Abstract
Objectives
Recent studies demonstrate vitamin D is inversely correlated with benign prostatic hyperplasia (BPH) and prostate cancer (PCa) incidence. We aim to clarify the associations of vitamin D with prostate volume.
Methods
This is an observational study investigating the associations of serum PSA, PSA Density (PSAD) and prostate volume with serum 25-hydroxyvitamin D (25-OH D) in PCa patients and men with negative biopsies seen in outpatient urology clinics in Chicago, IL. There were 571 men (40- to 79-years-old) with elevated PSA or abnormal digital rectal examination (DRE) with available prostate volume recorded from initial biopsy. The primary outcomes were the unadjusted associations of serum 25-hydroxyvitamin D deficiency with prostate volume. The secondary outcomes were the adjusted associations using linear and logistic regression analysis.
Results
On univariate analysis, serum 25-OH D < 20ng/ml inversely correlated with prostate volume among all men undergoing transrectal ultrasonography (p = 0.02), and this relationship remained significant for men with negative biopsy on stratified analysis. In adjusted models, controlling for age, serum PSA, 5-ARI use, obesity, and PCa diagnosis, prostate volume was inversely associated with vitamin D (p < 0.05) using serum vitamin D as a continuous and categorical variable. Logistic regression model also demonstrated an inverse association between vitamin D (continuous and categorical) and prostate volume ≥ 40 grams.
Conclusion
Serum 25-OH D levels are inversely associated with overall prostate volume and enlarged prostate gland (≥ 40 grams), especially in men with benign prostatic disease. Given the largely non-toxic effect of supplementation, consideration should be given to assessing vitamin D levels in men with benign prostatic disease in addition, to malignant prostatic disease.
Keywords: Vitamin D, Prostate Volume, Prostate Cancer
INTRODUCTION
Recent data have shown that serum vitamin D deficiency is associated with aggressive prostate cancer (PCa) diagnosis in men undergoing prostate biopsy (1,2) and adverse pathologic findings at radical prostatectomy (3). These findings are likely associated with epithelial proliferation and differentiation and angiogenesis based on molecular studies using 25-hydroxyvitamin D (25-OH D) or analogues in in vitro and in vivo prostate cancer experiments (4–6).
However, limited clinical data exists on the inverse relationship of vitamin D and benign prostate cell proliferation, which could manifest itself in increased prostate volume in men deficient of vitamin D (7). Moreover, little is known of the effect of serum 25-OH D on prostate volume in men with prostate cancer (PCa). The aim of this study is to evaluate the associations of serum 25-OH D and prostate volume in men undergoing transrectal ultrasound (TRUS)-guided prostate biopsy for elevated/rising PSA or suspicious digital rectal examination (DRE).
MATERIALS AND METHODS
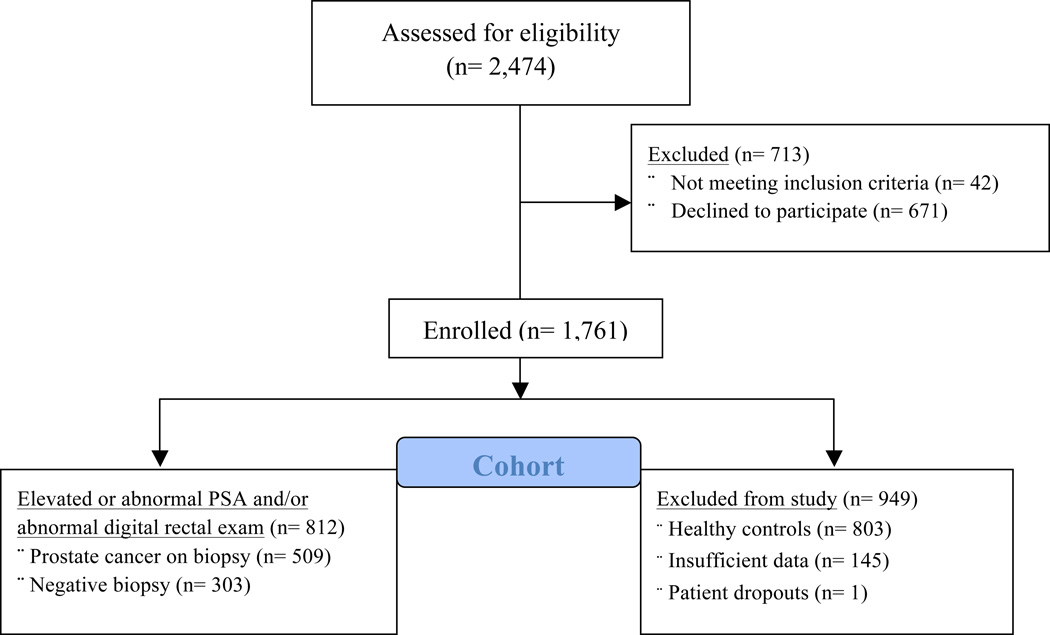
