Abstract
A major challenge in attaching fluorophores or other handles to proteins is the availability of a site-specific labeling strategy that provides stoichiometric modification without compromising protein integrity. We developed a simple approach that combines TEV protease cleavage, sortase modification and affinity purification to N-terminally label proteins. To achieve stoichiometrically-labeled protein, we included a short affinity tag in the fluorophore-containing peptide for post-labeling purification of the modified protein. This strategy can be easily applied to any recombinant protein with a TEV site and we demonstrate this on Epidermal Growth Factor Receptor (EGFR) and Membrane Scaffold Protein (MSP) constructs.
Keywords: sortase, post-labeling purification, TEV, LPXTG, affinity tag
Site-specific protein labeling is the method of choice for most biochemical and biophysical applications, as this offers a high level of precision for the attachment of a fluorophore or other chemical moiety [1,2]. Due to the relatively low abundance of cysteines in proteins [3], chemical labeling of proteins using maleimide chemistry is a common strategy for most applications. However, many proteins contain multiple cysteine residues and mutagenesis of these cysteines is time-consuming and may compromise protein function. An alternate approach is to label primary amines with N-hydroxysuccinimide ester-based fluorophores. However, the relatively high abundance of lysines and pKa requirements renders the utility of amino groups for protein modification a less commonly used strategy. These challenges are compounded by long reaction times to ensure complete modification of the protein. With fluorophores having a MW of <1kDa, separation of labeled products from the unlabeled protein can also present challenges. Sub-stoichiometric labeling often results in a diminished signal-to-noise ratio and impacts the utility of fluorophore-labeled proteins for biophysical studies.
Enzymatic approaches for site-specific incorporation of fluorophores are an alternative to these chemical labeling strategies. Sortases are membrane-associated transpeptidases that anchor Gram-positive bacterial surface proteins to their cell walls. Since the discovery of sortases, Staphylococcus aureus sortase A (SrtA) has been the prototype for understanding the mechanism of action of these enzymes [4]. Proteins anchored to the cell wall by SrtA possess a C-terminal sorting signal that contains a hydrophobic domain sandwiched between the conserved LPXTG recognition motif and a positively charged tail [4]. SrtA catalyses the hydrolysis of the peptide bond between the threonine and glycine residues to generate an acyl-enzyme intermediate that is subsequently attacked by an oligoglycine peptide in a nucleophilic attack [5]. This results in the formation of a new peptide bond between the incoming nucleophilic glycine-containing peptide and the protein. Seminal work by Schneewind and coworkers laid the ground for its utility in biochemical and biotechnological studies showed a recombinant peptide containing the LPXTG motif alone is sufficient for recognition and catalysis [6]. These studies also indicated that a peptide containing 1–3 N-terminal glycines could replace the peptidoglycan involved in the sortase-mediated reaction [6]. Current biochemical evidence has suggested that only one additional residue (preferably a glycine) is required at the C-terminus of the LPXTG recognition sequence for efficient sortase binding and catalysis [7].
Most recombinant proteins used for biochemical and biotechnological applications contain affinity tags that ensure their easy and efficient purification [8,9]. Sandwiched between the affinity tags and the proteins are protease recognition sites that offer the cleavage of the affinity tags following purification. Commonly used recognition sites include TEV, Factor Xa, and Thrombin protease cleavage sites. An important requirement for the sortase reaction is the generation of the N-terminal glycine residue which can be done by removing the initial methionine of an expressed protein using methionylaminopeptidase or engineering a thrombin or TEV protease recognition site that exposes an N-terminal glycine following cleavage [10].
Recent years have seen the development and utility of sortase to modify proteins at their carboxyl and amino termini in addition to internal loops [7,11]. Unlike traditional chemical strategies that are easy to use, protein modification employing short genetically encoded tags such as the LPXTG-tag, ACP-tag and LAP-tag offer a high degree of precision. However, back reaction from the final product (containing the LPXTG motif and therefore an efficient substrate for the sortase enzyme) and the reversible nature of the sortase reaction can lead to sub-stoichiometric protein modification and decreased labeling efficiencies. To address this challenge, the equilibrium of the reaction is driven towards product formation by increasing the fold excess of the fluorophore-containing peptide [7,11]. Recent methodologies to address this issue of irreversibility have included the use of a sortase-tagged expressed protein ligation (STEPL) system that circumvents the removal of unconjugated species [12], dialysis to remove reaction by-products [13] and the introduction of tryptophan-derived zippers around the SrtA recognition motif that induces the formation of a stable β-hairpin [14]. Other research groups have solved this problem by utilizing a depsipeptide, which replaces the amide bond between the threonine and glycine residues with an ester linkage [15]. These challenges make protein modification using sortase cumbersome and potentially expensive when the fluorophore-containing peptide is needed in many fold excess. The presence of reaction by-products as a result of back reaction and the reversible nature of the reaction affect the purity and degree of labeling of the final product, and subsequently present challenges in the utilization of fluorophore-labeled proteins.
