Abstract
Background
Existing clinical decision rules (CDR) to diagnose group A streptococcal (GAS) pharyngitis have not been validated in sub-Saharan Africa. We developed a locally applicable CDR while evaluating existing CDRs for diagnosing GAS pharyngitis in South African children.
Methods
We conducted a prospective cohort study and enrolled 997 children aged 3-15 years presenting to primary care clinics with a complaint of sore throat, and whose parents provided consent. Main outcome measures were signs and symptoms of pharyngitis, and a positive GAS culture from a throat swab. Bivariate and multivariate analyses were used to develop the clinical decision rule. In addition, the diagnostic effectiveness of six existing rules for predicting a positive culture in our cohort was assessed.
Results
206 of 982 children (21%) had a positive GAS culture. Tonsillar swelling, tonsillar exudates, tender or enlarged anterior cervical lymph nodes, absence of cough and absence of rhinorrhea were associated with positive cultures in bivariate and multivariate analyses. Four variables (tonsillar swelling and one of tonsillar exudate, no rhinorrhea, no cough), when used in a cumulative score, showed 83.7% sensitivity and 32.2% specificity for GAS pharyngitis. Of existing rules tested, the McIsaac rule had the highest positive predictive value (28%), but missed 49% of the culture-positive children who should have been treated.
Conclusion
The new four-variable clinical decision rule for GAS pharyngitis (i.e., tonsillar swelling and one of tonsillar exudate, no rhinorrhea, no cough) outperformed existing rules for GAS pharyngitis diagnosis in children with symptomatic sore throat in Cape Town.
Keywords: Group A Streptococcus, clinical prediction rule, pharyngitis, children
Introduction
Antibiotic treatment of pharyngitis with clinical features suggestive of a group A β-hemolytic streptococcus (GAS) etiology reduces the incidence of acute rheumatic fever (ARF) by 68% (1). The primary prevention of ARF with antibiotics represents an important strategy for reducing the burden of rheumatic heart disease (RHD)(2), which accounts for 14% of cases of acute heart failure in sub-Saharan Africa (3). The diagnosis of GAS pharyngitis can be made directly by microbiological culture (4), or indirectly based on rapid streptococcal assays (5), or through the use of a clinical decision rule (6). Clinical decision rules are practical decision tools to aid diagnostic evaluation, particularly in low-resourced settings where laboratory facilities may not be available. Clinical decision rules incorporate a limited number of clinical characteristics, to derive a probability score for directing a course of action (7, 8).
We have recently demonstrated that the use of a clinical decision rule is the most cost-effective strategy for the primary prevention of ARF in South African children with sore throat (9). Existing clinical decision rules to diagnose GAS pharyngitis have not been validated in some regions of the world, such as sub-Saharan Africa, where RHD is endemic. An Egyptian study showed that the diagnostic performance varies considerably in different communities and regions of the world (10), thus highlighting the importance of evaluating and validating clinical decision rules in local settings before they are rolled out as the standard of care (11).
We developed a locally applicable clinical decision rule for streptococcal pharyngitis in children with sore throat from Cape Town. In addition, we evaluated the performance of existing clinical decision rules for diagnosing streptococcal pharyngitis in South African children.
Materials and Methods
Study Site, Recruitment and Diagnosis of GAS Pharyngitis
This study was conducted from May 2008 to October 2013. We recruited children aged 3 to 15 years who presented with sore throat to three community clinics in Langa and Bonteheuwel, Cape Town, South Africa. Participants who had received a prescription of antibiotics, the last dose of which was taken within 30 days of enrolment, were excluded. Informed consent was obtained from the parent or legal guardian. Assent was also obtained from children ≥ 8 years of age. A medical history was taken and clinical examination of the child was performed by the trained study nurse. A throat swab was obtained as follows: a sterile cotton swab was slowly applied across one tonsil or tonsillar fossa, then across the posterior pharynx, and finally across the opposite tonsil or tonsillar fossa, without touching the tongue or mouth. Swabs with liquid media tips were used. Swabs were then returned to their sheaths, which contain a transport medium at the tip for the swab.
