Abstract
Background
Prolonged intensive care unit (ICU) stay of patients after cardiac surgery has a major impact on overall cost and resource utilization. The aim of this study was to identify perioperative factors which prolong stay in ICU.
Methods
All adult patients from a single, specialized cardiac center who were admitted to the ICU after cardiac surgery during a 2-month period were included. Demographic and clinical characteristics, comorbidities, preoperative use of drugs, intraoperative variables, and postoperative course were recorded. Hemodynamic and blood gas measurements were recorded at four time intervals during the first 24 postoperative hours. Routine hematologic and biochemical laboratory results were recorded preoperatively and in the first postoperative hours.
Results
During the study period 145 adult patients underwent cardiac surgery: 65 (45%) underwent coronary artery bypass graft surgery, 38 (26%) valve surgery, 26 (18%) combined surgery and 16 (11%) other types of cardiac operation. Seventy nine (54%) patients had an ICU stay of less than 24 hours. Random forests analysis identified four variables that had a major impact on the length of stay (LOS) in ICU; these variables were subsequently entered in a logistic regression model: preoperative hemoglobin [odds ratio (OR) =0.68], duration of aortic clamping (OR =1.01) and ratio of arterial oxygen partial pressure to inspired oxygen fraction (PaO2/FiO2) (OR =0.99) and blood glucose during the first four postoperative hours (OR =1.02). ROC curve analysis showed an AUC =0.79, P<0.001, 95% CI: 0.71–0.86.
Conclusions
Low preoperative hemoglobin, prolonged aortic clamping time and low PaO2/FiO2 ratio and blood glucose measured within the first postoperative hours, were strongly related with prolonged LOS in ICU.
Keywords: Surgery, cardiovascular, intensive care, heart, risk
Introduction
Cardiac surgery has substantially improved over time due to advances in medical and surgical practice and in cardiac surgical critical care. On the other hand, as a result of ageing of the population, cardiac surgery is being performed in older, more complicated patients, with increased numbers of comorbidities (1-3).
Several studies have attempted to identify current risk factors for complications and postoperative morbidity after cardiac surgery including the postoperative length of stay (LOS) in the intensive care unit (ICU) (4-8). Prolonged ICU stay is usually associated with increased hospital mortality, increased morbidity and hospital stay and poor long-term prognosis. Moreover, prolonged LOS in ICU after cardiac surgery has a major impact on overall cost and resource utilization (9-11).
Cardiac surgery differs from other types of operations owing to the use of cardiopulmonary bypass (CPB). This has predictable sequelae, mainly due to the interaction between blood and the artificial surface of the CPB circuit: systemic inflammatory response, coagulopathy, anemia and volume overload (1,2).
Among the postoperative complications, various factors have been described which specifically prolong mechanical ventilation after cardiac surgery (9,10,12). However, the determinants of a prolonged stay in ICU are not very clear. Moreover, the time course of usually monitored variables during the first 24 postoperative hours may differ in relation to LOS in ICU; this perspective is also not well studied. Predicting the risk of long postoperative stay in ICU is a critical issue in cardiac surgery, as it could help prognosis, early correction of underlying factors with the potential for improvement and, possibly, decision-making regarding provision and maintenance of care. The objective of this study was to identify preoperative, intraoperative and postoperative factors which are associated with prolonged ICU LOS after cardiac surgery in adult patients.
Methods
This was a prospective observational study, conducted in the surgical ICU at Onassis Cardiac Surgery Center, Athens, Greece. All consecutive adult patients (≥18 years) who were admitted to the postoperative cardiac surgical ICU after cardiovascular surgery during a 2-month period were included. The study protocol was approved by the Ethics Committee of “Onassis” Cardiac Surgery Center (Approval number: 444-21/10/10). Since it was an observational study and medical confidentiality and personal data were preserved, the requirement for written informed consent from patients or their relatives was waived.
Each patient had a central venous catheter in an internal jugular vein and an arterial catheter in a radial artery. Since not all patients had a pulmonary artery catheter in place, data derived from its use were not recorded in this study.
Demographic characteristics, the type of surgery along with intraoperative variables such as the CPB time, aortic clamping duration and number of red blood cell packs transfused were recorded. In addition, comorbidities, the preoperative use of drugs such as statins, beta-blockers and amiodarone, and postoperative complications [blood loss, defined as the need of RBC transfusion during the first 24 postoperative hours, pulmonary dysfunction, defined as the decrease of the arterial oxygen partial pressure to fractional inspired oxygen ratio (PaO2/FiO2) <200 mmHg, cardiac arrest, arrhythmia, re-intubation and myocardial infarction during the first 24 hours] were recorded. Hemodynamics and arterial and central venous blood gas measurements were recorded at the following time intervals: during the first hour in the ICU, after 1–4 hours, after 4–12 hours and after 12–24 hours. Arterial oxygen content (CaO2) and central venous oxygen content (CcvO2) were calculated by using the following equations:
CaO2 = (SaO2 × Hb × 1.39) + 0.0031 × PaO2, and CcvO2 = (ScvO2 × Hb × 1.39) +0.0031 × PcvO2, respectively. Moreover, the alveolar-arterial oxygen gradient and the difference of central venous to arterial carbon dioxide pressure were calculated. Laboratory results including complete blood count and routine biochemistry panel were recorded preoperatively and in the first postoperative hour. The Acute Physiology and Chronic Health Evaluation (APACHE) II (13) and the Sequential Organ Failure Assessment (SOFA) (14) scores were calculated for each patient upon completion of the first 24 hours postoperatively.
