Abstract
While gait variability may reflect subtle changes due to aging or cognitive impairment (CI), associated brain characteristics remain unclear. We summarize structural and functional neuroimaging findings associated with gait variability in older adults with and without CI and dementia.
We identified 17 eligible studies; all were cross-sectional; few examined multiple brain areas. In older adults, temporal gait variability was associated with structural differences in medial areas important for lower limb coordination and balance. Both temporal and spatial gait variability were associated with structural and functional differences in hippocampus and primary sensorimotor cortex and structural differences in anterior cingulate cortex, basal ganglia, association tracts, and posterior thalamic radiation. In CI or dementia, some associations were found in primary motor cortex, hippocampus, prefrontal cortex and basal ganglia.
In older adults, gait variability may be associated with areas important for sensorimotor integration and coordination. To comprehend the neural basis of gait variability with aging and CI, longitudinal studies of multiple brain areas are needed.
Keywords: gait variability, neuroimaging, older adults, aging, cognitive impairment, dementia
Introduction
While mean performance has been used to study the effects of age on movement and cognition (Beauchet et al. 2016), subtle aspects of development and aging are thought to be better captured by measures of intra-individual variability (Nesselroade 1991). Performance tends to become increasingly consistent through childhood and adolescence, but then becomes more inconsistent with aging (Williams et al. 2005). In aging, intra-individual variability in speed-related performance, especially during neuropsychological tasks, is a known strong predictor of cognitive decline, brain aging and risk of neurodegenerative disease (MacDonald et al. 2006, Bielak et al. 2010).
Mean performance during mobility and gait-related tasks is strongly associated with cognitive decline and predicts cognitive impairment and Alzheimer’s disease (Beauchet et al. 2016). Step-to-step gait variability, measured as fluctuations in the timing (i.e. temporal gait variability) and spacing (i.e. spatial gait variability) of steps, is a major predictor of fall risk and an indicator of impaired executive function and movement control (Hausdorff et al. 2009). As gait variability seems to reflect subclinical changes relevant to aging, cognitive decline and impairment, exploring the structural and functional alterations in the brain that are associated with gait variability might provide clinical and mechanistic insights. However, no systematic review has addressed the relationships between neuroimaging aspects of brain structure and function with gait variability, in usual aging, cognitive impairment or dementia.
Our goal is to review original research examining the relationship between neuroimaging indicators of brain health and gait variability in older adults with and without cognitive impairment and dementia, and to summarize prominent relationships for specific brain regions. While reviewing the literature, we identified gaps in knowledge and challenges in study design, neuroimaging approaches and gait variability assessment that should be addressed in future research.
2. Methods
2.1 Literature search
We searched existing literature through November 2016 using PubMed and PsycINFO databases, as well as references from published manuscripts. Studies were considered eligible if they were based on 1) original data on the relationship between neuroimaging measures of brain structure and function and gait variability, 2) included adults aged 50 and older, and 3) were written in English. Search terms included 1) older adults OR older persons OR older individuals OR elderly OR senior OR aged OR 50 years and older; 2) brain structure OR brain activity OR brain function OR brain metabolism OR neuroimaging marker OR cerebral perfusion OR cerebral blood flow OR amyloid OR gray matter OR white matter OR infarct OR leukoaraiosis OR default mode network OR functional connectivity OR magnetic resonance imaging OR diffusion tensor imaging OR near-infrared spectroscopy OR brain stimulation OR magnetoencephalography OR magnetic resonance spectroscopy; 3) gait variability OR spatiotemporal variability OR step length variability OR stride length variability OR step width variability OR stride width variability OR step time variability OR stride time variability OR stance time variability OR swing time variability OR single support time variability OR double support time variability OR step to step variability OR lap time variation. Studies were initially screened by title and abstract. All sources were merged to form a final listing.
2.2 Selection of Studies
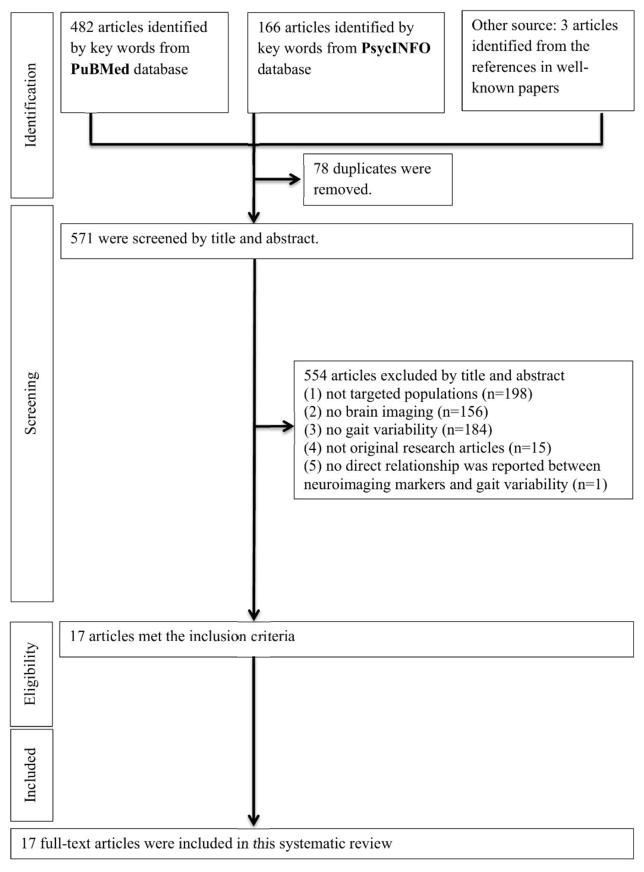
One author (QT) performed the initial search and two authors (QT and SAS) evaluated all articles based on title and abstract. All articles in which the two authors agreed on eligibility were included. There were five articles, all in persons with Parkinson disease, that used brain stimulation as an experimental maneuver to assess effects on gait variability (Hausdorff et al. 2009, Fasano et al. 2010, Thevathasan et al. 2012, Kaski et al. 2013, Vallabhajosula et al. 2015). After discussion, these studies were felt to deserve a separate evaluation and discussion due to the unique pathology and gait characteristics of the disease. In order to focus on older adults without diagnosed neurological conditions and those with cognitive impairment, we also excluded a study of peripheral neuropathy. Overall, of 571 studies initially identified, 555 were excluded by title and abstract because the study (1) did not focus on older adults, (2) lacked brain imaging data, (3) lacked information on gait variability, (4) was not original, or (5) did not assess the relationship between neuroimaging measures and gait variability. The remaining 17 met eligibility criteria and were included in this review (Figure 1).
Figure 1.
Flow chart of literature selection.
2.3 Analysis
For each study, the following data was abstracted (Table 1 and 2); sample characteristics, type of gait variability assessment, type of neuroimaging assessment, covariate adjustment, analytic approach, and a summary of findings. We did not perform statistical analyses or pool data for meta-analyses because the neuroimaging and gait variability methods were quite heterogeneous.
Table 1.