Throat swabs were transported in an insulated bag to the National Health Laboratory Service (NHLS) microbiology laboratory at Groote Schuur Hospital in Cape Town for incubation. Specimens received as appropriately labeled swabs were plated onto 4% sheep blood agar plates in a standard fashion. Every effort was made to place specimens in an incubator no later than 4 hours from the time of collection. Plates were inverted and incubated anaerobically at 35°C. After 18 to 24 hours, the plates were examined by the microbiology technician, under the supervision of one of us (ACW). Laboratory-confirmed GAS culture positivity was the reference standard for the diagnosis of GAS pharyngitis. Further confirmation was done through Gram stain, catalase, and sero-grouping, as appropriate. A single colony was picked off with a sterile wire loop, and sub-cultured for purity. Culture results from the study were returned to the clinic for filing in the patient's medical record.
Measurement of Potential Predictor Variables
The selection of potential predictor variables was based on the clinical decision rules that performed best in an Egyptian prospective study (sensitivity and specificity of more than 90% and 30% respectively) (6). Together with age, sex, race and place of residence, we recorded presence or absence of the following signs and symptoms: cough, rhinorrhoea, hoarseness, fever (>38°C), tonsillar erythema or swelling, pharyngeal or tonsillar exudate, tenderness of an anterior cervical lymph node on palpation, an anterior cervical lymph node >1.5 cm in diameter (i.e., swelling), and rash. In addition, the presence or absence of oropharyngeal candidiasis was recorded, in view of the high prevalence of human immune-deficiency virus (HIV) infection in South Africa (12).
Sample Size
For derivation of the clinical decision rule, we required 10 events per candidate predictor (13). The expected prevalence of GAS in children with pharyngitis was 30% (14, 15). Our initial estimate was that we would need to recruit 450 participants to obtain 150 GAS cases for the 15 candidate predictors (including age and sex). Preliminary analysis indicated a lower than expected prevalence of GAS (16%) in our study cohort. We therefore revised our target sample size to 940 participants.
Statistical Analysis
Statistical analysis was performed using Stata (version 11.2, StataCorp, College Station, Texas), SAS/STAT v.9.1 (SAS Institute Inc., NC, USA) and R version 3.0.0 [2013-04-03] (The R Foundation for statistical computing, Vienna, Austria). Proportions or means were calculated to describe the baseline characteristics of the study population. We generated scores according to the criteria contained in the existing rules to estimate the probability of a GAS positive culture. We aimed to develop a clinical decision rule with a sensitivity of ≥ 90% and specificity of ≥ 40%.
We calculated univariable odds ratios with 95% confidence intervals to examine the relationship between each individual predictor with the primary outcome measure, i.e., a laboratory-confirmed GAS culture from the throat swab specimen. For continuous predictors, the functional form of the association with the outcome was checked using restricted cubic splines, to find the proper transformation if needed (16). Variables were then selected for inclusion in the final logistic regression model following the stepwise approach proposed by Collett (17). The following interactions were tested in the final model:cough, rhinorrhoea, tonsillar swelling and tonsillar exudate.
Calibration was assessed graphically through calibration plots, and by computing the Hosmer and Lemeshow (HL) test. Discrimination was assessed by estimating the c-statistic. C-statistics vary from 0.5 (no discrimination) to 1 (perfect discrimination), with values of 0.7-0.8 deemed acceptable and 0.8–0.9 good (18). Internal validation of the model used 2000 bootstrap re-samples (16). To promote the uptake in routine settings, the new clinical decision rule was constructed incorporating previously described methods (19-21) where the logistic formula was transformed into a ready to use score chart. Beta coefficients were divided by a constant; predictors with a negative association were inverted and evaluated as positive entities; the total score was then compared to the observed proportion of GAS positivity in our cohort. Thereafter, receiver operating characteristic (ROC) curves were used to derive the optimal threshold point-score by applying the Youden's index approach (22).
We evaluated the diagnostic effectiveness of previously developed clinical decision rules (included in the Egyptian study) as well as additional clinical decision rules identified in a recent systematic review, against our data (23). We determined performance only for those clinical decision rules for which we had data on all predictors included in the clinical decision rule: the Abu Reesh (24), Wald (25), WHO (26), Steinhoff (6), McIsaac (20), and Attia (27) rules. We determined sensitivity, specificity, positive and negative predictive value, positive likelihood ratios, diagnostic odds ratio, area under the receiver operating curve and Youden's J statistic for each rule, at the cut point/range of cut points suggested by the authors. Performance of the derived clinical decision rule was compared using the ratio of missed streptococcal cases (missed diagnoses (MDx)) against unnecessarily treated non-cases (wrong diagnoses (WDx)) (6). We defined a successful clinical decision rule as one with a sensitivity of ≥90% and a specificity of ≥40%, which corresponds to a MDx/Wdx ratio of 0.044 in our cohort. Data were stored using Epi-Info software (version 3.5.3), and double entry verification was adopted to reduce data entry error.