Postoperative arrhythmia was defined as the occurrence of any of the following arrhythmias, during the first 24 hours postoperatively: atrial fibrillation, atrial flutter, broad or narrow QRS complex tachycardia and symptomatic bradycardia (15).
Vasoactive administration was defined as the use of either dopamine, dobutamine, epinephrine or norepinephrine, at any dose, for at least 1 hour during the first 24 postoperative hours. Prolonged LOS in the ICU was defined as a stay of more than 24 hours.
Statistical analysis
Univariate analyses were initially performed to identify the factors associated with the LOS in the ICU. For continuous variables we used the Mann-Whitney U test or the independent samples t-test, as appropriate. For categorical variables, the χ2 test was performed. For variables with sequential evaluations in the first 24 hours postoperatively, we used repeated measures analysis of variance to examine their time course in the two groups of patients.
Given the potentially large number of features associated with the outcome, we used random forests methodology to identify those with the greatest impact (16). Random forests is an ensemble statistical learning tool that is suitable for data sets with large number of predictors respective to data points, and is able to account for correlation as well as complex interactions among feature variables. They are less prone to overfitting compared to standard multivariable methods (17).
For each variable, we measured how well it can classify a data point, using a measure called the Gini index, and we ranked them according to their importance for the prediction of the outcome. We subsequently used these variables in a logistic regression model with ICU LOS as the outcome variable. This approach permits better interpretability of the role of each predictor using standard epidemiologic measures of association (18). Receiver operating characteristic (ROC) curve was used to evaluate the discriminative ability of the multivariate model.
We used IBM SPSS version 20 (IBM Corporation, Armonk, NY, USA) for data management and univariate analysis. We used the “randomForest” package of the R statistical environment version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) to implement the learning algorithm.
Results
During the study period, 145 adult patients underwent cardiac surgery in Onassis Cardiac Surgery Center. Operative characteristics are presented in Table 1. Preoperative, intraoperative and postoperative data of these patients in relation with LOS in ICU are shown in Table 2.
Table 1. Operative characteristics of the population studied (n=145).
| Type of surgery | N | Duration of CPB (min)a | Duration of aortic clamping (min)a |
|---|---|---|---|
| CABG [1] | 3† | 52±9 | 36±13 |
| CABG [2] | 25*,† | 83±42 | 56±38 |
| CABG [3] | 34†† | 108±44 | 77±44 |
| CABG [4] | 3† | 109±25 | 66±26 |
| Valve replacement or repair | |||
| Aortic | 35‡ | 103±42 | 78±28 |
| Mitral | 6§ | 117±53 | 95±48 |
| Aortic + mitral | 5 | 200±107 | 169±106 |
| Mitral + tricuspid | 2 | 158±15 | 132±11 |
| Combined surgery (CABG + valve) | 26 | 146±52 | 115±43 |
| AAA | 4 | 142±49 | 90±39 |
| Other | 2 | 119±77 | 87±83 |
| Total | 145 | 115±54 | 85±49 |
a, values are mean ± SD; †, one patient also had endarterectomy; *, one patient also had ventricular aneurysmectomy; ‡, ten patients also had ascending aorta aneurysm; §, one patient also had atrial septal defect. CPB, cardiopulmonary bypass; CABG, coronary artery bypass graft (No. of grafts); AAA, ascending aorta aneurysm.
Table 2. Characteristics and perioperative data of patients undergoing cardiac surgery (n=145), according to ICU length of stay.
