuvant therapy was associated with significantly improved survival in patients with complete resection and lymphadenectomy.
Keywords: Combined small cell lung cancer (c-SCLC), surgery, survival
Introduction
Small cell lung cancer (SCLC) is a relatively rare subtype of primary lung cancers, accounting for about 10–15% of all lung cancers (1-3). The World Health Organization (WHO) classification currently divides SCLC into two subtypes: pure and combined. Combined SCLC (c-SCLC) was defined as SCLC combined with any of non-small cell carcinoma (NSCLC) histologic type, such as large cell carcinoma, squamous cell carcinoma (SCC), and adenocarcinoma (AC). The incidence of pure SCLC is much more prevalent than its combined counterpart (4), which may be the result of insufficient diagnostic information provided by limited specimens such as cytological analysis or small biopsy. Also, few SCLC patients were undergoing resections due to the widely-accepted poor role of surgery in SCLCs (5).
Previous reports have demonstrated that c-SCLCs carry a different prognosis from pure ones, signifying that each may have a distinct biological behavior (6,7). However, most of the previously related studies regarding SCLCs only focused on pure SCLC while excluding the combined ones (8), or analyzed them as a whole instead of dividing them into the pure and combined parts (9,10). Given the very limited studies on combined SCLC histology, we aimed to report the clinical experience of two institutions. Herein, the characteristics and prognosis of c-SCLC of the lung are presented and discussed.
Methods
Patients
A retrospective review of SCLC patients from Shanghai Chest Hospital and The Affiliated Luoyang Central Hospital of Zhengzhou University between January 2009 and December 2013 was conducted. During this period, 16,806 patients underwent surgical resection of primary lung cancers at the Department of Thoracic Surgery of the two institutions, and of those, surgical records of 322 (1.9%) consecutive patients with pathologically-confirmed SCLC were retrospectively reviewed, based on the diagnostic criteria proposed by the 2015 edition of the WHO classification system (11). Of the 322 SCLCs, 97 (30.1%) patients were diagnosed as c-SCLC. Ten patients didn’t have a complete resection, of which five received surgical biopsies due to intraoperative unresectability, three had a microscopically positive margin, one had macroscopically positive margin, and one was performed wedge resection. In this study, we mainly focused on with complete tumor resection and lymphadenectomy (radical resection). SCLC staging was performed according to the 7th TNM (tumor, node, metastasis) classification (12). The Ethics Committee at these two hospitals approved this study [KS (Y) 1627 and 201600128].
All these patients had pre-operative examination to exclude distant metastasis, which included chest computed tomography (CT) scan, abdominal CT or ultrasonography examination, brain magnetic resonance imaging and whole-body bone scan. Mediastinoscopy (N=7) or (endobronchial ultrasound-guided trans-bronchial needle aspiration (EBUS-TBNA, N=7) or positron emission tomography (PET; only a few of patients had PET-CT examinations from other hospitals because the PET-CT was not available in the two hospitals during this periods, N=4) was performed to exclude mediastinal lymphatic metastasis when the mediastinal lymph node enlargement (size >1 cm). For a centrally-located tumor, bronchoscopy was commonly performed before surgery.
Each surgically resected tumor was systematically sampled according to standard principles. Paraffin-embedded tumor specimens, which included the widest cross-sections, were reassessed by one senior clinical pathologist in each hospital. Immunohistochemistry staining of surgically resected c-SCLC was used to for the modification of the classification of SCLC and non-SCLC components within c-SCLC. If the judgments from the pathologist in each hospital were not the same as before, then the slide will be jointly determined by two pathologists in each hospital after serious discussion.
Follow-up
All patients with tolerable status were suggested to receive adjuvant chemotherapy (CTx) at the first month follow-up after surgery. Methods to obtain follow-up information include: communication with physicians, looking up to inpatient or outpatient records, death certificates, and communication with patient or patient’s family. The duration of overall survival (OS) was defined as the interval between the day of surgery and the date of death by any cause or the last follow-up date. Disease free survival (DFS) was defined as the interval from the date of resection to the date of proven detection of local recurrence or metastasis. The primary end-point of the study was OS. Follow-up was complete up to May, 2016.
Statistical analysis
Normally distributed continuous variables are presented as the mean ± standard deviation (SD), otherwise as the median and range, while categorical variables are presented as numbers and percentages. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazards model was used for multivariate analysis. Clinical factors that potentially affect survival, such as age, gender, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), surgical approach, the type of resection, location, size, pathological stages, NSCLC components within c-SCLC tumors and adjuvant therapy were included in the Cox proportional hazards model. All tests were two-sided and a P value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS software (version 22.0; IBM-SPSS, Inc., Chicago, IL, USA).