Ethics Approval
The study was approved by the University of Cape Town's institutional review board and the department of health of the Western Cape. The consent forms were provided in the local languages of Afrikaans, English and isiXhosa.
Results
During the study period, 1055 children with sore throat presented to the participating community clinics. Of these, informed consent was obtained for 997 participants, of whom 993 met the inclusion criteria and were recruited into the study. Reasons for the 4 exclusions were antibiotic treatment in the preceding 30 days (n = 2), age greater than 15 years (n = 1) and refusal by the child to allow a throat swab to be taken (n = 1). GAS laboratory results were available for 982 children.
The demographic characteristics of the 982 children included in the analysis are presented in Table 1. A laboratory-confirmed diagnosis of GAS was reported in 206 (21%) children, which was higher than the proportion of 16% found at preliminary analysis.
Table 1. Characteristics of the 982 children presenting with pharyngitis.
| Characteristic | N (%) |
|---|---|
| Demographic variables | |
| Males | 429 (44) |
| Age, (median years (IQR)) | 8.3 (6.4 to 10.6) |
| Residence in Langa | 156 (16) |
| Black African | 286 (29) |
| Mixed Ancestry | 696 (71%) |
| Clinical Symptoms | |
| Cough | 661 (67) |
| Rhinorrhea | 573 (58) |
| Hoarseness | 165 (17) |
| Temperature > 38 °C | 347 (35) |
| Tonsillar erythema | 865 (88) |
| Tonsillar swelling | 864 (88) |
| Presence of exudate on the pharynx | 78 (8) |
| Presence of exudate on the tonsils | 507 (52) |
| Oropharyngeal candidiasis | 29 (3) |
| Tenderness of an anterior cervical node | 589 (60) |
| Presence of an anterior cervical lymph node greater than 1.5 cm in diameter | 398 (41) |
| Presence of a rash. | 120 (12) |
| Laboratory diagnosis | |
| Group A Streptococcus positive | 206 (21) |
N, number; °C, degrees Celsius;
Univariable Analysis
Table 2 shows the results of the association between throat culture results and individual variables. Tonsillar swelling was the univariable predictor most strongly associated with a positive GAS culture p<001. Five variables were significant predictors for GAS positivity: tonsillar swelling (p <0.001), a temperature >38°C (p=0.002), presence of exudate on the tonsils (p<0.001), tenderness of an anterior cervical node on palpation (p=0.002), and the presence of an anterior cervical lymph node >1.5 cm in diameter (p=0.028). Significant negative predictors of GAS positivity were the presence of cough (p <0.001) and rhinorrhoea (p=0.002).
Table 2. Univariable associations between candidate predictors and GAS in children with pharyngitis.
| Variable | GAS+ Participants with variable, n (%) | Unadjusted OR for GAS+ (95% CI) | p value | |
|---|---|---|---|---|
| Total | 206 (21) 1) | |||
| Male Gender | 87 (42) | 1.08 (0.79 – 1.48) | 0.5796 | |
| Cough | 115 (56) | 0.53 (0.39 – 0.73) | <0.001 | |
| Rhinorrhea | 100 (49) | 0.60 (0.44 – 0.82) | 0.002 | |
| Hoarseness | 31 (15) | 0.82 (0.53 – 1.25) | 0.4512 | |
| Pyrexia > 38 °C | 92 (45) | 1.62 (1.19 – 2.22) | 0.002 | |
| Tonsillitis | erythema | 188 (91) | 1.60 (0.95 – 2.71) | 0.076 |
| swelling | 195 (95) | 2.96 (1.56 – 5.62) | <0.001 | |
| exudate | 135 (66) | 2.03 (1.48 – 2.79) | <0.001 | |
| Exudate on the pharynx | 18 (9) | 0.12 (0.65 – 1.94) | 0.700 | |
| Oropharyngeal candidiasis | 6 (3) | 0.98 (0.39 – 2.44) | 0.958 | |
| Cervical glands | Enlarged | 98 (48) | 1.42 (1.05 – 1.94) | 0.028 |
| Tenderness | 143 (69) | 1.65 (1.19 – 2.28) | 0.002 | |
| Presence of a rash. | 17 (8) | 0.59 (0.34– 1.00) | 0.049 | |
GAS+, group A streptococcus positive; n, number; OR, odds ratio; CI, confidence interval; °C, degrees Celsius
Multivariable Model and Measures of Performance
Table 3 provides details of the multivariable analysis describing the variables in the final model. Our final multivariable logistic regression model consisted of the following four predictors: absence of cough [adjusted odds ratio (aOR) 1.59, 95% CI 1.15; 2.20], absence of rhinorrhoea (aOR 1.47, 95% CI 1.06; 2.02), presence of tonsillar swelling (aOR 2.06, 95% CI 1.05; 4.03), and presence of tonsillar exudate (aOR 1.71, 95% CI 1.22; 2.38). None of the interactions tested was significant.