| Variable | Patients with LOS ≤24 h (n=79) | Patients with LOS >24 h (n=66) | P value |
|---|---|---|---|
| Age, years | 66±11 | 70±9 | 0.015 |
| Sex, male, n (%) | 61 (77%) | 47 (71%) | 0.416 |
| Body mass index, kg/m2 | 28±4 | 28±5 | 0.721 |
| Preoperative | |||
| Laboratory values | |||
| Hemoglobin, mg/dL | 14±1 | 13±2 | <0.001 |
| Hematocrit, % | 42±4 | 39±6 | <0.001 |
| Urea, mg/dL | 44±19 | 56±28 | 0.003 |
| Creatinine, mg/dL | 0.99±0.3 | 1.33±1.5 | 0.047 |
| Glucose, mg/dL | 119±29 | 125±36 | 0.279 |
| Comorbidities, diagnosed with, n (%) | |||
| Hypertension | 61 (77%) | 58 (88%) | 0.097 |
| Diabetes | 23 (29%) | 31 (47%) | 0.027 |
| COPD | 8 (10%) | 12 (18%) | 0.163 |
| Dyslipidemia | 60 (76%) | 50 (76%) | 0.979 |
| Chronic renal failure | 1 (1%) | 6 (9%) | 0.029 |
| Arrhythmias | 4 (5%) | 5 (8%) | 0.536 |
| Preoperative medication, in treatment with, n (%) | |||
| Statin | 53 (67%) | 43 (65%) | 0.808 |
| Amiodarone | 0 (0%) | 1 (1.5%) | 0.275 |
| b-blockers | 46 (58%) | 43 (65%) | 0.396 |
| Intraoperative | |||
| Cardiopulmonary bypass time, min | 100±38 | 132±64 | <0.001 |
| Aortic clamping time, min | 73±36 | 100±57 | 0.001 |
| Allogeneic RBC transfusion, units | 1.5±1.3 | 2.7±2.3 | <0.001 |
| Postoperative | |||
| Laboratory values at ICU admission | |||
| Creatinine, mg/dL | 0.87±0.22 | 1.14±0.68 | 0.001 |
| Troponine I, mg/dL | 4.37±5.58 | 8.29±15.55 | 0.038 |
| Creatine kinase, mg/dL | 363±291.5 | 444.3±313.7 | 0.113 |
| Creatine kinase-MB, mg/dL | 24.95±15.68 | 37.17±40.33 | 0.014 |
| White blood cells, K/µL | 10.28±3.68 | 9.96±3.60 | 0.601 |
| Platelets, K/µL | 125.3±45.2 | 116.4±38.1 | 0.209 |
| Hematocrit, % | 29.92±3.26 | 28.86±3.75 | 0.075 |
| Bilirubin, mg/dL | 0.98±0.73 | 1.12±1.06 | 0.397 |
| Duration of mechanical ventilation in the ICUa, hours | 10±3 | 41±85 | 0.001 |
| Vasoactive agents, treated with, n (%)b | 43 (54%) | 51 (77%) | 0.001 |
| Severity scores | |||
| APACHE II score | 8.78±2.80 | 9.74±3.48 | <0.001 |
| SOFA score | 5.77±1.66 | 6.46±1.90 | <0.001 |
Values are mean ± SD. a, values are median ± SD; b, defined as the administration of either dopamine, dobutamine, epinephrine or norepinephrine, at any dose, for at least 1 hour during the first 24 postoperative hours. ICU, intensive care unit; LOS, length of stay; COPD, chronic obstructive pulmonary disease; RBC, red blood cell; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
Postoperative complications during the first 24 postoperative hours included blood loss (84 patients, 58%), pulmonary dysfunction (49 patients, 34%), arrhythmias (9 patients, 6%), re-intubation (5 patients, 3%) and cardiac arrest in one patient.
Sequentially recorded parameters in the first 24 postoperative hours, including hemodynamic and blood gases measurements, in relation with LOS in ICU, are shown in Table 3. Lactate levels differed consistently throughout the first 24 postoperative hours in the two groups; patients with a prolonged ICU stay had more elevated lactate concentration than those who were discharged within that period. In addition, the time course of parameters related to oxygen transport was clearly different in the two groups: hemoglobin levels, ratio of arterial oxygen partial pressure to inspired oxygen fraction, alveolar-arterial oxygen gradient, arterial oxygen content and arteriovenous oxygen content difference diverged early on during the immediate postoperative period, and continued to be markedly different in the first 24 hours.
Table 3. Sequentially recorded variables of the 145 patients in the first 24 postoperative hours according to ICU length of stay.