Results
Patient clinical characteristics
Patient clinical characteristics are reported in Table 1. The study cohort consisted of 80 men (82.5%) and 17 women (17.5%), with a median age of 53.0 (range, 31–71) years.
Table 1. Patients characteristics (N=97).
| Characteristics | N (%) |
|---|---|
| Gender | 97 (100.0) |
| Male | 80 (82.5) |
| Female | 17 (17.5) |
| Age (years) | |
| ≤53 | 42 (43.3) |
| >53 | 55 (56.7) |
| ECOG PS | |
| 0–1 | 89 (91.8) |
| ≥2 | 8 (8.2) |
| Smoking | |
| No | 18 (18.6) |
| Yes | 79 (81.4) |
| Location | |
| Central | 66 (68.0) |
| Peripheral | 31 (32.0) |
| Surgical approach | |
| Thoracotomy | 75 (77.3) |
| VATS | 22 (22.7) |
| Type of resection | |
| Lobectomy | 60 (61.9) |
| Standard resection | 52 |
| Sleeve resection | 8 |
| Extented resection | 27 (27.8) |
| Bilobectomy | 16 |
| Pneumonectomy | 11 |
| Incomplete resection | 10 (10.3) |
| Combined components | |
| LCNEC | 46 (47.4) |
| Non-LCNEC | 51 (52.6) |
| Size | |
| ≤4 cm | 52 (53.6) |
| >4 cm | 45 (46.4) |
| N stage | |
| N0 | 26 (26 .8) |
| N1 | 25 (25.8) |
| N2 | 36 (37.1) |
| Multiple | 19 |
| Single | 17 |
| Nx | 10 (10.3) |
| Stage | |
| I | 17 (17.5) |
| II | 23 (23.7) |
| IIIA | 47 (48.5) |
| Unknown | 10 (10.3) |
ECOG PS, Eastern Cooperative Oncology Group performance status; VATS, video-assisted thoracoscopic surgery; LCNEC, large cell neuroendocrine carcinoma; Nx, unknown nodal status.
Forty-nine patients (50.57%) received a pathologic diagnosis prior to surgery. Of those, 28 patients were diagnosed pre-operatively with pure SCLC, nine with poorly differentiated carcinoma, eight with SCC, three with necrosis, and one with AC, according to cytologic and/or histologic analysis, and finally diagnosed as c-SCLC according to histopathologic analysis of the surgical specimens. The postoperatively pathologically-confirmed c-SCLC showed that SCLC/large cell neuroendocrine carcinoma (LCNEC) was the most common (N=46), followed by SCLC/SCC (N=32), SCLC/AC (N=12), and adenosquamous carcinoma (ASC, N=7).
Adjuvant therapy
Sixty-one patients (62.9%) were treated with combination CTx, prophylactic cranial irradiation, and thoracic radiotherapy. Sixteen patients (18.4%) received adjuvant CTx only after resection, 17 (17.5%) received surgery alone, and 3 received thoracic radiotherapy alone. The most commonly used CTx regimen was etoposide and platinum salts (EP; N=72), followed by vinorelbine, ifosfamide and cisplatin (NIP; N=5). Of the 20 patients who did not receive adjuvant chemotherapy, eight discontinued the treatment because of the intolerable side effects. Seven patients did not receive chemotherapy due to relatively poor conditions. Three of them did not follow the doctors’ suggestions, due to fear of the potential side effects caused by chemotherapy. Two patients were unwilling to receive chemotherapy because of poor economic status.
Survival analysis
The median follow-up period was 28.0 (range, 6–81) months. Fifty-eight (59.8%) patients had died at the last follow-up visit. The OS rates of the entire cohort were 42.4% and 35.2% at 3 and 5 years, respectively. The OS rates of c-SCLCs with complete resection and lymphadenectomy were 58.6% and 42.3% at 3 and 5 years, respectively. The corresponding DFS of the entire cohort and those with complete resection and lymphadenectomy were 32.9% and 27.0%, and 30.9% and 25.4%, respectively. The median OS of patients with stage I, II, IIIA and unknown was non-estimated, 27.0, 19.0 and 23.0 months, respectively.