Table 3. Multivariable logistic regression model for the diagnosis of GAS in children with pharyngitis.
| Variable | Adjusted Odds Ratio (95% CI) | Beta | p value | Point scores |
|---|---|---|---|---|
| Absence of cough | 1.59 (1.15 – 2.20) | - 0.2298 | 0.0059 | 1 |
| Absence of rhinorrhea | 1.47 (1.06 – 2.02) | - 0.1899 | 0.0200 | 1 |
| Presence of tonsillar swelling | 2.06 (1.05 – 4.03) | 0.3606 | 0.0036 | 2 |
| Presence of tonsillar exudate | 1.71 (1.22 – 2.38) | 0.2674 | 0.0017 | 1 |
CI, confidence interval;
The calibration curve from the final model (Figure 1, Panel a) followed the ideal calibration line, indicating a near-perfect agreement between the rate of streptococcal pharyngitis estimated by the model, and the streptococcal pharyngitis frequency observed in the study population. This near-perfect agreement was confirmed by the HL test: Hl-χ2=1.30 (p=0.99). The model's discriminatory performance as demonstrated in a plotting of sensitivity versus 1 – specificity (receiver operating characteristic curve) was quantified by the area under the curve, c-statistic = 0.638 (95%CI, 0.597-0.678) (Figure 1, Panel b). The equivalents in bootstrap validation were 0.633 (95% CI 0.625; 0.641); the optimism estimate was 0.0086 (95% CI -0.0304; 0.0476; p-value = 0.666), indicating good stability of the model in bootstrap internal validation.
Figure 1.
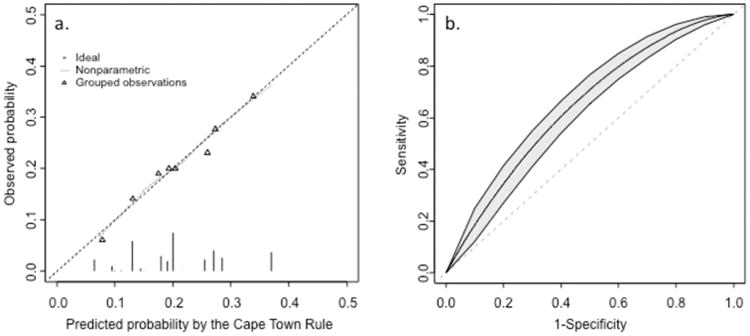
(a) Calibration plot for the new Cape Town clinical decision rule. Triangles indicate the observed frequency of streptococcal pharyngitis per decile of increasing predicted probability of streptococcal pharyngitis by the Cape Town rule. The dotted line shows the relation between observed streptococcal pharyngitis and predicted streptococcal pharyngitis, from non-parametric methods. Ideally, this line equals the dashed line that represents perfect calibration. (b) Receiver-operating curve (ROC) for predicting group A streptococcal positivity in pharyngitis. The diagonal line represents zero discriminative value and corresponds to an AUC of 0.50. The three curves above the diagonal line are the ROC curve (middle line) and the lower and upper bounds of the 95% confidence interval around the ROC curves.
Derivation of the Score and Management Recommendations
Table 3 illustrates the scoring system of the Cape Town clinical decision rule developed to calculate the risk of GAS positivity in an individual participant. Clinical predictors were converted into points allocated for each predictor value, and summed into a total score of 5.