Studies examining the relationship between brain structure and function and gait variability in older adults without diagnosed neurological diseases
| Author, yr | Sample (N, Female%, Age, usual gait) | Gait variability assessment | Neuroimaging assessment | covariates | Analysis | Main findings |
|---|---|---|---|---|---|---|
| Rosano et al. 2007 | N=331 78.3 (4.0) NS |
Step length variability, step width variability, stance time variability (CoV) using the GaitMat II | MRI: infarcts and WM hyperintensities | Age, sex, cognitive function, cardiovascular disease | General | Higher step length variability and stance time variability were associated with greater brain infarcts, asal ganglia infarcts, and severity of WM hyperintensities. There were no significant associations of step width variability with these neuroimaging markers. |
| Srikanth et al. 2009 | N=294, 44.6%F 72.3 (7.0) 1.13m/s |
A summary score consisted of stride length variability, stride time variability, and step width variability (SD) using the GAITRite system | MRI: WM hyperintensities | Age, sex, total brain volume, other physiological variables | General | A higher gait variability score was associated with greater WM lesions. |
| Annweiler et al. 2014 | N=115, 43.5%F 70.4 (4.4) 1.08m/s |
Stride time variability (CoV) using the GAITRite system | MRI: ventricle sub-volumes | Age, sex, cumulative illness rating scale, mini-mental state exam score, go/no-go, brain vascular burden, depressive symptoms, psychoactive drugs, vision, proprioception, body mass index, muscle strength, and gait velocity | General | Higher stride time variability was associated with larger temporal horns. Those in the top tertile of stride time variability had larger middle portions of ventricular bodies than those in the middle tertile of stride time variability. |
| Beauchet et al. 2014 | N=71, 59.7%F 69.0 (0.8) NS |
Stride time variability (CoV) using footswitches | MRI: whole brain GM and WM volumes | Age, sex, body mass index, and total brain matter volume | VBM | Higher stride time variability was associated with smaller right angular gyrus. |
| Beauchet et al. 2015 | N=47, 48.9%F 69.7 (3.6) 1.13m/s |
Stride time variability, swing time variability, and stride width variability (CoV) using the GAITRite system | MRI: Hippocampal volume | Age, sex, body mass index, drugs taken daily, mini-mental state exam score, history of falls, gait speed, and white matter signal-intensity abnormality scoring. | One ROI | Higher stride time variability was associated with greater hippocampal volume. There were no significant associations of swing time variability or stride width variability with hippocampal volume. |
| Manor et al. 2012 | without diabetes: N=89, 51.7%F 63.5(8.2), NS; with type 2 diabetes: N=68, 45.6%F 63.6(7.8), NS. |
Stride time variability (CoV) using footswitches | MRI: Global and 7 ROIs of GM volumes (left and right precentral, postcentral, dorsolateral prefrontal cortex, and cerebellum | Age, sex, body mass | General and multiple ROIs | In those without diabetes, stride time variability was not associated with global or regional volumes. In those with diabetes and without diabetic peripheral neuropathy, higher stride time variability was associated with smaller GM volume and cerebellum. |
| De Laat et al. 2011 | N=484, 43.4% F 65.6 (8.9) 1.3 m/s |
Stride length variability, stride time variability, and stride width variability (CoV) using the GAITRite system | DTI: Fractional anisotropy and mean diffusivity of total WM lesions and normal appearing WM MRI: total brain volume |
Age, sex, height, and total brain volume, additional adjustment for WM lesions | General | Higher stride length variability was associated with higher mean diffusivity of normal appearing WM and smaller brain volume. Higher stride time variability was associated with lower fractional anisotropy and higher mean diffusivity of normal appearing WM and smaller brain volume. Higher stride width variability was associated with higher mean diffusivity of WM lesions. |
| Rosso et al. 2014 | N=265, 57.4%F 82.9 (2.7) 0.91 m/s |
Step length variability (CoV) using the GaitMat II | DTI: Mean diffusivity of 17 GM ROIs (pre- and postcentral gyri, putamen, caudate, thalamus, supplementary motor, precuneus, inferior parietal, pallidum, anterior cingulate, middle frontal gyrus, superior parietal, hippocampus, entorhinal cortex, parahippocampal gyrus, amygdala, posterior cingulate) | Gait speed, demographic, health, functional covariates | Multiple ROIs | The association between step length variability and GM integrity was strongest for the hippocampus and anterior cingulate cortex compared to other ROIs. |
| Tian et al. 2015 | Young-old: N=209, 65.1%F 62.1 (4.9) NS. Old-old: N=233, 48.9%F 78.2 (5.4) NS. |
Lap time variation (detrended SD) from the 400m walk test | DTI: Fractional anisotropy of 9 WM tracts of interest (superior longitudinal, inferior fronto-occipital, and uncinate fasciculi, corpus callosum, anterior limb of the internal capsule, and the anterior corona radiata) | Age, sex, height, weight, global white matter hyperintensities, and mean lap time | Multiple tracts of interest | Independent of WM hyperintensities, higher lap time variation was associated with lower fractional anisotropy in the body of the corpus callosum only in the young-old. |
| Tian et al. 2016 | N=449, 56.8%F 70.8 (9.7) NS. |
Lap time variation (detrended SD) from the 400m walk test | DTI: Mean diffusivity of 16 GM ROIs (pre- and postcentral gyri, supplementary motor cortex, putamen, caudate, thalamus proper, middle frontal gyrus, superior parietal lobe, hippocampus, parahippocampus, entorhinal cortex, amygdala, precuneus, posterior cingulate cortex, anterior and middle cingulate cortices) | Age, sex, height, weight, mean lap time, and additional adjustment for hypertension and diabetes | Multiple ROIs and VBM | Higher lap time variation was associated with higher mean diffusivity of the precuneus, the middle cingulate cortex and the anterior cingulate cortex. |
| Verlinden et al. 2016 | N=2330, 55.1% F 65.9 (9.2) NS |
Stride length variability and stride time variability obtained from principle components analysis (SD) using the GAITRite system | DTI: Fractional anisotropy, mean diffusivity, radial diffusivity, axial diffusivity in 14 WM tracts of interest that were categorized into brainstem, projection, association, limbic, and callosal tracts | Age, age2, sex, height, weight, education, interval between MRI and gait assessment, phase- and frequency-encoding direction of the diffusion scan, intracranial volume, lacunar infarcts, and tract-specific WM and WM lesion volumes | Multiple tracts of interest | Higher stride length variability was associated with higher mean diffusivity across association tracts and the posterior thalamic radiation. |
| Zimmerm an et al. 2009 | N=34, 55.9% F 80.37 (5.77) 0.96m/s |
Stride length variability (SD) using the GAITRite system | MRI: hippocampal volume MRS: NAA/Cr of the hippocampus |
Age, sex, education, ethnicity, weight, midsagittal area, gait speed | One ROI | There were no significant associations between stride length variability and neuronal integrity of the hippocampus or hippocampal volume. |
| Wennberg et al. 2016 | N=611, 49.3% F 62.7 1.22 m/s |
Stance time variability (CoV) using the GAITRite system | PiB-PET: amyloid deposition in 8 ROIs (prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, posterior cingulate, and motor-specific region) | Age, sex, body mass index, education, ApoE ε4 allele, Charlson comorbidity index, depression, and AD-signature neurodegeneration. | Multiple ROI | Higher stance time variability was associated with higher amyloid deposition in all ROIs. After stratification by sex, these associations were only present in women. |
| Shimada et al. 2013 | N=24, 100% F 75–82 NS |
Step length variability (CoV) assessed on a treadmill using an infrared ray device | FDG-PET: brain glucose uptake | None. | Multiple ROIs | The primary sensorimotor cortex was activated more during treadmill walking than the resting condition in low than high variability group. The hippocampus and WM of the middle and superior temporal gyrus was deactivated more during treadmill waking than the resting condition in high than low variability group. |
| Rochester et al. 2012 | N=22, 59.1%F 67.43(8.43) 1.31m/s |
Gait speed variability, stride time variability, stride length variability and step width variability (SD) using the GAITRite system | TMS: Cholinergic function of the motor cortex | Age, motor disease severity, and cognition | One ROI | There was no significant association between short-latency afferent inhibition and gait variability. |
Note: NS=not specified; ROI=regions of interest; SD=standard deviation; CoV=coefficient of variance; NS=not specified; MRS= magnetic resonance spectroscopy; MRI= magnetic resonance imaging; DTI=diffusion tensor imaging; SPECT=Single photon emission computed tomography; VBM=Voxel-based morphometry; WM=white matter; GM=gray matter; TMS=transcranial magnetic stimulation, FDG-PET=fluorodeoxyglucose-positron emission tomography; NAA/Cr=N-acetylaspartate/creatine ratio.
Table 2.