| Variable | ICU LOS | Time elapsed after ICU admission (hours) | Interaction P value | |||
|---|---|---|---|---|---|---|
| 0–1 | 1–4 | 4–12 | 12–24 | |||
| Temperature, °C | N | 36.2±0.7 | 36.8±0.8* | 37.6±0.7† | 37.4±0.5 | 0.009 |
| P | 36.1±0.8 | 36.5±0.9* | 37.3±0.7† | 37.3±0.6 | ||
| HR, beats/min | N | 86±13 | 86±15 | 88±13 | 87±12 | 0.4 |
| P | 87±15 | 88±16 | 89±13 | 90±13 | ||
| SAP, mmHg | N | 123±14 | 118±15 | 120±14 | 126±13 | 0.254 |
| P | 121±18 | 119±17 | 117±13 | 124±17 | ||
| DAP, mmHg | N | 65±10* | 62±9 | 62±7 | 65±7† | 0.008 |
| P | 61±9* | 60±9 | 61±9 | 61±9† | ||
| MAP, mmHg | N | 84±11* | 80±11 | 81±8 | 84±8* | 0.024 |
| P | 80±11* | 79±10 | 79±10 | 80±11* | ||
| CVP, mmHg | N | 14±3 | 14±3 | 14±3* | 13±3 | 0.432 |
| P | 14±4 | 16±4 | 15±4* | 15±5 | ||
| Blood gases | ||||||
| pH | N | 7.42±0.05 | 7.44±0.05 | 7.41±0.04 | 7.40±0.03 | 0.365 |
| P | 7.41±0.05 | 7.42±0.06 | 7.41±0.05 | 7.41±0.05 | ||
| PaO2, mmHg | N | 285±105 | 186±43† | 147±35* | 117±27 | 0.039 |
| P | 269±108 | 164±48† | 135±34* | 111±30 | ||
| PaCO2, mmHg | N | 35.1±4.7 | 33.6±4.7* | 36.0±4.9 | 37.5±4.1 | 0.105 |
| P | 36.7±5.8 | 35.3±4.8* | 35.7±4.7 | 37.9±5.3 | ||
| HCO3−, mmol/L | N | 22.8±1.2 | 22.7±1.2 | 22.6±1.5 | 23.1±1.6 | 0.301 |
| P | 23.0±1.8 | 23.1±2.28 | 22.8±2.6 | 23.4±2.6 | ||
| SaO2, % | N | 99.6±0.4 | 99.2±0.6* | 98.8±0.9 | 98.1±1.3 | 0.041 |
| P | 99.4±0.5 | 98.9±0.8* | 98.7±0.9 | 97.8±1.7 | ||
| PcvO2, mmHg | N | 36.9±6.1 | 34.5±4.2 | 37.2±5.2 | 35.9±4.7 | 0.779 |
| P | 39.1±14.3 | 34.5±5.1 | 35.6±5.8 | 36.1±4.4 | ||
| ScvO2, % | N | 67.8±9.9 | 63.6±7.2 | 66.6±7.9 | 65.0±7.8 | 0.335 |
| P | 70.5±8.1 | 64.4±8.1 | 65.8±9.1 | 66.0±7.1 | ||
| PcvCO2, mmHg | N | 41.3±5.2 | 42.6±6.1 | 44.0±5.2 | 44.4±4.4 | 0.504 |
| P | 42.7±6.3 | 43.2±5.6 | 43.0±5.5 | 45.2±5.6 | ||
| Lactate, mmol/L | N | 1.89±0.84* | 1.69±0.93 | 1.55±0.76* | 1.49±0.70† | 0.008 |
| P | 2.38±1.81* | 2.18±2.05 | 2.29±2.41* | 1.97±1.17† | ||
| Hb, g/dL | N | 10.09±1.12 | 10.39±1.01* | 10.89±1.00† | 11.18±0.86* | 0.009 |
| P | 9.88±1.26 | 10.02±1.14* | 10.36±1.09† | 10.78±1.15* | ||
| Glucose, mg/dL | N | 147±27* | 141±28ǂ | 167±33* | 168±29* | 0.001 |
| P | 163±43* | 163±43ǂ | 182±41* | 182±36* | ||
| PaO2/FiO2, mmHg | N | 336±114* | 358±98† | 333±85ǂ | 359±92ǂ | <0.001 |
| P | 292±103* | 304±85† | 277±80ǂ | 259±100ǂ | ||
| D(A-a)O2, mmHg | N | 288±113* | 149±67 | 129±57ǂ | 75±57ǂ | <0.001 |
| P | 340±110* | 183±70 | 178±69ǂ | 170±89ǂ | ||
| P(cv-a)CO2, mmHg | N | 6.26±4.82 | 9.16±3.78 | 7.84±4.30 | 6.97±3.15 | 0.501 |
| P | 6.03±5.87 | 8.15±3.79 | 7.53±3.69 | 7.32±3.47 | ||
| CaO2, mL/dL | N | 14.56±1.51 | 14.59±1.37* | 15.09±1.33† | 15.29±1.24* | 0.003 |
| P | 14.20±1.66 | 13.99±1.57* | 14.33±1.46† | 14.70±1.60* | ||
| CcvO2, mL/dL | N | 9.42±1.70 | 9.08±1.25 | 9.98±1.50* | 10.02±1.56 | 0.24 |
| P | 9.60±1.60 | 8.90±1.57 | 9.40±1.73* | 9.82±1.62 | ||
| C(a-cv)O2, mL/dL | N | 5.13±1.40* | 5.51±1.23* | 5.11±1.29 | 5.27±1.11* | 0.005 |
| P | 4.60±1.26* | 5.09±1.15* | 4.93±1.32 | 4.86±1.00* | ||
Values are mean ± SD. *, P<0.05 for differences between groups; †, P<0.01 for differences between groups; ǂ, P<0.001 for differences between groups. ICU, intensive care unit; LOS, length of stay; N, normal; P, prolonged; HR, heart rate; SAP, systolic arterial blood pressure; MAP, mean arterial blood pressure; DAP, diastolic arterial blood pressure; CVP, central venous pressure; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; HCO3−, bicarbonate; SaO2, hemoglobin oxygen saturation of arterial blood; PcvO2, partial pressure of central venous oxygen; ScvO2, hemoglobin oxygen saturation in central venous blood; PcvCO2, partial pressure of central venous carbon dioxide; Hb, hemoglobin; PaO2/FiO2, ratio of arterial oxygen partial pressure to inspired oxygen fraction; D(A-a)O2, alveolar-arterial oxygen gradient; P(cv-a)CO2, central venous to arterial carbon dioxide partial pressure difference; CaO2, arterial oxygen content; CcvO2, central venous oxygen content; C(a-cv)O2, arteriovenous oxygen content difference.