We have developed a simple approach that combines TEV protease cleavage, sortase modification and affinity purification to N-terminally label proteins. To achieve stoichiometrically-labeled product, a short affinity tag is included in the fluorophore-containing peptide so that post-labeling affinity purification of only the labeled protein can be performed.
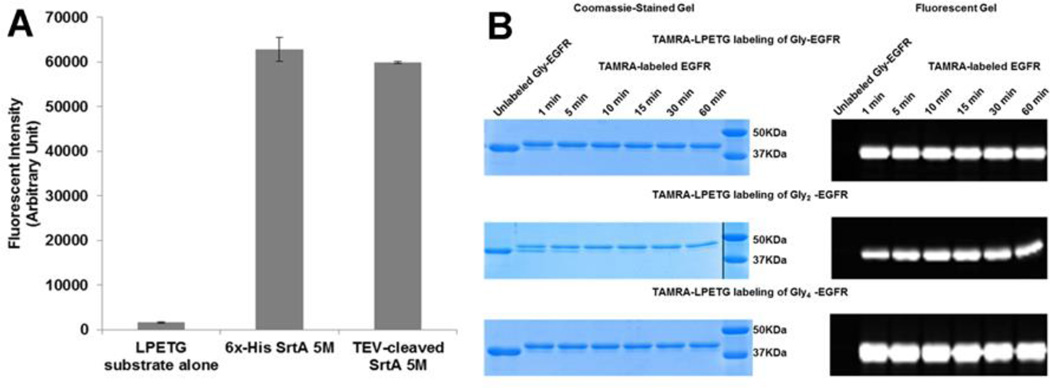
We used a Staphylococcus aureus Sortase pentamutant (SrtA 5M) that had previously undergone directed evolution to be catalytically more efficient [16]. SrtA 5M contains five mutations and has a 140-fold increase in transpeptidase activity over wildtype SrtA. We engineered SrtA 5M to have a TEV cleavage site before the C-terminal histidine tag, in order to facilitate our downstream purification strategy. This TEV-cleaved SrtA 5M (lacking a histidine tag) has catalytic activity essentially identical to the original SrtA 5M construct (Fig. 1A).
Fig. 1. The use of TEV-cleaved SrtA 5M to label EGFR containing varying N-terminal glycines.
A. Comparing sortase activity of 6x-His SrtA 5M and TEV-cleaved SrtA 5M. B. Comparison of labeling of Gly-EGFR, Gly2-EGFR and Gly4-EGFR using an LPETG peptide substrate containing the TAMRA fluorophore.
We expressed and purified a recombinant Epidermal Growth Factor Receptor kinase domain (EGFR KD) that contains an N-terminal polyhistidine tag and a TEV cleavage site, ENLYFQG. It is important to note that the ENLYFQS sequence for the TEV protease should not be used in this approach because the resulting N-terminal serine residue is not an effective substrate for SrtA 5M. TEV protease cleavage of this EGFR construct yields a glycine residue at the N-terminus and we will call this protein, Gly-EGFR. We have optimized the TEV protease cleavage procedure to give complete cleavage of this EGFR construct in 6 to 8 hours. We tested whether additional N-terminal glycines (Gly2-EGFR and Gly4-EGFR) are better substrates in the sortase-mediated reaction. Our results using a TAMRA-labeled LPETGG peptide showed that the labeling of Gly-EGFR, Gly2-EGFR, and Gly4-EGFR were very similar (Fig. 1B). The addition of several N-terminal glycine residues had no effect on the efficiency of the reaction and all future experiments utilized one N-terminal glycine for the sortase-mediated labeling reaction.