Studies examining the relationship between brain structure and function and gait variability in older adults with cognitive impairment or dementia
| Author, yr | Sample(N, Female%, Age, usual gait) | Gait variability assessment | Neuroimaging assessment | covariates | Analysis | Main findings |
|---|---|---|---|---|---|---|
| Beauchet et al. 2015 | MCI: N=43, 62.8%F. 70.2 (3.7) 1.10 m/s |
Stride time variability, swing time variability, and stride width variability (CoV) using the GAITRite system | MRI: Hippocampal volume | Age, sex, body mass index, drugs taken daily, mini-mental state exam score, history of falls, gait speed, and white matter signal-intensity abnormality scoring. | One ROI | There was no significant association of these gait variability measures with hippocampal volume. |
| Annweiler et al. 2013 | MCI: N=20, 30%F. 76 (11) 1.19 m/s single task; 0.96 m/s dual task |
Stride time variability during single and dual tasking (CoV) using the GAITRite system | MRI: volume of primary motor cortex MRS: NAA/Cr and Cho/Cr of the primary motor cortex. |
Age, sex, body mass index, cognition, education, subcortical vascular burden | One ROI | Higher stride time variability while single tasking was associated with smaller primary motor cortex. Higher stride time variability while dual tasking was associated with loss of neuronal integrity of the primary motor cortex, but not the cell-membrane metabolism. |
| Zimmerman et al. 2009 | Mild memory impairment: N=14, 28.6%F. 83.14 (4.2) 0.88m/s |
Stride length variability (SD) using the GAITRite system | MRI: hippocampal volume MRS: NAA/Cr of the hippocampus |
Age, sex, education, ethnicity, weight, midsagittal area, gait speed | One ROI | Higher stride length variability was associated with loss of neuronal integrity of the hippocampus, independent of gait speed. Stride length variability was not associated with hippocampal volume. |
| Nakamura et al. 1997 | AD: CDR1:N=15,66.7%F. 75.9 (3.6). 0.93m/s CDR2:N=15,73.3%F. 77.5 (4.0). 0.65m/s CDR3:N=15,73.3%F. 78.1 (3.2). 0.57m/s |
Stride length variability (CoV) using reflective markers and a motion analyzer system | SPECT: Regional cerebral blood flow (rCBF) in 12 ROIs (bilateral upper frontal, lower frontal, parietal, occipital, upper temporal, lower temporal lobes, basal ganglia, thalamus, brainstem, cerebellum) | Age, sex, clinical dementia rating, mini-mental state exam score, and disease duration. | Multiple ROIs | At CDR=1, there was no significant association between stride length variability and rCBF. At CDR=2, higher stride length variability was associated with lower rCBF in the cortex and upper and lower frontal lobe. At CDR=3, higher stride length variability was associated with lower rCBF in the cortex, upper and lower frontal lobe and basal ganglia. |
Note: ROI=regions of interest; SD=standard deviation; CoV=coefficient of variance; NS=not specified; MRS= magnetic resonance spectroscopy; MRI= magnetic resonance imaging; DTI=diffusion tensor imaging; SPECT=Single photon emission computed tomography; VBM= Voxel-based morphometry; MCI=mild cognitive impairment; AD=Alzheimer’s disease; CDR=clinical dementia rating; NAA/Cr= N-acetylaspartate/creatine ratio; Cho/Cr=Choline/creatine ratio.
3. Results
The 17 studies are presented in two groups; 1) those focusing on older adults without diagnosed neurological disease and 2) studies of persons with cognitive impairment and dementia (Tables 1 and 2). In four studies, cognitive status was not explicitly characterized as impaired, so were assigned to the group of older adults without neurological disease (Rosano et al. 2007, Srikanth et al. 2009, de Laat et al. 2011, Verlinden et al. 2016). Within each study population type, studies are listed by whether gait variability was characterized by temporal or spatial factors, and then by type of neuroimaging approach. Findings are then summarized according to brain areas associated with temporal and spatial gait variability, respectively (Table 3, Figure 2 and 3).
Table 3.
Global and regional associations between brain structure (underline) and function (italics) with gait variability
| Gait variability (use) | Older adults without diagnosed neurological diseases | Older adults with cognitive impairment or dementia | ||
|---|---|---|---|---|
| Temporal gait variability | General measures | Regional measures | General measures | Regional measures |
| Stride time variability (7) | Total WMH (Srikanth et al. 2009), temporal horns, middle portion of the ventricular bodies (Annweiler et al. 2014), total gray matter volume (Manor et al. 2012). Integrity of normal appearing WM and total brain volume (de Laat et al. 2011) | Volumes of right angular gyrus (Beauchet et al. 2014), hippocampus (counterintuitive) (Beauchet et al. 2015), and cerebellum (Manor et al. 2012). Microstructural integrity of association tracts and the posterior thalamic radiation (Verlinden et al. 2016). | Volume of primary motor cortex (Annweiler et al. 2013), neuronal integrity of
primary motor cortex (Annweiler et al.2013). NS with hippocampal volume (Beauchet et al. 2015). NS with cell-membrane metabolism of the primary motor cortex (Annweiler et al. 2013) |
|
| Lap time variation (2) | Microstructural integrity of anterior cingulate cortex, middle cingulate cortex, precuneus (Tian et al. 2016), body of the corpus callosum (Tian et al. 2015) | |||
| Swing time variability (1) | NS with hippocampal volume (Beauchet et al. 2015) | NS with hippocampal volume (Beauchet et al. 2015) | ||
| Gait speed variability (1) | NS with cholinergic activity of motor cortex (Rochester et al. 2012) | |||
| Stance time variability (2) | Total brain infarcts (Rosano et al. 2007), total WMH (Rosano et al. 2007) | Basal ganglia infarcts (Rosano et al. 2007), amyloid deposition in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, posterior cingulate, and motor-specific regions (primary sensorimotor cortex, supplementary motor cortex, Rolandic operculum) (Wennberg et al. 2016) | ||
| Spatial gait variability | ||||
| Stride length variability (6) | Total WMH (Srikanth et al. 2009). Integrity of normal appearing WM and total brain volume (de Laat et al. 2011) | Microstructural integrity of association tracts and the posterior thalamic radiation (Verlinden et al. 2016). NS with hippocampal volume, NS with neuronal integrity of hippocampus (Zimmerman et al. 2009), NS with cholinergic function of motor cortex (Rochester et al. 2012). | Neuronal integrity of hippocampus (Zimmerman et al. 2009), cerebral blood flow of prefrontal cortex and basal ganglia (Nakamura et al. 1997). NS with hippocampal volume (Zimmerman et al. 2009) | |
| Step length variability (3) | Total brain infarcts, total WMH (Rosano et al. 2007) | Basal ganglia infarcts (Rosano et al. 2007). Integrity of hippocampus and anterior cingulate cortex (Rosso et al. 2014), glucose uptake of primary sensorimotor cortex, hippocampus, WM of the middle and superior temporal gyrus (Shimada et al. 2013) | ||
| Step width variability (3) | Total WMH (Srikanth et al. 2009) NS with brain infarcts or total WMH (Rosano et al. 2007) |
NS with basal ganglia infarcts (Rosano et al. 2007) or with cholinergic function of motor cortex (Rochester et al. 2012) | ||
| Stride width variability (4) | Integrity of WML (de Laat et al. 2011) | NS with hippocampal volume (Beauchet et al. 2015) | NS with hippocampal volume (Beauchet et al. 2015) | |
Note: WM=white matter; WMH=white matter hyperintensities; WML=white matter lesions; NS=not significant. Bold text reflects regional areas that are associated with gait variability.