Univariate analysis identified 25 variables that significantly differed between patients with and without prolonged ICU stay. Specifically, patients with prolonged ICU stay were older (70±9 vs. 66±11 years, P=0.015) and they most likely had combined surgery (P=0.049). Other variables were: chronic renal failure (P=0.029), diabetes mellitus (P=0.027), hemoglobin concentration (P<0.001), creatinine level (P=0.047), three intraoperative [CPB time (P<0.001), aortic cross clamp time (P=0.001), RBC units transfused (P<0.001)], three measured at ICU admission [creatinine level (P=0.001), troponin level (P=0.038), creatine kinase-MB level (P=0.014)], seven measured during the first postoperative hour [diastolic arterial pressure (P=0.015), mean arterial pressure (P=0.037), PaO2/FiO2 ratio (P=0.016), alveolar-arterial oxygen gradient (P=0.04), arterial lactate level (P=0.034), glucose level (P=0.01), arteriovenous oxygen content difference (P=0.018)] and six measured from 1 to 4 postoperative hours [core body temperature (P=0.025), PaO2/FiO2 ratio (P=0.001), partial pressure of arterial carbon dioxide (P=0.03), hemoglobin concentration (P=0.046), glucose level (P<0.001), arterial oxygen content (P=0.016)].
From the results of random forests analysis, four variables ranked higher: preoperative hemoglobin concentration, aortic cross clamp time, PaO2/FiO2 ratio and blood glucose measured during the first 1 to 4 postoperative hours. The logistic regression model constructed with these variables is shown in Table 4. It had a moderate to good discriminatory ability, with an area under its ROC curve of 0.79 (P<0.001, 95% CI: 0.71–0.86, Figure 1).
Table 4. Logistic regression model with the predictors identified by the random forest algorithm.
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Preoperative hemoglobin, g/dL | 0.68 | 0.54–0.86 | 0.01 |
| Aortic cross clamp time, min | 1.01 | 0.99–1.02 | 0.06 |
| PaO2/FiO2, mmHg (1–4 postoperative hours) | 0.99 | 0.98–0.99 | 0.03 |
| Glucose, mg/dL (1–4 postoperative hours) | 1.02 | 1.01–1.02 | 0.009 |
PaO2/FiO2, ratio of arterial oxygen partial pressure to inspired oxygen fraction.
Figure 1.
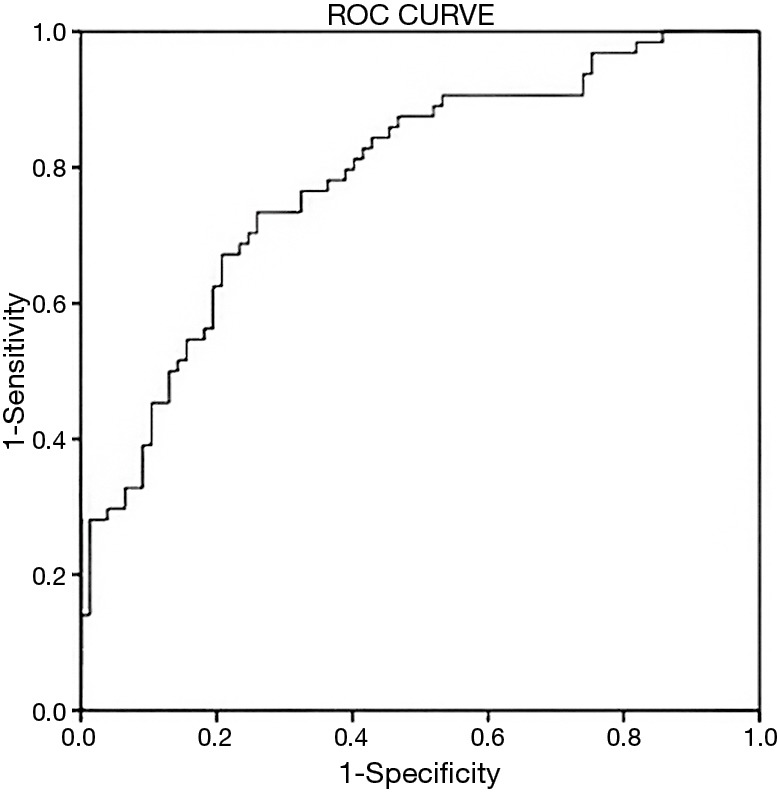
Receiver operating characteristic (ROC) curve of random forest analysis, evaluating the discriminative ability of the multivariate model.