N-terminal labeling of Gly-EGFR using sortase and a short peptide (that contains the fluorophore and the sortase recognition motif) initially resulted in <60% modification of the protein with the labeled peptide (data not shown). This was unsatisfactory as unlabeled protein decreases the signal-to-noise ratio and complicates data analysis and experimental interpretation. We hypothesized that the addition of an affinity tag to the short peptide would enable the efficient purification of the labeled EGFR. We designed a peptide that had a 6x histidine tag (for purification of labeled EGFR from unlabeled EGFR), a cysteine residue for maleimide labeling (we also utilized the NH2-amino group of the peptide for labeling with NHS-succinimidyl esters) and an LPETGG motif at the C-terminus. This 25-mer peptide (MSYYHHHHHHDYDIPTCENLPETGG or H6-LPETGG peptide, hereafter) was fluorophore-labeled at either the N-terminal methionine residue or at the internal cysteine residue. Labeling of Gly-EGFR with the H6-LPETGG peptide results in the regeneration of the sequence N-terminal to the EGFR that was cleaved off during TEV protease digestion (albeit subtle mutations at the C-terminus of the peptide, ENLPETG instead of the original ENLYFQG).
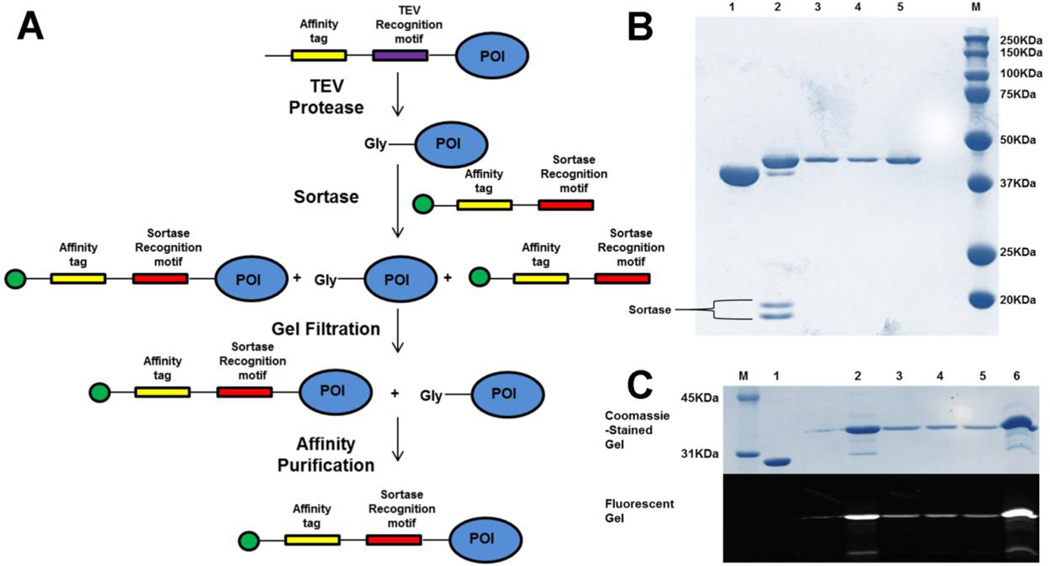
Our proposed labeling strategy (Fig. 2A) proceeds by mixing the 0.5mM fluorophore-labeled H6-LPETGG peptide with 25uM Gly-EGFR (300ug) and 1uM SrtA 5M (lacking a 6× Histidine tag) in a sortase buffer containing 50mM Tris-HCl pH 7.5, 150mM NaCl, and 10mM CaCl2. Following incubation at 4°C for 30minutes, the reaction is quenched with 10mM EDTA. We tested the chelating effect of EDTA and EGTA and showed that both chelators at 10mM concentration were sufficient in quenching the activity of sortase. The reaction mixture which contains labeled EGFR, unlabeled EGFR, the H6-LPETGG peptide and the sortase enzyme is loaded onto a Superdex 75 10/30 GL column (GE Healthcare Life Sciences, Pittsburg, PA) to remove the excess H6-LPETGG peptide and sortase enzyme. Fractions containing labeled and unlabeled EGFR are pooled together and incubated with Nickel NTA beads. After 20 minutes, the flowthrough (containing unlabeled EGFR) is discarded while the bound labeled EGFR is eluted with imidazole. Fig. 2B shows the use of this strategy to site specifically label TEV protease-cleaved EGFR KD with the H6-LPETGG peptide labeled with a quencher (CruzQuencher™1 Maleimide, from Santa Cruz Biotechnology, Santa Cruz, CA). Our protein recovery from labeling 300ug of EGFR was 140ug, resulting in ~50% protein yield. The absorbance of the fluorophore (Amax) and the labeled protein (A280) together with the molar extinction coefficients of the fluorophore (εmax) and protein (εprotein) allow us to calculate the moles of dye per mole protein or the degree of labeling (DOL) using the equation below;
The correction factor (CF) is included in this equation to account for the absorption of fluorophore at 280 nm and equals the A280 of the dye divided by the Amax of the dye. We have consistently achieved >0.95 moles of dye per mole of protein in our purified labeled EGFR. Following labeling and purification, in vitro kinase assays of unlabeled and fluorophore-labeled EGFR kinase domains show essentially identical enzyme activity (data not shown).