Table 4 depicts the performance of the Cape Town clinical decision rule for GAS pharyngitis for scores ranging from one to five out of a total of five. A score of 3.5 had the most discriminatory ability with a sensitivity of 51.2, specificity of 68.2%, positive predictive value of 30.1% and negative predictive value of 83.9%. A score of 3 incorporating the presence of tonsillar swelling had a sensitivity of 83.7%, specificity of 32.2%, positive predictive value of 24.8% and negative predictive value of 88.1%. A summary of the diagnostic performance of the Cape Town clinical decision rule (at a threshold score of 3 points or more) evaluated by comparing the MDx/WDx ratio (missed diagnoses (MDx) versus unnecessarily treated non-cases (wrong diagnoses (WDx)) (6) against existing clinical decision rules in our cohort is provided in Table 5. The Cape Town rule proved to have a MDx/WDx ratio closest to 0.044, which met our target parameters of a sensitivity of ≥90% and a specificity of ≥40%.
Table 4. Performance of the Cape Town clinical decision rule for streptococcal pharyngitis according to scores ranging from 1 to 5.
| Treat if Score: | Sens (%) | Spec (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| 1 | 99.0 | 8.1 | 22.3 | 96.9 |
| 2 | 96.6 | 12.4 | 22.8 | 93.3 |
| 3 | 83.7 | 32.2 | 24.8 | 88.1 |
| 4 | 51.2 | 68.2 | 30.1 | 83.9 |
| 5 | 18.2 | 91.4 | 36.2 | 80.7 |
Sens, sensitivity; Spec, specificity; PPV, positive predictor value; NPV, negative predictive value all expressed as percentages
Table 5. Performance of clinical decision rules for streptococcal pharyngitis in children from Cape Town.
| Clinical Decision Rule | Sens | Spec | PPV | NPV | LR+ | MDx (%) | WDx (%) | MDx/WDx |
|---|---|---|---|---|---|---|---|---|
| Abu Reesh | 75.2 | 39.4 | 24.8 | 85.7 | 1.24 | 51 (25) | 470 (61) | 0.11 |
| Wald | 99 | 3.1 | 21.3 | 92.1 | 1.21 | 2 (1) | 752 (97) | 0.002 |
| Steinhoff | 79.8 | 34.1 | 24.4 | 86.4 | 1.21 | 42 (20) | 511 (66) | 0.08 |
| McIsaac | 51.4 | 64.7 | 27.9 | 83.4 | 1.46 | 100 (49) | 274 (35) | 0.37 |
| WHO | 36.5 | 73.8 | 27.0 | 81.4 | 1.39 | 131 (64) | 203 (26) | 0.64 |
| Attia | 76.2 | 36.3 | 24.1 | 85.2 | 1.20 | 49 (24) | 494 (64) | 0.10 |
| Cape Towna | 83.7 | 32.2 | 24.8 | 88.1 | 1.24 | 33 (16) | 525 (68) | 0.06 |
Sens, sensitivity; Spec, specificity; PPV, positive predictor value; NPV, negative predictive value all expressed as percentages; LR+, positive likelihood ratio; MDx (%), number of missed diagnoses of streptococcal pharyngitis and percentage of all true strep cases (=1-sensitivity); WDx (%), number of wrong diagnoses (unnecessarily treated non-cases) and percentage of all non-strep cases (=1-specificity).
Evaluated at the suggested score of 3/5 incorporating tonsillar swelling as compulsory symptom
Discussion
We developed a new clinical decision rule for streptococcal pharyngitis in children from Cape Town (the Cape Town clinical decision rule) by incorporating the presence of tonsillar exudate and swelling, and absence of both cough and rhinorrhoea to determine the likelihood of GAS positivity in children with pharyngitis. The Cape Town clinical decision rule has a maximum score of five points with 2 points allocated to the presence of tonsillar swelling and 1 point each for the remaining variables. It has a sensitivity of > 80% when incorporating tonsillar swelling together with any one of the remaining clinical symptoms. Only 15.9% of GAS-positive cases would have been missed using this approach.
We evaluated the Cape Town clinical decision rule against existing clinical decision rules purported to identify individuals with GAS positive pharyngitis. Compared with laboratory culture results, the McIsaac rule (20) had the highest positive predictive value (28%), but missed 49% of the culture-positive children who should have been treated. Evaluation of the MDx/WDx ratios revealed the best performing clinical decision rules to be the Cape Town rule. The WHO guideline, with a sensitivity of 36.5% was the worst performing rule (see Table, Supplemental Digital Content 1).