This is a cross-sectional, observational study evaluating the associations of serum 25-OH D status and prostate volume. It is nested within a large epidemiologic study of men undergoing prostate cancer biopsy and healthy controls evaluating the environmental and biological mediators of vitamin D and PCa risk. Study participants were prospectively enrolled through outpatient urology clinics from 3 academic (Northwestern University, University of Chicago, University of Illinois at Chicago) and 2 public institutions (Jesse Brown Veteran Affairs and Cook County Hospitals) in Chicago, IL from 2009 to 2014. The study population is composed of men between the age of 40–79 years old, undergoing TRUS prostate biopsy at one of the participating institutions (Figure 1).
Figure 1.
CONSORT diagram demonstrating the inclusion and exclusion of men in our study cohort
Of the 2,474 men initially surveyed, 1,761 (72.4%) were enrolled in our study. Forty-two men initially surveyed met exclusion criteria for the study—diseases known to affect vitamin D metabolism including hyperparathyroidism, severe liver or renal dysfunction, rickets disease, and history of inborn error of vitamin D metabolism. One patient dropped out from the study, and the 803 healthy controls were excluded since they did not undergo transrectal ultrasound. There was incomplete data on prostate volume for 145 men who were referred from other institutions, and did not have their prostate biopsy performed at one of the study sites.
Ultimately, 812 men were enrolled prospectively and underwent initial TRUS-guided prostate core-needle biopsy (minimum of 10 cores) for rising/elevated PSA or abnormal/suspicious finding on DRE (Figure 1). 571 (70.3%) of the 812 patients had a documented prostate volume. The cancer cases are overrepresented among the men undergoing prostate biopsy as a result of 168 of the 571 patients being enrolled in our study cohort within 1 month following their PCa diagnosis. We continue to review the electronic medical records of all the men with negative biopsies for at least two years after the biopsy to ensure they remained free of PCa diagnosis. Men in the cohort routinely had their prostate volume estimated during TRUS using the prolate ellipsoid formula. The sample size for the overall epidemiological study was calculated for the genetic analysis if vitamin D pathway SNPs and associations with aggressive prostate cancer. This study is powered at 85.3 % to detect a difference of 15% in the mean gland volume between men with 25-OH D < 20ng/ml versus 25-OH D > 20ng/ml. All study participants provided written consent, and the institutional review board at each participating institution approved the protocol.
Clinical and Environmental Data
Clinical and demographic data were collected by patient administered questionnaires and independent chart review by trained research coordinators. A peripheral serum sample was obtained to measure 25 OH-D at time of enrollment. Serum PSA levels that prompted the prostate biopsy were obtained from the medical record in accordance with institutional practice at each facility. Relevant clinical covariates including age, first degree family history of PCa, and alcohol- and tobacco- use, current use of 5-α reductase inhibitors (5-ARI), clinical history of lower urinary tract syndrome were all collected based on patient report and validated by the medical record, when appropriate.