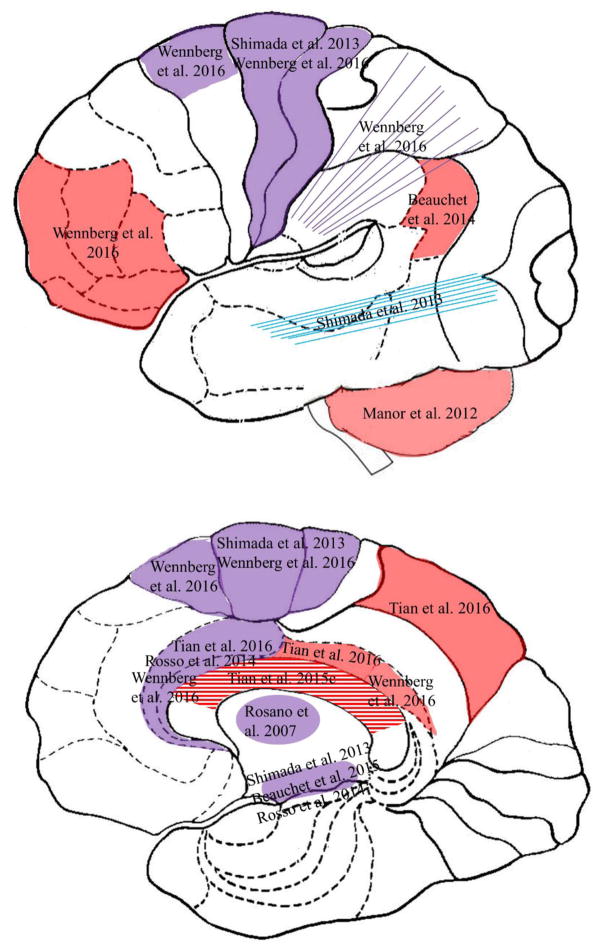
Figure 2. The brain map of gait variabiliy in aging.
Caption: Brain gray matter (solid fill) and white matter (line fill) on sagittal view of the lateral cortex (top) and the medial cortex (bottom), that are associated with temporal gait variability (red), spatial gait variability (blue), and both (purple) in older adults without diagnosed neurological disease.
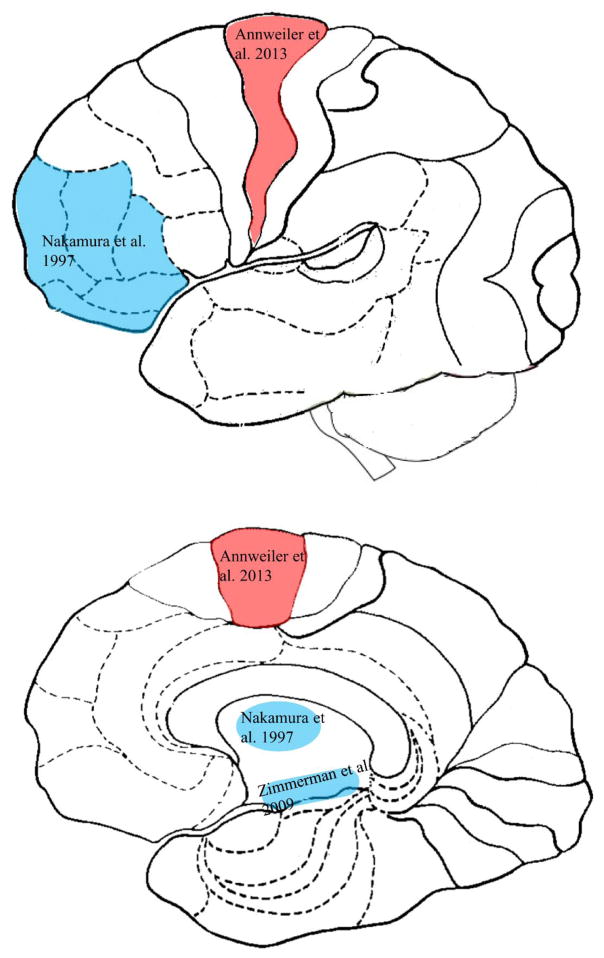
Figure 3. The brain map of gait variability in cognitive impairment or dementia.
Caption: Brain gray matter areas on sagittal view of the lateral cortex (top) and the medial cortex (bottom), that are associated with temporal gait variability (red) and spatial gait variability (blue) in older adults with cognitive impairment or dementia.
3.1 Overview
3.1.1 Study populations and study designs
Of 17 studies, 13 focused on older adults without diagnosed neurological disease (Rosano et al. 2007, Srikanth et al. 2009, de Laat et al. 2011, Manor et al. 2012, Rochester et al. 2012, Shimada et al. 2013, Annweiler et al. 2014, Beauchet et al. 2014, Rosso et al. 2014, Tian et al. 2015, Tian et al. 2016, Verlinden et al. 2016, Wennberg et al. 2016). Two studies reported both older populations without diagnosed neurological disease and with mild memory impairment (Zimmerman et al. 2009) or mild cognitive impairment (MCI) (Beauchet et al. 2015). Two focused on a diagnosed cognitive condition; MCI (Annweiler et al. 2013) or Alzheimer’s disease (AD) (Nakamura et al. 1997). All 17 studies were cross-sectional observational designs.
3.1.2. Assessment of gait variability
Based on the unit of the measurement, we categorized gait variability into temporal and spatial forms. The most frequently used temporal and spatial measures were stride time variability (seven times) and stride length variability (five times). Other temporal gait variability measures included lap time variation (twice), swing time variability (once), gait speed variability (once), and stance time variability (twice). Spatial gait variability measures included step length variability (three times), step width variability (three times), and stride width variability (twice).
Eleven of the 17 studies expressed variability using the coefficient of variation (standard deviation divided by the mean, multiplied by 100) (Nakamura et al. 1997, Rosano et al. 2007, de Laat et al. 2011, Annweiler et al. 2013, Shimada et al. 2013, Annweiler et al. 2014, Beauchet et al. 2014, Rosso et al. 2014, Beauchet et al. 2015, Wennberg et al. 2016), while the remaining six expressed variability using the standard deviation (Srikanth et al. 2009, Zimmerman et al. 2009, Rochester et al. 2012, Tian et al. 2015, Tian et al. 2016, Verlinden et al. 2016).
3.1.3 Assessment of brain structure and function
These studies used diverse imaging techniques. MRI provides volumetric measures of gray matter, white matter, and white matter hyperintensities. DTI quantifies the microstructural integrity of white matter tracts as well as gray matter regions. Brain MRS assesses metabolism in specific brain areas. PET-FDG is widely used to assess glucose uptake in the brain. PiB-PET is used to measure β amyloid deposition, while SPECT quantifies cerebral blood flow (Holtzer et al. 2014).
Of 17 studies, 12 examined brain structure using magnetic resonance imaging (MRI) (Rosano et al. 2007, Srikanth et al. 2009, de Laat et al. 2011, Manor et al. 2012, Annweiler et al. 2014, Beauchet et al. 2014, Beauchet et al. 2015), diffusion tensor imaging (DTI) (de Laat et al. 2011, Rosso et al. 2014, Tian et al. 2016, Tian et al. 2016, Verlinden et al. 2016), and PiB positron emission tomography (PiB-PET) (Wennberg et al. 2016). Five examined brain function using magnetic resonance spectroscopy (MRS) (Zimmerman et al. 2009, Annweiler et al. 2013), positron emission tomography with fluorodeoxyglucose (FDG-PET) (Shimada et al. 2013), single-photon emission computed tomography (SPECT) (Nakamura et al. 1997), or transcranial magnetic stimulation (Rochester et al. 2012).
Of the 17, eight focused on neuroimaging markers of either the brain as a whole (Rosano et al. 2007, Srikanth et al. 2009, de Laat et al. 2011, Annweiler et al. 2014) or one specific brain area (Zimmerman et al. 2009, Rochester et al. 2012, Annweiler et al. 2013, Beauchet et al. 2015). The remaining nine either examined multiple brain areas of interest (Nakamura et al. 1997, Manor et al. 2012, Shimada et al. 2013, Rosso et al. 2014, Tian et al. 2015, Verlinden et al. 2016, Wennberg et al. 2016), or conducted voxel based whole brain analyses (Beauchet et al. 2014), or did both (Tian et al. 2016).