There is debate about the acceptable level of false-negative rates of clinical decision rules for GAS pharyngitis (28). Taking into account the rarity of reports of penicillin anaphylaxis and absence of GAS penicillin resistance to date, it is desirable to employ clinical decision rules with a low MDx/WDx ratio, which indicates favouring less missed diagnoses at the expense of over-treating non-cases (6). In our study of the existing clinical decision rules for streptococcal pharyngitis, the Steinhoff rule had a favourable MDx/WDx ratio, thereby confirming its suitability for our context despite the potential selection bias in their cohort where pharyngeal erythema needed to be present. The Cape Town rule had the lowest MDx/WDx ratio overall. The four clinical parameters identified by the Cape Town rule were largely similar to those incorporated in the clinical decision rules evaluated. However, the presence of pharyngeal exudate and a temperature >38°C, criteria in the WHO guideline and the McIsaac rule respectively, were absent from our final model.
Our study had a number of strengths: sufficient events were included in developing the clinical decision rule, and the datasets were complete, therefore no imputation for missing data was needed. Furthermore, given that participants were symptomatic individuals seeking care at community clinics, there was no need to establish the carrier rate in asymptomatic individuals from the sampled population (11). Prescribing antibiotic therapy for Cape Town clinical decision rule scores ≥2 would have meant that in excess of 96% of GAS-positive individuals would have been correctly treated in our setting. A sensitive “rule-out” clinical decision rule score was shown to be cost effective in a recent study (9); therefore, a Cape Town clinical decision rule score ≥2 may be an appropriate threshold for antibiotic use in routine clinical practice. However, a limitation of our study was the poor overall diagnostic performance of the new and existing clinical decision rules for streptococcal pharyngitis. An additional limitation was that we could not test performance of some previous clinical decision rules using our data as we had not collected information on some of the predictors included in those clinical decision rules, such as white blood cell count and headache (29), since full blood counts are not available at the clinic as a routine test.
The wide application of the Cape Town clinical decision rule, which incorporated many of the recommendations for building a multivariate prognostic model using regression techniques with empirical data (30) (31), could reduce the incidence of ARF and RHD in sub-Saharan Africa (2, 32).
Acknowledgments
The authors thank the children and their parents for participating in this study, and the local authorities in the Department of Health for permission to conduct this project.
Sources of support: This work was supported by the USPHS National Institutes of Health, UO1AI060592 and RO1AI010085 to J.B.D., the Medical Research Council of South Africa grant to M.E.E. and B.M.M, the Lily and Ernst Hausmann Research Trust, and the Medtronic Foundation.
Footnotes
Authors' Contributions: GM conceived the study and together with JD and BMM gave conceptual advice, interpreted the data and edited the manuscript; MEE, RG and KC wrote the protocol and designed the study. MEE implemented and managed the study, conducted the analysis with APK, MB AS and KC, interpreted the data and wrote the manuscript; KC contributed to the design of the study; VF recruited participants; DDB collected and managed the data and assisted with the analysis. All authors read and approved the final draft of the manuscript.