Among patients with complete resection and lymphadenectomy (N=87), a poorer OS could be observed in SCLC/LCNEC patients, compared to those with SCLC/non-LCNEC (P=0.045; Figure 1) after adjusting for the potentially-influential factors. Also, significant differences could be found between c-SCLCs managed with surgery alone and those with surgery plus adjuvant therapy (P=0.002, Figure 2). However, there was no significant difference between patients with and without radical resection (P=0.661).
Figure 1.
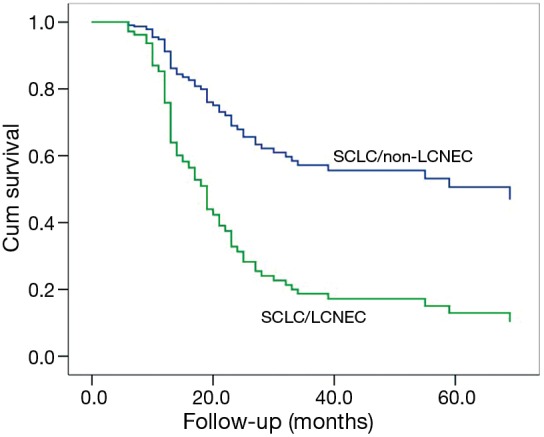
Among patients with radical resections (N=87), better overall survival (OS) could be observed in patients mixed with large cell neuroendocrine carcinoma (LCNEC) compared to those with non-LCNEC after adjusting potentially influential factors [LCNEC vs. non-LCNEC, hazard ratio (HR) =3.00; 95% confidence interval (CI): 1.03–8.76; P=0.045].
Figure 2.
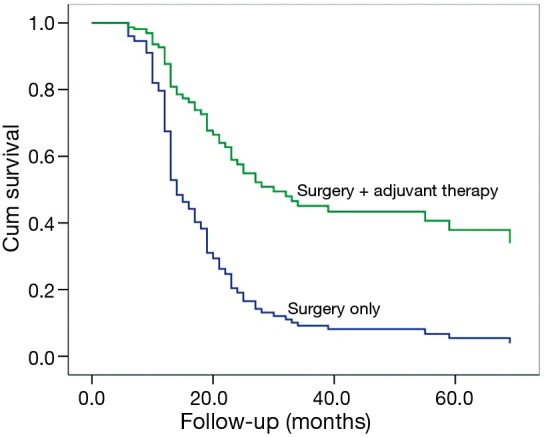
Among patients with radical resections (N=87), improved overall survival (OS) could be observed in patients managed with surgery plus adjuvant chemotherapy compared to those with surgery alone after adjustment [hazard ratio (HR) =0.47; 95% confidence interval (CI): 0.24–0.93; P=0.002].
The associations of various prognostic factors with DFS and OS using univariate analysis are presented in Table 2. Multivariate analysis using Cox’s proportional hazards model identified sex [female vs. male, hazards ratio (HR) =0.38; 95% confidence interval (CI): 0.19–0.79; P=0.010], age (≤53 vs. >53 years, HR =0.28; 95% CI: 0.09–0.81; P=0.019), ECOG PS (<2 vs. ≥2, HR =0.08; 95% CI: 0.02–0.32; P<0.001), combined NSCLC components (LCNEC vs. non-LCNEC, HR =3.00; 95% CI: 1.03–8.76; P=0.045), adjuvant therapy (yes vs. no, HR =0.33; 95% CI: 0.17–0.67; P=0.002) as significantly prognostic factors of OS in patients with complete resection and lymphadenectomy (N=87). ECOG PS (HR =0.28; 95% CI: 0.08–0.98; P=0.046), visceral pleural invasion (yes vs. no, HR =1.87; 95% CI: 1.06–3.29; P=0.030), pathologic stage (stage IIIA vs. stage I: HR =2.22; 95% CI: 0.96–5.10; P=0.061; stage II vs. I, HR =2.68; 95% CI: 1.26–5.73; P=0.011), adjuvant therapy (HR =0.47; 95% CI: 0.24–0.93; P=0.030) were corresponding factors for DFS.