3.1.4 Gait speed adjustment
Six of 17 studies adjusted for gait speed in the analyses; adjustment did not substantially alter the associations between gait variability and brain structure (Annweiler et al. 2014, Rosso et al. 2014, Beauchet et al. 2015, Tian et al. 2015, Tian et al. 2016) or activity (Zimmerman et al. 2009).
3.2 The brain map of gait variability
3.2.1 General neuroimaging findings in older adults without diagnosed neurological disease
None of the studies reported here assessed global neuroimaging findings with gait variability in older adults with cognitive impairment or dementia.
In older adults without diagnosed neurological disease, higher temporal gait variability was associated with several overall volumetric neuroimaging markers, including greater prevalence of silent infarcts (Rosano et al. 2007), greater severity of white matter hyperintensities (Rosano et al. 2007, Srikanth et al. 2009), larger temporal horns, and larger middle portions of the ventricular bodies (Annweiler et al. 2014), smaller total brain volume, and lower microstructural integrity in normal appearing white matter and in white matter lesions (de Laat et al. 2011). One reported that spatial gait variability was associated with smaller gray matter volume in those with type 2 diabetes without diabetic peripheral neuropathy, but not in those without diabetes (Manor et al. 2012). Higher spatial gait variability was associated with greater brain infarcts, increased white matter hyperintensities (Rosano et al. 2007), smaller brain volume, and lower microstructural integrity of normal appearing white matter and white matter lesions (de Laat et al. 2011). Higher step width variability was shown to be associated with greater white matter hyperintensities (Srikanth et al. 2009), while another study did not find this association (Rosano et al. 2007).
3.2.2. Regional neuroimaging findings
3.2.2.1 Older adults without diagnosed neurological disease
Findings about specific regional brain volumes in relation to temporal gait variability were somewhat diverse. Some found that higher temporal gait variability was associated with smaller right angular gyrus (Beauchet et al. 2014) but with greater hippocampal volume, a somewhat counterintuitive finding (Beauchet et al. 2015), while others found no significant associations with regional gray matter volumes, including hippocampus, primary sensorimotor cortex and dorsolateral prefrontal cortex (Manor et al. 2012, Beauchet et al. 2015). In cognitively normal older adults with type 2 diabetes without diabetic peripheral neuropathy, higher temporal gait variability was associated with a smaller cerebellum (Manor et al. 2012). One recent study found that higher temporal gait variability was associated with greater amyloid deposition in the prefrontal, orbitofrontal, parietal, and temporal lobes, anterior and posterior cingulate cortices, and motor-specific regions (primary sensorimotor cortex, supplementary motor cortex, Rolandic operculum (Wennberg et al. 2016). Data on regional microstructural integrity of the brain in relation to temporal gait variability were sparse. Higher temporal gait variability was associated with lower gray matter integrity localized to the anterior cingulate cortex, middle cingulate cortex and precuneus (Tian et al. 2016). Higher temporal gait variability was also associated with lower white matter integrity in the body of the corpus callosum, particularly among the young-old (Tian et al. 2015), as well as lower integrity in the association tracts, and the posterior thalamic radiation (Verlinden et al. 2016). There was no significant association between temporal gait variability and cholinergic function of the motor cortex indicated by short-latency afferent inhibition based on TMS in clinically normal older adults (Rochester et al. 2012). Notably, two studies found non-linear relationships between brain structure and gait variability (Rosano et al. 2007, Srikanth et al. 2009). Those with very high and very low levels of gait variability tended to have greater brain infarcts and larger sub-volume of the middle portion of the ventricular bodies compared to those with intermediate gait variability.
Some studies reported regional associations with spatial gait variability. Higher step length variability but not step width variability, was associated with greater basal ganglia infarcts (Rosano et al. 2007). Several studies examined associations of spatial gait variability with structure and function of the hippocampus. Higher spatial gait variability was associated with lower integrity of the hippocampus (Rosso et al. 2014), but not with hippocampal volume (Beauchet et al. 2015) or neuronal function of the hippocampus (Zimmerman et al. 2009). Higher spatial gait variability was also associated with lower microstructural integrity of the anterior cingulate cortex (Rosso et al. 2014) as well as lower integrity in the association tracts, and the posterior thalamic radiation (Verlinden et al. 2016). There were no significant associations between spatial gait variability and cholinergic function of the motor cortex indicated by short-latency afferent inhibition based on TMS (Rochester et al. 2012). One study of older adults characterized by high and low spatial gait variability compared the change in brain glucose uptake immediately after treadmill walking to a resting condition. During treadmill walking compared to the resting condition, and compared to those with low spatial gait variability, those with high variability showed a greater deactivation in the hippocampus and in the white matter of the middle and superior temporal gyrus (Shimada et al. 2013). In addition, during treadmill walking compared to the resting condition, and compared to those with high gait variability, those with low variability showed more activation in the primary sensorimotor cortex (Shimada et al. 2013).
3.2.2.2 Older adults with cognitive impairment or dementia
Among studies of older persons with mild cognitive impairment, higher temporal gait variability was associated with smaller volume of the primary motor cortex (Annweiler et al. 2013), but not with hippocampal volume (Beauchet et al. 2015). Higher temporal gait variability while performing a dual task was associated with loss of neuronal integrity of the primary motor cortex, indicated by MRS-based NAA/Cr ratio, but not with cell-membrane metabolism indicated by Cho/Cr ratio (Annweiler et al. 2013). In those with cognitive impairment, there was no significant association of spatial gait variability with hippocampal volume (Zimmerman et al. 2009, Beauchet et al. 2015).
In older adults with mild memory impairment, higher spatial gait variability was associated with loss of neuronal integrity of the hippocampus, indicated by MRS-based NAA/Cr ratio (Zimmerman et al. 2009). In a study using SPECT imaging to evaluate cerebral blood flow, older adults with Alzheimer’s disease were classified by clinical dementia rating (CDR) scale as level 2 or 3. In those with a CDR of 2, higher spatial gait variability was associated with lower cerebral blood flow in the prefrontal cortex and in those with a CDR of 3, higher spatial gait variability was associated with lower cerebral blood flow in the basal ganglia and prefrontal cortex (Nakamura et al. 1997).
3.2.2.3 Structured Quality Assessment
Key quality factors assessed included study objectives, study design, representation of the general older populations, older adults with cognitive impairment or dementia, characteristics of study population, sample size, neuroimaging analyses, gait variability assessments, selection of covariates, agreement/disagreement with the literature, strengths and limitations (van Uem et al. 2016).
Overall, the quality of these studies is limited due to their cross-sectional designs, small to moderate sample sizes, and lack of whole-brain analysis and comprehensive gait assessment (Supplementary Table 1).
4. Discussion
In order to characterize the state of knowledge about the relationship between gait variability and alterations in brain structure and function in older adults without diagnosed neurological disease and in those with cognitive impairment or dementia, we identified 17 studies and characterized them in terms of samples, measures and findings. Here we interpret our findings from the perspective of general and regional neuroimaging findings, appraisal of study quality, followed by methodological issues, gaps and next steps.
In older adults without diagnosed neurological disease, general neuroimaging findings suggest that spatial and temporal gait variability is associated with white matter hyperintensities, brain infarcts, larger temporal horns, smaller middle portion of ventricular bodies, reduced total brain volume and, smaller brain volume, and lower microstructural integrity in normal appearing white matter and in white matter lesions (Rosano et al. 2007, Srikanth et al. 2009, de Laat et al. 2011, Manor et al. 2012, Annweiler et al. 2014). No general neuroimaging findings were reported in cognitive impairment or dementia. Regionally, higher temporal gait variability was associated with smaller medial brain areas important for lower limb coordination (middle cingulate cortex, precuneus, body of the corpus callosum) and maintaining balance (angular gyrus, cerebellum, parietal and temporal lobes, supplementary motor cortex, and Rolandic operculum). Both temporal and spatial gait variability was associated with structural and functional differences in the hippocampus and primary sensorimotor cortex, and structural differences in the anterior cingulate cortex, basal ganglia, association tracts, and posterior thalamic radiation. Limited evidence in cognitive impairment and dementia suggests that primary motor cortex, prefrontal cortex, basal ganglia, and hippocampus were important for gait variability.