References
- 1.Robertson KA, Volmink JA, Mayosi BM. Antibiotics for the primary prevention of acute rheumatic fever: a meta-analysis. BMC Cardiovasc Disord. 2005;5:11. doi: 10.1186/1471-2261-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karthikeyan G, Mayosi BM. Is Primary Prevention of Rheumatic Fever the Missing Link in the Control of Rheumatic Heart Disease in Africa? Circulation. 2009;120:709–713. doi: 10.1161/CIRCULATIONAHA.108.836510. [DOI] [PubMed] [Google Scholar]
- 3.Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–1394. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg P, McIsaac W, Macintosh D, Kroll M. Diagnosing streptococcal pharyngitis in the emergency department: Is a sore throat score approach better than rapid streptococcal antigen testing? CJEM. 2002;4:178–184. doi: 10.1017/s1481803500006357. [DOI] [PubMed] [Google Scholar]
- 6.Steinhoff MC, Walker CF, Rimoin AW, Hamza HS. A clinical decision rule for management of streptococcal pharyngitis in low-resource settings. Acta Paediatr. 2005;94:1038–1042. doi: 10.1111/j.1651-2227.2005.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 7.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA : the journal of the American Medical Association. 1997;277:488–494. [PubMed] [Google Scholar]
- 8.Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313:793–799. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 9.Irlam J, Mayosi BM, Engel M, Gaziano TA. Primary prevention of acute rheumatic Fever and rheumatic heart disease with penicillin in South african children with pharyngitis: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2013;6:343–351. doi: 10.1161/CIRCOUTCOMES.111.000032. [DOI] [PubMed] [Google Scholar]
- 10.Fischer Walker CL, Rimoin AW, Hamza HS, Steinhoff MC. Comparison of clinical prediction rules for management of pharyngitis in settings with limited resources. J Pediatr. 2006;149:64–71. doi: 10.1016/j.jpeds.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM. Health in South Africa: changes and challenges since 2009. Lancet. 2012;380:2029–2043. doi: 10.1016/S0140-6736(12)61814-5. [DOI] [PubMed] [Google Scholar]
- 13.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 14.Jasir A, Noorani A, Mirsalehian A, Schalen C. Isolation rates of Streptococcus pyogenes in patients with acute pharyngotonsillitis and among healthy school children in Iran. Epidemiol Infect. 2000;124:47–51. doi: 10.1017/s0950268899003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omurzakova NA, Yamano Y, Sato T, et al. Increased prevalence of group A β-hemolytic streptococcus among an ethnic population in Kyrgyzstan detected by the rapid antigen detection test. Molecular Medicine Reports. 2008:869–874. doi: 10.3892/mmr_00000043. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York; London: Springer; 2001. [Google Scholar]
- 17.Collett D. Modelling binary data. 2nd. New York: Chapman & Hall/CRC; 2003. [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 19.Zuithoff NP, Vergouwe Y, King M, et al. A clinical prediction rule for detecting major depressive disorder in primary care: the PREDICT-NL study. Fam Pract. 2009;26:241–250. doi: 10.1093/fampra/cmp036. [DOI] [PubMed] [Google Scholar]
- 20.McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158:75–83. [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Statistics in medicine. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JF, Cohen R, Levy C, et al. Selective testing strategies for diagnosing group A streptococcal infection in children with pharyngitis: a systematic review and prospective multicentre external validation study. CMAJ. 2015;187:23–32. doi: 10.1503/cmaj.140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinhoff MC, Abd el Khalek MK, Khallaf N, et al. Effectiveness of clinical guidelines for the presumptive treatment of streptococcal pharyngitis in Egyptian children. Lancet. 1997;350:918–921. doi: 10.1016/s0140-6736(97)03317-5. [DOI] [PubMed] [Google Scholar]
- 25.Wald ER, Green MD, Schwartz B, Barbadora K. A streptococcal score card revisited. Pediatr Emerg Care. 1998;14:109–111. doi: 10.1097/00006565-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 26.The management of acute respiratory infections in children : practical guidelines for outpatient care. Geneva: World Health Organization; 1995. [Google Scholar]
- 27.Attia M, Zaoutis T, Eppes S, Klein J, Meier F. Multivariate predictive models for group A beta-hemolytic streptococcal pharyngitis in children. Acad Emerg Med. 1999;6:8–13. doi: 10.1111/j.1553-2712.1999.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 28.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279–1282. doi: 10.1093/cid/cis847. [DOI] [PubMed] [Google Scholar]
- 29.Breese BB. A simple scorecard for the tentative diagnosis of streptococcal pharyngitis. Am J Dis Child. 1977;131:514–517. doi: 10.1001/archpedi.1977.02120180028003. [DOI] [PubMed] [Google Scholar]
- 30.Janssen KJ, Moons KG, Harrell FE., Jr Prediction rules must be developed according to methodological guidelines. Annals of Internal Medicine. 2010;152:263. doi: 10.7326/0003-4819-152-4-201002160-00014. author reply 263-264. [DOI] [PubMed] [Google Scholar]
- 31.Bleeker SE, Moll HA, Steyerberg EW, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol. 2003;56:826–832. doi: 10.1016/s0895-4356(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 32.Watkins DA, Mvundura M, Nordet P, Mayosi BM. A Cost-Effectiveness Analysis of a Program to Control Rheumatic Fever and Rheumatic Heart Disease in Pinar del Rio, Cuba. PLoS ONE. 2015;10:e0121363. doi: 10.1371/journal.pone.0121363. [DOI] [PMC free article] [PubMed] [Google Scholar]