Discussion
Our findings suggest that low preoperative hemoglobin concentration, prolonged aortic cross clamp time, low early postoperative PaO2/FiO2 ratio and high early postoperative blood glucose have significant prognostic value in prolonged ICU stay after cardiac surgery.
Our results on the importance of baseline hemoglobin levels come to support earlier findings. Lower preoperative hemoglobin increases the likelihood of perioperative allogeneic blood transfusion (19), a procedure that may lead to adverse outcomes (20,21). A recent study by Hallward et al. (22) examined the relationship between preoperative hemoglobin levels and utilization of hospital resources and adverse outcomes, including LOS, in a cohort of 1,972 patients undergoing cardiac surgery. They concluded that each g/dL fall in preoperative hemoglobin concentration results in increased use of hospital resources, increased blood transfusion requirements and increased ICU and hospital LOS. Ranucci et al. (23) showed that preoperative anemia is associated with adverse outcomes, relating mortality and morbidity, after cardiovascular surgery. Time on mechanical ventilation and ICU stay were significantly longer in severely anemic patients as a result of the higher rate of major complications. Additionally, when they compared patients with any kind of anemia versus non-anemic patients, the differences in outcome became even more relevant, including a higher rate of low cardiac output and bloodstream infection. Differences between anemic and non-anemic patients were also found by Williams et al. (24), who investigated preoperative hematocrit from data collected on 182,699 patients and showed that it is a powerful predictor of adverse outcomes in coronary artery bypass graft surgery. The two groups had significant differences in mortality, renal failure, stroke incidence, prolonged ventilation and deep sternal wound infection.
Al-Sarraf et al. (25) assessed the effect of aortic cross-clamp time on outcome following cardiac surgery in 3,799 patients. They concluded that prolonged cross-clamp time correlates with major post-operative morbidity and mortality. Their analysis showed that aortic cross-clamp time of more than 60 minutes was an independent risk factor for low cardiac output, prolonged ventilation, renal complications, blood transfusion, mortality and prolonged stay. Longer aortic cross-clamp time obviously relates to prolonged CPB. From a data set of 5,006 patients, Salis et al. (6) showed that prolonged CPB duration independently predicts postoperative morbidity and mortality after cardiac surgery. Additionally, Nissinen et al. (26) studied 3,280 patients and found that safe time limits of aortic cross-clamping (<150 min) and CPB (<240 min) were associated with a rather low risk of immediate postoperative adverse events. Prolongation of CPB time consequently affects ICU stay, as it was also shown by Rosenfeld et al. in 9,869 patients undergoing CABG surgery (27).
The PaO2/FiO2 ratio is an indicator of oxygenation status that is widely used in ICUs. Low values may arise from pathological conditions, mainly of respiratory etiology (atelectasis, acute respiratory distress syndrome), as well as due to cardiovascular disorders (cardiogenic shock and pulmonary edema). After cardiac surgery, inadequate oxygen delivery has been observed, due to low cardiac index, compromising tissue oxygenation and leading to higher oxygen extraction in the first 24 h after surgery (28). Also, restrictive lung disorders, atelectasis, pulmonary inflammation and decreased lung compliance may occur even in patients without preexisting lung pathology (1). Thus, a low PaO2/FiO2 ratio may indicate a persistent cardio-respiratory dysfunction which can affect patient outcome. In the study by Trouillet et al. (29), with 2,620 patients, lung injury was one of the six factors associated with very prolonged mechanical ventilation after cardiac surgery. In our study, the PaO2/FiO2 ratio measured as early as within the first 4 hours of ICU admission significantly differed between the two groups of patients. This difference further increased after the first 12 postoperative hours. This finding concurs with a recent prospective study on 2,725 patients by Esteve et al. (30), who evaluated the PaO2/FiO2 ratio after cardiac surgery as a predictor of outcome. According to their results, the value of PaO2/FiO2 at 3 hours after ICU admission was the best predictor of longer ICU LOS and higher mortality.
Adverse effects of elevated blood glucose after CPB that lead to increased morbidity and mortality have long been documented (31,32). According to Reddy et al. (33), patients submitted to cardiac surgery may have undiagnosed diabetes or metabolic syndrome. These patients may have abnormally high blood glucose levels in the perioperative period and an increased risk for adverse outcome. In our study, mean glucose levels postoperatively, presented a constant difference of approximately 20 mg/dL between patients with normal and prolonged ICU stay. Gandhi et al. (34) analyzed glucose measurements from 409 cardiac surgery patients and found that a 20 mg/dL increase in mean intraoperative glucose level was associated with a 30% increase of adverse events. In a retrospective review of 8,727 cardiac surgery patients Ascione et al. (35), showed that glucose level >200 mg/dL, at any time during the first five postoperative days, was associated with an increased likelihood of in-hospital morbidity and mortality. Similar results were also found by Duncan et al. (36). Since these findings were mutually consistent, the Society of Thoracic Surgeons recommends that patients with persistently elevated serum glucose (>180 mg/dL), regardless of their diabetic status, should receive IV insulin infusions to maintain a serum glucose <180 mg/dL for the duration of their ICU stay (37).