4.1 Brain areas that are only associated with temporal gait variability
In usual aging, brain areas associated with temporal gait variability were found in medial areas, including the middle cingulate cortex, posterior cingulate cortex, precuneus, and the body of the corpus callosum (Tian et al. 2016, Wennberg et al. 2016). These medial areas play a critical role in bilateral lower limb coordination. Notably, these areas except posterior cingulate cortex were identified by DTI, but not by volumetric MRI. One MRI study using voxel-wise analyses did not find a similar pattern (Beauchet et al. 2014). Prior studies suggest that neuroimaging markers using DTI are considered more sensitive to early brain structural abnormalities compared to conventional volumetric measures (Moseley 2002).
Temporal gait variability was also found to be associated with brain areas important for maintaining balance, including angular gyrus, cerebellum, parietal and temporal lobes, supplementary motor cortex, and Rolandic operculum (Manor et al. 2012, Beauchet et al. 2014, Wennberg et al. 2016). The angular gyrus is located in the posterior parietal lobe, which is involved in the processing of visual, vestibular, and proprioceptive information to maintain balance. It is worth noting that the right hemisphere has consistently shown a relationship with stride time variability; it was associated with the volumes of the right angular gyrus in older adults without diagnosed neurological disease (Beauchet et al. 2014) and of the right basal ganglia in those with diabetic peripheral neuropathy (not included in this review due to the diagnosed neurological condition) (Manor et al. 2012). Prior studies suggest that the right hemisphere is critical for motor control, specifically in monitoring sensorimotor information between the cortex and basal ganglia (Serrien et al. 2006). Several prior reports show that slower gait and poorer balance are associated with smaller cerebellum volume in older adults, suggesting the aging cerebellum plays a critical role in motor control and balance (Rosano et al. 2007, Rosano et al. 2008, Nadkarni et al. 2014). To date, only one study assessed the relationship between gait variability and cerebellar volume (Manor et al. 2012). Findings suggest that the relationship between cerebellar volume and gait variability is affected by the presence of type 2 diabetes.
In mild cognitive impairment, gait variability during a single task is associated with the volume of the primary motor cortex, while gait variability during a dual task is associated with poorer neuronal integrity of the primary motor cortex measured by MRS-based NAA/Cr ratio (Annweiler et al. 2013). Perhaps the dual task condition captures subtle variations in neuronal integrity in MCI, a transitional stage between usual aging and dementia.
4.2 Brain areas that are only associated with spatial gait variability
Areas related only to spatial gait variability were found in the white matter of the middle and superior temporal gyrus among older adults without diagnosed neurological disease (Shimada et al. 2013), and in the prefrontal cortex in AD (Nakamura et al. 1997). White matter of the middle and superior temporal gyrus connects the temporal and occipital lobes which contain visual areas and hippocampus. The functional role of this white matter area in gait is less clear and requires further investigation. The prefrontal cortex is important for executive function and attention. In older adults, poorer executive function and attention were associated with higher spatial gait variability (Holtzer et al. 2012).
4.3 Brain areas in relation to both temporal and spatial gait variability
Several brain areas are associated with both temporal and spatial gait variability, including the primary sensorimotor cortex, hippocampus, anterior cingulate cortex, basal ganglia, association tracts, and posterior thalamic radiation (Rosano et al. 2007, Zimmerman et al. 2009, Rosso et al. 2014, Beauchet et al. 2015, Tian et al. 2016).
The primary sensorimotor cortex contains the primary motor cortex and somatosensory cortex. The primary motor cortex is essential for planning and executing movements, while the somatosensory cortex receives and processes all sensory information, such as visual and auditory stimuli. This brain area plays a key role in movement planning and spatial navigation. The hippocampus is involved in the integration of sensory and motor information. There is also a functional relationship between the hippocampus and the prefrontal cortex through the entorhinal cortex and the nigrostriatal system. The only significant association between temporal gait variability and hippocampal volume in older adults without diagnosed neurological disease is counterintuitive in that larger hippocampal volume was associated with higher variability (Beauchet et al. 2015). One possible explanation is that greater hippocampal volume compensates for higher gait variability in usual aging. Others did not find significant associations between temporal gait variability and hippocampal volume in usual aging (Beauchet et al. 2015), cognitive impairment or dementia (Zimmerman et al. 2009, Beauchet et al. 2015). Spatial gait variability was found to be associated with microstructural integrity and glucose uptake, but not volume or neuronal integrity, of the hippocampus in usual aging (Zimmerman et al. 2009, Shimada et al. 2013, Rosso et al. 2014, Beauchet et al. 2015). Perhaps disrupted microstructural integrity and reduced glucose uptake are early causes of high gait variability.
The anterior cingulate cortex has widespread anatomical projections to the prefrontal cortex, parietal lobe, premotor area, amygdala, and hypothalamus. It subserves a variety of movement-relevant functions, including executive function, attention, decision making, and performance monitoring. The basal ganglia have strong anatomical and functional connections with the cortex, thalamus, and brain stem and play a predominant role in initiating and controlling movement.
Association tracts and the posterior thalamic radiation play an important role in communication among several cortical areas important for gait control, such as frontal, posterior parietal, and occipital cortices (Aralasmak et al. 2006).
4.4 Study Quality
All studies are limited due to their cross-sectional designs and small to moderate sample sizes except Verlinden et al. (Verlinden et al. 2016). Although the Verlinden et al.’s study had a large sample size (2330 dementia-free older adults), there was a gap of up to three years between gait and neuroimaging data collection. Findings are fragmented due to a lack of whole-brain analyses and gait assessment. Only two studies applied whole-brain analyses (Beauchet et al. 2014, Tian et al. 2016). Of 17, six studies examined both temporal and spatial gait variability (Rosano et al. 2007, Srikanth et al. 2009, Rochester et al. 2012, Beauchet et al. 2015, Verlinden et al. 2016).
Without specific detail about cognitive status, four study samples could not be defined as having cognitive impairment so were classified as older adults without diagnosed neurological disease (Rosano et al. 2007, Srikanth et al. 2009, de Laat et al. 2011, Verlinden et al. 2016).
4.5 Gaps in knowledge and future directions
4.5.1 What characteristics of gait variability are needed to understand their relationships to the brain?
Gait variability is a global term that reflects diverse measures that are often related. One way to summarize gait variability is by time and space. The studies in this review often selected specific measures of temporal and spatial gait variability; some provided an explicit rationale (Zimmerman et al. 2009) or clinical justification (Rosano et al. 2007, Annweiler et al. 2014). In general, there is a limited theoretical framework to guide selection of gait variability measures (Lord et al. 2011). While temporal and spatial gait variability might be assessed separately in order to detect underlying mechanisms, some recent studies have used dimension-reduction techniques to generate a global measure (Balasubramanian et al. 2015, Gouelle et al. 2015), or to reduce the number of measures (Verghese et al. 2007, Lord et al. 2013). The global measure has been reported to have high inter-session reliability and is thought to be more sensitive than individual measures (Gouelle et al. 2013).
Classifying gait variability also has analytical challenges. First, gait variability is assessed either from stride-to-stride or from step-to-step. Prior research shows that step-to-step variability has high reliability (Galna et al. 2013) and suggests that step-to-step variability may reveal early functional decline in neurodegenerative diseases (Lord et al. 2011, Lord et al. 2013). Perhaps step-to-step gait variability is more sensitive to early abnormal gait variability because stride-to-stride variability cannot capture the side-to side asymmetry that is commonly seen in usual aging. Future studies should also determine how many steps are needed to assess gait variability with high reliability and validity. Second, either the standard deviation or coefficient of variation is used to reflect variability, but there is no consensus. The standard deviation is sensitive to the scale while the coefficient of variance tends to infinity when the mean is close to zero (Gouelle et al. 2013). Thus, gait variability with low mean values tends to have high coefficient variance (Gabell and Nayak 1984). Both standard deviation and coefficient of variation should be reported until a consensus is reached (Lord et al. 2011, Lord et al. 2013). More cutting edge measures of gait variability are being developed including coefficients reflecting loss of complexity (Ihlen et al. 2016) or smoothness (Brach et al. 2011). Third, gait speed may confound the relationship between neuroimaging findings and gait variability because gait speed is related to both brain health and gait variability. One prior study suggests that stance time variability may depend on gait speed while swing time variability is insensitive to gait speed (Frenkel-Toledo et al. 2005). Future studies, particularly using stance time variability, should evaluate gait speed as a potential confounder.