Table 2. Univariate analysis in patients with radical resection (N=87).
| Characteristics | N | DFS | OS | |||
|---|---|---|---|---|---|---|
| Median survival (months) | P | Median survival (months) | P | |||
| Sex | 0.036* | 0.107 | ||||
| Male | 73 | 9.0±1.9 | 14.0±3.7 | |||
| Female | 14 | 16.0±2.5 | 30.0±4.6 | |||
| Age (years) | 0.889 | 0.476 | ||||
| ≤53 | 39 | 13.0±1.2 | 25.0±3.1 | |||
| >53 | 48 | 13.0±3.0 | 28.0±10.2 | |||
| ECOG PS | <0.001* | <0.001* | ||||
| 0–1 | 83 | 14.0±1.7 | 28.0±4.9 | |||
| ≥2 | 4 | 5.0 | 9.0±1.3 | |||
| Smoking | 0.343 | 0.518 | ||||
| No | 14 | 14.0±1.9 | 27.0±5.2 | |||
| Yes | 73 | 9.0±1.9 | 18.0±3.7 | |||
| Location | 0.130 | 0.107 | ||||
| Central | 58 | 12.0±1.4 | 23.0±2.5 | |||
| Peripheral | 29 | 24.0±9.0 | 55.0±19.6 | |||
| Surgical approach | 0.101 | 0.106 | ||||
| Thoracotomy | 67 | 13.0±1.4 | 23.0±2.6 | |||
| VATS | 20 | 25.0±1.6 | 55.0±16.1 | |||
| Type of resection | 0.138 | 0.190 | ||||
| Lobectomy | 60 | 14.0±1.9 | 30.0±5.3 | |||
| Extented resection | 27 | 7.0±2.6 | 17.0±3.5 | |||
| Combined components | 0.594 | 0.654 | ||||
| LCNEC | 43 | 13.0±1.3 | 25.0±3.3 | |||
| Non-LCNEC | 44 | 15.0±3.9 | 28.0±9.6 | |||
| Size (cm) | 0.044* | 0.115 | ||||
| ≤4 | 50 | 16.0±6.6 | 34.0±14.1 | |||
| >4 | 37 | 11.0±2.4 | 21.0±3.6 | |||
| VPI | 0.177 | 0.294 | ||||
| Yes | 29 | 13.0±1.3 | 55.0±19.0 | |||
| No | 58 | 24.0±11.7 | 23.0±2.7 | |||
| Stage | 0.026* | 0.038* | ||||
| I | 17 | –# | –# | |||
| II | 23 | 13.0±1.4 | 27.0±5.6 | |||
| IIIA | 47 | 10.0±1.4 | 19.0±5.1 | |||
| Adjuvant therapy | <0.001* | <0.001* | ||||
| Yes | 71 | 17.0±4.6 | 39.0±14.9 | |||
| No | 16 | 5.0±1.0 | 12.0±1.5 | |||
*, statistically significant; #, cannot be estimated; DFS, disease free survival; OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; VATS, video-assisted thoracoscopic surgery; LCNEC, large cell neuroendocrine carcinoma; non-LCNEC, included squamous cell carcinoma (SCC), adenocarcinoma (AC) and adenosquamous carcinoma (ASC); VPI, visceral pleural invasion.
Discussion
SCLC represents a distinct pathologic and clinical entity, accounting for approximately 15% of all primary lung cancers. c-SCLC is the one subtype of SCLC that has a less frequency and different prognosis compared to its pure SCLC counterpart. However, studies regarding c-SCLC are very limited. This was a retrospective analysis of surgically resected c-SCLC histology patients, showing that the mixed NSCLC components and postoperative adjuvant therapy had a significant influence on its prognosis.
Combined SCLC has been reported to account for 2–24% of all SCLC cases (7,13,14). However, previous reports showed that up to 28% of SCLC patients who underwent surgical resection were combined SCLC (4). In our surgically treated SCLC database, 30.4% were combined, suggesting that specimens obtained via surgery more accurately reflect the pathologic features of the tumor than the non-surgical approaches, and the real incidence of c-SCLC is much greater than expected. The mixed LCENC within c-SCLCs was the most common, followed by SCC and AC, which was similar to previous study (7,15).
Surgery plays an increasing role in limited-stage SCLCs (8-10), and was preferred in c-SCLCs (7). Resection allows a sufficient number of specimens for diagnosis, while cytological analysis or small biopsy can only provide limited diagnostic information and thus may not be truly representative of lung cancer, especially those with a mixed histology (16,17). SCLC can be readily and accurately diagnosed in biopsy or cytological specimens; however, in selected cases, especially combined ones, it can pose difficult diagnostic dilemmas (5,18,19). Forty-nine patients in the present study with postoperatively pathologically proven SCLCs were preoperatively misdiagnosed as pure SCLCs (N=28), poorly differentiated carcinoma (N=9), SCC (N=8), necrosis (N=3) or AC (N=1) through cytology (diagnostic fine needle aspiration or bronchial brush and lavage) or bronchial biopsy. These revealed discrepancies regarding the diagnostic outcomes of c-SCLCs between preoperative evaluation, via cytology or bronchial biopsy and postoperative diagnosis by immunohistochemistry, suggest a diagnostic dilemma for c-SCLC cases treated by non-resection approaches. Preoperative diagnostic outcomes mainly depend on morphologic identification via light microscope. Tumors with poor differentiation or mixed components might lead to difficulties in preoperative diagnosis. Therefore, it is important to consider this diagnostic dilemma for potential cases of c-SCLCs that are not surgical candidates because optimal treatment regimens are essential.