Low SvO2 values in the early postoperative period after cardiac surgery have been related to worse short- and long-term outcome (28,38). In the present study the ScvO2 value, though it has been proposed as an alternative variable to SvO2 in the critically ill (39), did not differ significantly between patients with and without prolonged ICU stay. However, this finding is not surprising because the accuracy of ScvO2 to predict SvO2 among patients undergoing cardiac surgery is unreliable (40).
There are potential limitations that should be addressed: Our data comes from a single hospital, highly specialized in cardiac surgery. The sample size could be considered relatively small; however it allowed significant factors to emerge from the several variables monitored perioperatively, related to the risk for prolonged ICU stay after cardiac surgery. Furthermore, some significant variables could have been missed as important predictors because of the observed low frequency of their occurrence in this study.
Conclusions
Multiple factors are associated with prolonged stay in the ICU after cardiac surgery operations. Specifically, low preoperative hemoglobin concentration, prolonged aortic cross clamping time, and low PaO2/FiO2 value and high level of blood glucose, both at the early postoperative hours were strongly related with prolonged LOS in ICU.
These findings may have clinical implications in the identification of cardiac surgical patients who are at increased risk for prolonged ICU stay.
Acknowledgements
The authors would like to thank Mr. Anastasios Stefos, BA in English and Greek Language and Literature for the linguistic revision of this manuscript.
Funding: This work was supported by the Special Account for Research Grants (grant number: 11596) of the National and Kapodistrian University of Athens, Greece.
Ethical Statement: The study was approved by the Ethics Committee of “Onassis” Cardiac Surgery Center (No. 444-21/10/10). Since it was an observational study and medical confidentiality and personal data were preserved, the requirement for written informed consent from patients or their relatives was waived.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Stephens RS, Whitman GJ. Postoperative Critical Care of the Adult Cardiac Surgical Patient. Part I: Routine Postoperative Care. Crit Care Med 2015;43:1477-97. 10.1097/CCM.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 2.Stephens RS, Whitman GJ. Postoperative Critical Care of the Adult Cardiac Surgical Patient: Part II: Procedure-Specific Considerations, Management of Complications, and Quality Improvement. Crit Care Med 2015;43:1995-2014. 10.1097/CCM.0000000000001171 [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Li S, Rankin JS, et al. Fifteen-year outcome trends for valve surgery in North America. Ann Thorac Surg 2011;91:677-84; discussion p 684. [DOI] [PubMed]
- 4.Sanders J, Keogh BE, Van der Meulen J, et al. The development of a postoperative morbidity score to assess total morbidity burden after cardiac surgery. J Clin Epidemiol 2012;65:423-33. 10.1016/j.jclinepi.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Galas FR, Almeida JP, Fukushima JT, et al. Blood transfusion in cardiac surgery is a risk factor for increased hospital length of stay in adult patients. J Cardiothorac Surg 2013;8:54. 10.1186/1749-8090-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814-22. 10.1053/j.jvca.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Croal BL, Hillis GS, Gibson PH, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 2006;114:1468-75. 10.1161/CIRCULATIONAHA.105.602370 [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos A, Tzelepis G, Dafni U, et al. Determinants of hospital mortality after coronary artery bypass grafting. Chest 1999;115:1598-603. 10.1378/chest.115.6.1598 [DOI] [PubMed] [Google Scholar]
- 9.Cohn LH, Rosborough D, Fernandez J. Reducing costs and length of stay and improving efficiency and quality of care in cardiac surgery. Ann Thorac Surg 1997;64:S58-60; discussion S80-2. [DOI] [PubMed]
- 10.Williams MR, Wellner RB, Hartnett EA, et al. Long-term survival and quality of life in cardiac surgical patients with prolonged intensive care unit length of stay. Ann Thorac Surg 2002;73:1472-8. 10.1016/S0003-4975(02)03464-1 [DOI] [PubMed] [Google Scholar]
- 11.Doering LV, Esmailian F, Laks H. Perioperative predictors of ICU and hospital costs in coronary artery bypass graft surgery. Chest 2000;118:736-43. 10.1378/chest.118.3.736 [DOI] [PubMed] [Google Scholar]
- 12.Engoren M, Buderer NF, Zacharias A. Long-term survival and health status after prolonged mechanical ventilation after cardiac surgery. Crit Care Med 2000;28:2742-9. 10.1097/00003246-200008000-00010 [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 15.Peretto G, Durante A, Limite LR, et al. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract 2014;2014:615987. [DOI] [PMC free article] [PubMed]