Traditionally, gait variability is assessed with video motion analysis and/or force plates, systems that are time consuming, cost ineffective, and not applicable in clinical settings. Simpler techniques using accelerometers and foot switches are now available (Lord et al. 2011). An especially simple approach is to assess variability across repeated laps of a long walk, such as lap time variation, which is clinically accessible (Tian et al. 2015, Tian et al. 2016). The relationship between traditional step-to-step variability and lap time variation needs further investigation.
4.5.2 What is the best way to detect subtle abnormal gait variability?
Prior studies suggest that more challenging mobility performance tasks, such as fast-paced or dual task walking, may better capture early brain abnormalities. For example, gait variability at a fast but not usual pace, is associated with mild cognitive impairment (Beauchet et al. 2013). In one study, walking speed at fast not usual pace, predicted future cognitive function (Deshpande et al. 2009). Fast walking may capture further subtle differences in fitness and function (Fitzpatrick et al. 2007, Deshpande et al. 2009, Beauchet et al. 2013). Dual-tasks are also forms of challenge. The dual-task cost (difference in performance between single and dual conditions) may be an early indicator of a functional deficit of high-level cortical motor control (Beurskens and Bock 2012). In the future, dual tasks might help unmask the earliest brain changes associated with high gait variability.
4.5.3 What are early, sensitive neuroimaging markers to indicate abnormal gait variability?
Our understanding of the spatial distribution of brain alterations underlying high gait variability depends on neuroimaging modalities and their interpretations. The structural data are primarily obtained by MRI and DTI, while functional data can be obtained by various neuroimaging techniques, such as fMRI, MRS, MEG, and PET. Only a few studies applied multimodality techniques to assess the relationship of brain structure and activity simultaneously with gait variability (Zimmerman et al. 2009, Annweiler et al. 2013). Studies in this review suggest that microstructural integrity of selected brain areas, rather than the volume, is associated with gait variability (Zimmerman et al. 2009, Rosso et al. 2014, Beauchet et al. 2015). Neuronal integrity indicated by MRS-based NAA/Cr ratio, as opposed to macroscopic structure, is associated with gait variability (Zimmerman et al. 2009). To detect early brain changes, future studies should focus on microstructure, neuronal integrity, and glucose uptake in the brain.
Two studies reported non-linear trends of brain structure and temporal gait variability (Rosano et al. 2007, Annweiler et al. 2014). One author suggested that very high and very low levels of gait variability might indicate abnormal cortical control due to brain abnormalities while intermediate gait variability is considered “normal” (Rosano et al. 2007). Another study suggested that those with intermediate gait variability who had better brain structure are compensating for increased gait variability compared to those with low gait variability (Annweiler et al. 2014). Future longitudinal studies with neuroimaging assessments of both brain structure and function, testing for nonlinear gait variability are needed to understand potential compensatory mechanisms.
Brain changes are important because they may affect the approach to treatment of gait abnormalities. In one clinical trial, older individuals with white matter disease benefitted more from a motor learning than a traditional exercise intervention (Brach et al. 2013). Knowledge gained from neuroimaging assessment may help formulate a tailored, individualized intervention strategy.
4.5.4 Is there a brain network underlying high gait variability?
The bulk of neuroimaging evidence is limited to either a global assessment or a specific brain region. Could there be one or more brain networks where changes affect gait variability? In order to determine which brain areas and networks are associated with gait variability, a broader evaluation of multiple brain regions is needed. Even if there is a predefined region-specific hypothesis, it is still useful to examine other brain areas. Since there is a real risk of false positive findings due to multiple testing, a sensitivity analysis, such as voxel-based morphometry (VBM), might provide complementary evidence. To date, only our group has applied both regions-of-interest (ROI) and VBM approaches to explicitly confirm findings related to the pattern of relationships (Tian et al. 2016). Regarding brain function, only one study applied a voxel-based approach (Shimada et al. 2013). Future studies should consider examining multiple brain areas or applying a voxel-based approach to identify whether there are brain networks underlying high gait variability. Research on functional connectivity of the brain using resting state functional MRI (rs-fMRI) is advancing rapidly (van den Heuvel and Hulshoff Pol 2010). Whether functional connectivity of specific brain areas or networks is associated with high gait variability has not been explored. This neuroimaging tool may add value in understanding brain networks underlying abnormal gait variability. Some imaging approaches have constraints as well as advantages. For example, magnetic resonance spectroscopy (MRS) is limited in the ability to assess multiple brain regions because data acquisition takes a long time.
4.5.5 How does brain function during walking relate to gait variability?
The ability to assess brain function during walking rather than separately is evolving. Initial neuroimaging evidence shows greater activation in selected cortical areas during imagined walking in older adults compared to young adults (Hamacher et al. 2015). Imagined walking may induce brain activations similar to real world walking but we do not yet know how brain function during walking relates to gait variability. Some investigators now use near infrared spectroscopy (NIRS), which allows simultaneous assessment of brain activity and gait, but this technique is often limited to surface brain areas and sometimes only the prefrontal areas (Hoshi 2003).
4.5.6 Can future clinical trials help elucidate the role of the brain in gait variability?
Interventions that affect brain function might be used as probes into the causes and treatment of gait variability. One brain area, particularly in Parkinson’s disease, that plays a key role in gait and falls, is the brainstem pedunculopontine nucleus (PPN). Cholinergic deficits in PPN and the thalamus are associated with gait problems and falls (Bohnen et al. 2009, Ferraye et al. 2010, Kucinski and Sarter 2015). Promoting cholinergic function might be a strategy to treat gait problems. A recent clinical trial suggests that anti-dementia treatment (i.e. acetylcholinesterase inhibitors or memantine, an NMDA (N-methyl-D-aspartate) receptor blocker) improves gait variability in older adults with possible or probable AD (Beauchet et al. 2014). Another clinical trial suggests that an acetylcholinesterase inhibitor improves step time variability in patients with Parkinson’s disease (Henderson et al. 2016). Given the high priority placed on developing interventions to prevent or delay cognitive impairment and dementia, future clinical trials in this area should incorporate measures of gait and gait variability.
Supplementary Material
Highlights.
Gait variability in aging is associated with altered brain structure and function.
Sensorimotor integration and coordination areas are most often altered.
In cognitive impairment and dementia, general and regional findings are limited.
Longitudinal studies of multiple brain areas are warranted to understand mechanisms.
Acknowledgments
Funding:
This research was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (grant number: 03-AG-0325).