Out results showed that the majority of c-SCLC patients had nodal positive disease. Previous report showed that PET-CT was more sensitive than other imaging modalities with respect to the pretreatment staging of SCLCs (20,21). A retrospectively nationwide study showed that clinical stage had a poor consistency with pathological stage (9). All of the results suggested that highly malignant SCLCs had a micro-metastatic tendency even at clinically early-stage. Thus, surgery played an important role in both accurate diagnosis and staging. However, this didn’t mean that we recommended surgical resections in SCLC cases with nodal disease, although some studies showed that even in SCLCs with stage II–III, surgery could provide better survival benefits than the non-surgical treatment (8,10). Of course, we should have more carefully pre-operative examinations in order to avoid operating on patients with locally advanced stages.
Currently, the standard CTx regimen for SCLC is etoposide and cisplatin (EP), while histologically-mixed tumors with both SCLC and NSCLC components may fail EP protocols since there is less sensitivity of the NSCLC component to this regimen. The present study showed that adjuvant therapy after resection could improve the prognosis, consistent with previous studies (22-24). In a study by Luo and colleagues (25), the efficacy and safety of NIP with EP in the treatment of advanced c-SCLCs were compared, which demonstrated that an EP regimen presented a survival benefit, although the difference was not statistically significant. However, the adverse effect was less in the EP group than the NIP counterpart. Li et al showed that paclitaxel plus the EP regimen failed to show additional significant survival benefits compared to the EP regimen alone, although a better objective response rate could be observed (26). In this study, whether the different CTx protocols for c-SCLCs had an effect on treatment response was not addressed due to the very limited size of non-EP samples.
Whether the NSCLC components within c-SCLCs shared the same clone origin remains unclear. In an analysis of seven cases of SCLC combined with either AC, SCC, or LCNEC, Wagner et al. reported a shared identical immunophenotype in six cases between the SCLC and non-SCLC components of individual cases. They argued that both histologic components share the same clonal origin, and, therefore, should not be classified as a subtype, but as pure SCLC (27). In our present study, the LCNEC component within SCLCs had a worse survival than the other mixed NSCLC components, suggesting that the NSCLC components within c-SCLCs could affect the prognosis, which was in accord with some study (6), while inconsistent with some other (15). It will be interesting for future studies to investigate factors that determine the differential directions of mixed pure SCLC and NSCLC components and the transformation mechanism between SCLC and NSCLC in the context of survival differences in larger samples.
Some of the major limitations to this study included the retrospective analysis and the small sample size. Also, we could not detect whether the NSCLC components within c-SCLCs harbored the same molecular features with the pure SCLC counterparts because we did not perform microdissection and assess the two components separately. Whether the thoracic or prophylactic cranial irradiation could provide survival benefit was not studied due to the very limited samples. Last but not least, given the existed NSCLC components, whether the different CTx protocols for c-SCLCs had an effect on the treatment response was not addressed due to the very limited size of samples. Therefore, further studies are warranted.
Acknowledgements
The authors are indebted to Keke Yu (Department of Pathology of Shanghai Chest Hospital, Shanghai Jiao Tong University) and Huixing Zhou (Department of Pathology of the Affiliated Luoyang Central Hospital of Zhengzhou University) for pathological re-assessment.