Fig. 2. Strategy for N-terminal labeling of TEV-cleavable proteins using sortase.
A. Schematic representation for stoichiometric N-terminal labeling of proteins. Green circle at the N-terminus of peptide indicates the fluorophore or any other desired moiety. Affinity tag used in here was 6x-His. However, this tag can be varied and the purification process can be made more stringent by using two different affinity tags. As noted in the text, the TEV recognition motif used must be ENLYFQG rather than ENLYFQS. B. Coomassie-stained gel showing the labeling and purification of EGFR kinase domain using an LPETG peptide substrate containing CruzQuencher maleimide. Lanes; 1-Unlabeled EGFR KD, 2-Reaction mix after 30mins, 3-Fraction from Gel filtration, 4-Eluted protein from Ni-NTA beads, 5-Final concentrated labeled protein. C. Coomassie and fluorescent gels showing the labeling and purification of MSP protein using the H6-LPETGG peptide substrate containing fluorescein. Lanes; 1-Unlabeled MSP, 2-Reaction mix after 30mins, 3,4-Fractions from Gel filtration, 5-Eluted protein from Ni-NTA beads, 6-Final concentrated labeled protein.
To show the generality and applicability of this labeling strategy for other proteins, we labeled the membrane scaffold protein MSP1E3D1 using sortase. MSP constructs are used to make nanodiscs and MSP1D1 has been site-specifically labeled using sortase and a fluorescein-labeled depsipeptide [17]. MSP1E3D1 in particular generates a 12.1 nm bilayer disc and has been useful in solubilizing and studying membrane proteins in their functional forms [18]. TEV protease cleaved MSP1E3D1 (25uM) was labeled with 1uM sortase and a 20-fold molar excess of the fluorescein-labeled H6-LPETGG peptide (reaction time: 30 minutes at 4°C), resulting in >95% purity after post-labeling purification (Fig. 2C).
We have demonstrated that TEV-cleaved recombinant proteins are good substrates for SrtA. Addition of an affinity tag to the peptide substrate enables the efficient purification of labeled product, resulting in >95% purity. The ability to site-specifically modify proteins with fluorophores to stoichiometric proportions is highly desirable for downstream biophysical studies.
The epidermal growth factor receptor, EGFR has been the archetypical protein for studying receptor tyrosine kinases. EGFR and its orthologues (HER2, HER3 and HER4) have been known to play significant roles in tumorigenesis. Our site-specific fluorophore-labeled EGFR kinase domain offers a biological tool that can be used to answer mechanistic questions regarding protein-lipid and protein-protein interactions by receptor tyrosine kinases. Further, the development of the nanodisc technology by Sligar and colleagues has allowed for biophysical studies of proteins in a native bilayer environment. Our fluorescein-labeled MSP1E3D1 broadens the utility and applicability of nanodiscs by providing a toolbox to use bulk and single molecule fluorescent spectroscopy to study conformational dynamics and macromolecular interactions between lipids and proteins.
In conclusion, the N-terminal amino group offers unique functional properties for site-specific labeling of proteins. Our N-terminal labeling strategy provides a robust and straightforward protocol for functionalizing TEV protease cleaved recombinant proteins via a sortase-mediated reaction.
Highlights.
TEV protease cleaved proteins are amenable to sortase-mediated labeling
Post-labeling purification using an affinity tag in the peptide substrate results in >95% purity of the labeled protein
Acknowledgments
This work was supported by National Institutes of Health Grants R01 CA161001 (to R.B.). We thank Dr. Hidde L. Ploegh for providing the SrtA 5M plasmid and Drs. Thomas E. Kraft and Paul W. Hruz for providing purified MSP1E3D1 protein. We also want to thank Dr. James W. Janetka for assistance with peptide synthesis. The plasmid for SrtA 5M with TEV cleavage site has been deposited at Addgene (plasmid number 86962 https://www.addgene.org/Ron_Bose/).