Footnotes
Conflicts of Interest:
No conflicts of interest are declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annweiler C, et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain. 2013;136(Pt 3):859–871. doi: 10.1093/brain/aws373. [DOI] [PubMed] [Google Scholar]
- Annweiler C, et al. Motor cortex and gait in mild cognitive impairment: A magnetic resonance spectroscopy and volumetric imaging study. Brain: A Journal of Neurology. 2013;136(3):859–871. doi: 10.1093/brain/aws373. [DOI] [PubMed] [Google Scholar]
- Annweiler C, et al. Association between gait variability and brain ventricle attributes: a brain mapping study. Exp Gerontol. 2014;57:256–263. doi: 10.1016/j.exger.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Aralasmak A, et al. Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr. 2006;30(5):695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, et al. Validity of the gait variability index in older adults: effect of aging and mobility impairments. Gait Posture. 2015;41(4):941–946. doi: 10.1016/j.gaitpost.2015.03.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, et al. Gait variability at fast-pace walking speed: a biomarker of mild cognitive impairment? J Nutr Health Aging. 2013;17(3):235–239. doi: 10.1007/s12603-012-0394-4. [DOI] [PubMed] [Google Scholar]
- Beauchet O, et al. Poor Gait Performance and Prediction of Dementia: Results From a Meta-Analysis. J Am Med Dir Assoc. 2016 doi: 10.1016/j.jamda.2015.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, et al. Higher gait variability is associated with decreased parietal gray matter volume among healthy older adults. Brain Topography. 2014;27(2):293–295. doi: 10.1007/s10548-013-0293-y. [DOI] [PubMed] [Google Scholar]
- Beauchet O, et al. Higher gait variability is associated with decreased parietal gray matter volume among healthy older adults. Brain Topogr. 2014;27(2):293–295. doi: 10.1007/s10548-013-0293-y. [DOI] [PubMed] [Google Scholar]
- Beauchet O, et al. Gait changes with anti-dementia drugs: a prospective, open-label study combining single and dual task assessments in patients with Alzheimer’s disease. Drugs Aging. 2014;31(5):363–372. doi: 10.1007/s40266-014-0175-3. [DOI] [PubMed] [Google Scholar]
- Beauchet O, et al. Hippocampal volume, early cognitive decline and gait variability: which association? Exp Gerontol. 2015;61:98–104. doi: 10.1016/j.exger.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Beurskens R, Bock O. Age-related deficits of dual-task walking: a review. Neural Plast. 2012;2012:131608. doi: 10.1155/2012/131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak AA, et al. Intraindividual variability is related to cognitive change in older adults: evidence for within-person coupling. Psychol Aging. 2010;25(3):575–586. doi: 10.1037/a0019503. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, et al. Validation of a measure of smoothness of walking. J Gerontol A Biol Sci Med Sci. 2011;66(1):136–141. doi: 10.1093/gerona/glq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, et al. Motor learning versus standard walking exercise in older adults with subclinical gait dysfunction: a randomized clinical trial. J Am Geriatr Soc. 2013;61(11):1879–1886. doi: 10.1111/jgs.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat KF, et al. Diffusion tensor imaging and gait in elderly persons with cerebral small vessel disease. Stroke. 2011;42(2):373–379. doi: 10.1161/STROKEAHA.110.596502. [DOI] [PubMed] [Google Scholar]
- Deshpande N, et al. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38(5):509–514. doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain. 2010;133(Pt 12):3635–3648. doi: 10.1093/brain/awq267. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133(Pt 1):205–214. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- Frenkel-Toledo S, et al. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. J Neuroeng Rehabil. 2005;2:23. doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabell A, Nayak US. The effect of age on variability in gait. J Gerontol. 1984;39(6):662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- Galna B, et al. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture. 2013;37(4):580–585. doi: 10.1016/j.gaitpost.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Gouelle A, et al. Changes in Gait Variability From First Steps to Adulthood: Normative Data for the Gait Variability Index. J Mot Behav. 2015:1–7. doi: 10.1080/00222895.2015.1084986. [DOI] [PubMed] [Google Scholar]
- Gouelle A, et al. The gait variability index: a new way to quantify fluctuation magnitude of spatiotemporal parameters during gait. Gait Posture. 2013;38(3):461–465. doi: 10.1016/j.gaitpost.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Hamacher D, et al. Brain activity during walking: A systematic review. Neurosci Biobehav Rev. 2015;57:310–327. doi: 10.1016/j.neubiorev.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, et al. Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov Disord. 2009;24(11):1688–1692. doi: 10.1002/mds.22554. [DOI] [PubMed] [Google Scholar]
- Henderson EJ, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(3):249–258. doi: 10.1016/S1474-4422(15)00389-0. [DOI] [PubMed] [Google Scholar]
- Holtzer R, et al. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69(11):1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, et al. The relationship between attention and gait in aging: facts and fallacies. Motor Control. 2012;16(1):64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40(4):511–520. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- Ihlen EA, et al. The complexity of daily life walking in older adult community-dwelling fallers and non-fallers. J Biomech. 2016;49(9):1420–1428. doi: 10.1016/j.jbiomech.2016.02.055. [DOI] [PubMed] [Google Scholar]
- Kaski D, et al. Improving gait and balance in patients with leukoaraiosis using transcranial direct current stimulation and physical training: an exploratory study. Neurorehabil Neural Repair. 2013;27(9):864–871. doi: 10.1177/1545968313496328. [DOI] [PubMed] [Google Scholar]
- Kucinski A, Sarter M. Modeling Parkinson’s disease falls associated with brainstem cholinergic systems decline. Behav Neurosci. 2015;129(2):96–104. doi: 10.1037/bne0000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord S, et al. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68(7):820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- Lord S, et al. Gait variability in older adults: a structured review of testing protocol and clinimetric properties. Gait Posture. 2011;34(4):443–450. doi: 10.1016/j.gaitpost.2011.07.010. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, et al. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29(8):474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Manor B, et al. The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy. Diabetes Care. 2012;35(9):1907–1912. doi: 10.2337/dc11-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15(7–8):553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Nadkarni NK, et al. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2014;69(8):996–1003. doi: 10.1093/gerona/glt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11(3):132–139. doi: 10.1097/00002093-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Nakamura T, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Disease and Associated Disorders. 1997;11(3):132–139. doi: 10.1097/00002093-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR. The warp and the woof of the developmental fabric. In: Downs RLL, Palermo DS, editors. Visions of aesthetics, the environment, and development: The legacy of Joachim F. Wohlwill. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Rochester L, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain: A Journal of Neurology. 2012;135(9):2779–2788. doi: 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 2012;135(Pt 9):2779–2788. doi: 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, et al. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, et al. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rosano C, et al. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3–4):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, et al. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40(1):225–230. doi: 10.1016/j.gaitpost.2014.03.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, et al. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40(1):225–230. doi: 10.1016/j.gaitpost.2014.03.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, et al. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006;7(2):160–166. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Shimada H, et al. Gait adaptability and brain activity during unaccustomed treadmill walking in healthy elderly females. Gait Posture. 2013;38(2):203–208. doi: 10.1016/j.gaitpost.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Srikanth V, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40(1):175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, et al. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain. 2012;135(Pt 5):1446–1454. doi: 10.1093/brain/aws039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, et al. The effect of age and microstructural white matter integrity on lap time variation and fast-paced walking speed. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, et al. The effect of age and microstructural white matter integrity on lap time variation and fast-paced walking speed. Brain Imaging and Behavior. 2016;10(3):697–706. doi: 10.1007/s11682-015-9449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, et al. Lower gray matter integrity is associated with greater lap time variation in high-functioning older adults. Exp Gerontol. 2016;77:46–51. doi: 10.1016/j.exger.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhajosula S, et al. Low-frequency versus high-frequency subthalamic nucleus deep brain stimulation on postural control and gait in Parkinson’s disease: a quantitative study. Brain Stimul. 2015;8(1):64–75. doi: 10.1016/j.brs.2014.10.011. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van Uem JM, et al. Health-Related Quality of Life in patients with Parkinson’s disease--A systematic review based on the ICF model. Neurosci Biobehav Rev. 2016;61:26–34. doi: 10.1016/j.neubiorev.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Verghese J, et al. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden VJA, et al. Tract-specific white matter microstructure and gait in humans. Neurobiology of Aging. 2016;43:164–173. doi: 10.1016/j.neurobiolaging.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Wennberg AM, et al. Cerebral Amyloid Deposition Is Associated with Gait Parameters in the Mayo Clinic Study of Aging. J Am Geriatr Soc. 2016 doi: 10.1111/jgs.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, et al. Inconsistency in reaction time across the life span. Neuropsychology. 2005;19(1):88–96. doi: 10.1037/0894-4105.19.1.88. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, et al. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Research. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, et al. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.