Ethical Statement: The Ethics Committee at these two hospitals approved this study [KS (Y) 1627 and 201600128] and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 2.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. 10.1200/JCO.2007.12.5435 [DOI] [PubMed] [Google Scholar]
- 3.Eskandar A, Ahmed A, Daughtey M, et al. Racial and sex differences in presentation and outcomes of small cell lung cancer in the United States: 1973 to 2010. Chest 2015;147:e164-5. 10.1378/chest.14-3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. 10.1097/00000478-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Adelstein DJ, Tomashefski JF, Jr, Snow NJ, et al. Mixed small cell and non-small cell lung cancer. Chest 1986;89:699-704. 10.1378/chest.89.5.699 [DOI] [PubMed] [Google Scholar]
- 6.Radice PA, Matthews MJ, Ihde DC, et al. The clinical behavior of "mixed" small cell/large cell bronchogenic carcinoma compared to "pure" small cell subtypes. Cancer 1982;50:2894-902. [DOI] [PubMed] [Google Scholar]
- 7.Babakoohi S, Fu P, Yang M, et al. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer 2013;14:113-9. 10.1016/j.cllc.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. 10.1097/JTO.0000000000000402 [DOI] [PubMed] [Google Scholar]
- 9.Takei H, Kondo H, Miyaoka E, et al. Surgery for small cell lung cancer: a retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2014;9:1140-5. 10.1097/JTO.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 10.Takenaka T, Takenoyama M, Inamasu E, et al. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung Cancer 2015;88:52-6. 10.1016/j.lungcan.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 11.Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Fourth edition. Lyon: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Osterlind K, Hansen HH. The prognostic significance of histopathologic subtyping of small cell carcinoma of the lung according to the classification of the World Health Organization. A study of 375 consecutive patients. Cancer 1983;52:2144-50. [DOI] [PubMed] [Google Scholar]
- 14.Mangum MD, Greco FA, Hainsworth JD, et al. Combined small-cell and non-small-cell lung cancer. J Clin Oncol 1989;7:607-12. [DOI] [PubMed] [Google Scholar]
- 15.Wallace AS, Arya M, Frazier SR, et al. Combined small-cell lung carcinoma: An institutional experience. Thorac Cancer 2014;5:57-62. 10.1111/1759-7714.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song TN, Zhang JH, Li B, et al. Misdiagnosis of a small cell lung cancer resulting from inaccurate pathology. Ann Thorac Surg 2015;99:e125-7. 10.1016/j.athoracsur.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Gao YD, Cao Y, et al. Surgical specimen histology revealed inadequacy of conventional transbronchial needle aspiration sample in the diagnosis of adenosquamous lung carcinoma. J Thorac Dis 2015;7:680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchevsky AM, Wick MR. Diagnostic difficulties with the diagnosis of small cell carcinoma of the lung. Semin Diagn Pathol 2015;32:480-8. 10.1053/j.semdp.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 19.Iwata T, Nishiyama N, Nagano K, et al. Role of pulmonary resection in the diagnosis and treatment of limited-stage small cell lung cancer: revision of clinical diagnosis based on findings of resected specimen and its influence on survival. Gen Thorac Cardiovasc Surg 2012;60:43-52. 10.1007/s11748-011-0847-4 [DOI] [PubMed] [Google Scholar]
- 20.Bradley JD, Dehdashti F, Mintun MA, et al. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol 2004;22:3248-54. 10.1200/JCO.2004.11.089 [DOI] [PubMed] [Google Scholar]
- 21.Nobashi T, Koyasu S, Nakamoto Y, et al. Prognostic value of fluorine-18 fludeoxyglucose positron emission tomography parameters differs according to primary tumour location in small-cell lung cancer. Br J Radiol 2016;89:20150618. 10.1259/bjr.20150618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corso CD, Rutter CE, Park HS, et al. Role of Chemoradiotherapy in Elderly Patients With Limited-Stage Small-Cell Lung Cancer. J Clin Oncol 2015;33:4240-6. 10.1200/JCO.2015.62.4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behera M, Ragin C, Kim S, et al. Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer 2016;122:50-60. 10.1002/cncr.29674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CF, Chan DY, Speicher PJ, et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1057-64. 10.1200/JCO.2015.63.8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, Wu FY, Li AW, et al. Comparison of vinorelbine, ifosfamide and cisplatin (NIP) and etoposide and cisplatin (EP) for treatment of advanced combined small cell lung cancer (cSCLC) patients: a retrospective study. Asian Pac J Cancer Prev 2012;13:4703-6. 10.7314/APJCP.2012.13.9.4703 [DOI] [PubMed] [Google Scholar]
- 26.Li YY, Zhou C, Yang DX, et al. Paclitaxel-etoposide-carboplatin/cisplatin versus etoposide-carboplatin/cisplatin as first-line treatment for combined small-cell lung cancer: a retrospective analysis of 62 cases. Cancer Biol Med 2015;12:117-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner PL, Kitabayashi N, Chen YT, et al. Combined small cell lung carcinomas: genotypic and immunophenotypic analysis of the separate morphologic components. Am J Clin Pathol 2009;131:376-82. 10.1309/AJCPYNPFL56POZQY [DOI] [PubMed] [Google Scholar]