Abbreviations used
- TEV
Tobacco Etch Virus
- EGFR
epidermal growth factor receptor
- MSP
membrane scaffold protein
- HER
Human Epidermal growth factor Receptor
- KD
kinase domain
- SrtA
Sortase A
- ACP
acyl carrier protein
- LAP
Lipoid acid ligase Acceptor Protein
- TAMRA
5-carboxytetramethylrhodamine
- EDTA
Ethylenediaminetetraacetic acid
- EGTA
Ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
- NTA
nitrilotriacetic acid
References
- 1.Carrico IS. Chemoselective modification of proteins: hitting the target. Chem. Soc. Rev. 2008;37:1423–1431. doi: 10.1039/b703364h. [DOI] [PubMed] [Google Scholar]
- 2.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miseta A, Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol. Biol. Evol. 2000;17:1232–1239. doi: 10.1093/oxfordjournals.molbev.a026406. [DOI] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 5.Ton-That H, Liu G, Mazmanian SK, Faull KF, Schneewind O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA. 1999;96:12424–12429. doi: 10.1073/pnas.96.22.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ton-That H, Mazmanian SK, Faull KF, Schneewind O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. J. Biol. Chem. 2000;275:9876–9881. doi: 10.1074/jbc.275.13.9876. [DOI] [PubMed] [Google Scholar]
- 7.Theile CS, Witte MD, Blom AE, Kundrat L, Ploegh HL, Guimaraes CP. Site-specific N-terminal labeling of proteins using sortase-mediated reactions. Nat. Protoc. 2013;8:1800–1807. doi: 10.1038/nprot.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichty JJ, Malecki JL, Agnew HD, Michelson-Horowitz DJ, Tan S. Comparison of affinity tags for protein purification. Protein Expr. Purif. 2005;41:98–105. doi: 10.1016/j.pep.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson J, Stahl S, Lundeberg J, Uhlen M, Nygren PA. Affinity fusion strategies for detection, purification, and immobilization of recombinant proteins. Protein Expr. Purif. 1997;11:1–16. doi: 10.1006/prep.1997.0767. [DOI] [PubMed] [Google Scholar]
- 10.Arnau J, Lauritzen C, Petersen GE, Pedersen J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 2006;48:1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Guimaraes CP, Witte MD, Theile CS, Bozkurt G, Kundrat L, Blom AE, Ploegh HL. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc. 2013;8:1787–1799. doi: 10.1038/nprot.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warden-Rothman R, Caturegli I, Popik V, Tsourkas A. Sortase-tag expressed protein ligation: combining protein purification and site-specific bioconjugation into a single step. Anal Chem. 2013;85:11090–11097. doi: 10.1021/ac402871k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobashigawa Y, Kumeta H, Ogura K, Inagaki F. Attachment of an NMR-invisible solubility enhancement tag using a sortase-mediated protein ligation method. J. Biomol. NMR. 2009;43:145–150. doi: 10.1007/s10858-008-9296-5. [DOI] [PubMed] [Google Scholar]
- 14.Yamamura Y, Hirakawa H, Yamaguchi S, Nagamune T. Enhancement of sortase A-mediated protein ligation by inducing a β-hairpin structure around the ligation site. Chem. Commun. 2011;47:4742–4744. doi: 10.1039/c0cc05334a. [DOI] [PubMed] [Google Scholar]
- 15.Williamson DJ, Fascione MA, Webb ME, Turnbull WB. Efficient N-terminal labeling of proteins by use of sortase. Angew Chem. Int. Ed. 2012;51:9377–9380. doi: 10.1002/anie.201204538. [DOI] [PubMed] [Google Scholar]
- 16.Chen I, Dorr BM, Liu DR. A general strategy for the evolution of bond-forming enzymes using yeast display. Proc. Natl. Acad. Sci. USA. 2011;108:11399–11404. doi: 10.1073/pnas.1101046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrache AI, Machin DC, Williamson DJ, Webb ME, Beales PA. Sortase-mediated labeling of lipid nanodiscs for cellular tracing. Mol. Biosyst. 2016;12:1760–1763. doi: 10.1039/c6mb00126b. [DOI] [PubMed] [Google Scholar]
- 18.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. AM. Chem. Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]