- 16.Breiman L. Random Forests. Mach Learn 2001;45:5-32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 17.Hastie T, Tibshirani R, Friedman J. editors. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd edition. Springer: New York, 2009. [Google Scholar]
- 18.Malley JD, Malley KG, Pajevic S. editors. Statistical Learning for Biomedical Data. New York: Cambridge University Press, 2011. [Google Scholar]
- 19.Hung M, Besser M, Sharples LD, et al. The prevalence and association with transfusion, intensive care unit stay and mortality of pre-operative anaemia in a cohort of cardiac surgery patients. Anaesthesia 2011;66:812-8. 10.1111/j.1365-2044.2011.06819.x [DOI] [PubMed] [Google Scholar]
- 20.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559-67. 10.1001/jama.2010.1446 [DOI] [PubMed] [Google Scholar]
- 21.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 2006;34:1608-16. 10.1097/01.CCM.0000217920.48559.D8 [DOI] [PubMed] [Google Scholar]
- 22.Hallward G, Balani N, McCorkell S, et al. The Relationship Between Preoperative Hemoglobin Concentration, Use of Hospital Resources, and Outcomes in Cardiac Surgery. J Cardiothorac Vasc Anesth 2016;30:901-8. 10.1053/j.jvca.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 23.Ranucci M, Di Dedda U, Castelvecchio S, et al. Impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg 2012;94:1134-41. 10.1016/j.athoracsur.2012.04.042 [DOI] [PubMed] [Google Scholar]
- 24.Williams ML, He X, Rankin JS, et al. Preoperative hematocrit is a powerful predictor of adverse outcomes in coronary artery bypass graft surgery: a report from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2013;96:1628-34; discussion 1634. 10.1016/j.athoracsur.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 25.Al-Sarraf N, Thalib L, Hughes A, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104-9. 10.1016/j.ijsu.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 26.Nissinen J, Biancari F, Wistbacka JO, et al. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion 2009;24:297-305. 10.1177/0267659109354656 [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld R, Smith JM, Woods SE, et al. Predictors and outcomes of extended intensive care unit length of stay in patients undergoing coronary artery bypass graft surgery. J Card Surg 2006;21:146-50. 10.1111/j.1540-8191.2006.00196.x [DOI] [PubMed] [Google Scholar]
- 28.Routsi C, Vincent JL, Bakker J, et al. Relation between oxygen consumption and oxygen delivery in patients after cardiac surgery. Anesth Analg 1993;77:1104-10. 10.1213/00000539-199312000-00004 [DOI] [PubMed] [Google Scholar]
- 29.Trouillet JL, Combes A, Vaissier E, et al. Prolonged mechanical ventilation after cardiac surgery: outcome and predictors. J Thorac Cardiovasc Surg 2009;138:948-53. 10.1016/j.jtcvs.2009.05.034 [DOI] [PubMed] [Google Scholar]
- 30.Esteve F, Lopez-Delgado JC, Javierre C, et al. Evaluation of the PaO2/FiO2 ratio after cardiac surgery as a predictor of outcome during hospital stay. BMC Anesthesiol 2014;14:83. 10.1186/1471-2253-14-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills NL, Beaudet RL, Isom OW, et al. Hyperglycemia during cardiopulmonary bypass. Ann Surg 1973;177:203-5. 10.1097/00000658-197302000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes 1974;23:9-15. 10.2337/diab.23.1.9 [DOI] [PubMed] [Google Scholar]
- 33.Reddy P, Duggar B, Butterworth J. Blood glucose management in the patient undergoing cardiac surgery: A review. World J Cardiol 2014;6:1209-17. 10.4330/wjc.v6.i11.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med 2007;146:233-43. 10.7326/0003-4819-146-4-200702200-00002 [DOI] [PubMed] [Google Scholar]
- 35.Ascione R, Rogers CA, Rajakaruna C, et al. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation 2008;118:113-23. 10.1161/CIRCULATIONAHA.107.706416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan AE, Abd-Elsayed A, Maheshwari A, et al. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology 2010;112:860-71. 10.1097/ALN.0b013e3181d3d4b4 [DOI] [PubMed] [Google Scholar]
- 37.Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009;87:663-9. 10.1016/j.athoracsur.2008.11.011 [DOI] [PubMed] [Google Scholar]
- 38.Holm J, Håkanson E, Vánky F, et al. Mixed venous oxygen saturation predicts short- and long-term outcome after coronary artery bypass grafting surgery: a retrospective cohort analysis. Br J Anaesth 2011;107:344-50. 10.1093/bja/aer166 [DOI] [PubMed] [Google Scholar]
- 39.Reinhart K, Kuhn HJ, Hartog C, et al. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med 2004;30:1572-8. 10.1007/s00134-004-2337-y [DOI] [PubMed] [Google Scholar]
- 40.Gasparovic H, Gabelica R, Ostojic Z, et al. Diagnostic accuracy of central venous saturation in estimating mixed venous saturation is proportional to cardiac performance among cardiac surgical patients. J Crit Care 2014;29:828-34. 10.1016/j.jcrc.2014.04.012 [DOI] [PubMed] [Google Scholar]