Indicators of socioeconomic status were collected through questionnaire at enrollment and review of subject medical records. Ethnicity/race was determined by self-identification among participants and placed into classification of African American (AA), European American (EA), or other. Standing height (m) and weight (kg) measurements were used to calculate body-mass-index (BMI) on all subjects. Genitourinary pathologists confirmed all PCa diagnoses from core biopsy specimens.
Outcomes Measurements & Statistical Analysis
Descriptive statistics between PCa and negative biopsies were tested for significance using non-parametric testing with Wilcoxon rank-sum test for continuous variables and χ2 test analysis for categorical traits. The primary outcome of interest was the association of prostate volume with serum 25-OH D level. The primary outcome was assessed using a univariate sensitivity analysis of prostate volume and PSA using several cut-points for 25-OH D deficiency derived from the literature including < 30 ng/ml (8), < 20 ng/ml (9), and < 12 ng/ml (10), which were stratified by biopsy result. Season of blood draw was defined as a categorical variable defined as high ultraviolet exposure (May-October) and low ultraviolet exposure (November-April) months.
Adjusted models were constructed with multivariable linear regression to evaluate the associations of serum 25-OH D with prostate volume among men in the study. The model was constructed by sequential addition of each covariate. The beta coefficients represented the predicted change in prostate volume per 1 unit change in each variable. A multivariable logistic model was also constructed to evaluate the relationship between vitamin D and a prostate volume greater than 40 grams, which was chosen because of its established relevance in the response to medical therapy with combined 5-ARI and selective alpha-blockade therapy (11,12). We evaluated most of the known covariates known to affect vitamin D levels and prostate volume in both the linear regression and logistic models, but excluded from our best fit model variables with a p-value greater than 0.10. All statistical tests were two-sided, with significance defined at 0.05. Statistical analyses were conducted with STATA 12.1 (StataCorp 2011, College Station, TX).
RESULTS
Table 1 shows the baseline characteristics of the study participants. The median age of prostate cancer cases was 62.8 years and 61.4 years for negative biopsies (p = 0.005). Prostate cancer cases differed from patients with a negative biopsy by median prostate volume (35.5 vs. 50.0 cm3, p < 0.001) and PSA density (0.18 vs. 0.12 ng/ml/cm3, p < 0.001). AA men comprised 42.9 % of PCa cases and 35.2 % of negative biopsies. Additionally, PCa cases were less likely to use 5-ARIs (5.4 % vs. 17.3 %, p < 0.001).
Table 1.
Demographic and clinical characteristics of patients after radical prostatectomy
| Prostate Cancer (N =374) |
Negative Biopsy (N = 197) |
||
|---|---|---|---|
| Continuous variables | Median (IQR) | Median (IQR) | p-valuea |
| Age, years | 62.8 (58.5–67.7) | 61.4 (56.4–66.1) | 0.005 |
| 25-OH D serum level, ng/ml | 22.0 (15.0–29.0) | 21.0 (13.0–28.0) | 0.36 |
| Body mass indexc, kg/m2 | 27.9 (24.9–31.2) | 27.8 (24.7–31.0) | 0.62 |
| Serum PSA, ng/ml | 6.2 (4.4–10.2) | 6.2 (4.3–9.3) | 0.38 |
| Prostate volume, cm3 | 35.5 (29.1–49.4) | 50.0 (34.5–66.0) | < 0.001 |
| PSA density, ng/ml/cm3 | 0.18 (0.11–0.29) | 0.12 (0.08–0.20) | < 0.001 |
| Categorical variables | % | % | p-valueb |
|
1st degree family history of PCa (n = 570) |
26.5 | 15.8 | 0.004 |
| Suspicious Findings on DRE, yes | 33.4 | 35.0 | 0.70 |
| BPH/LUTS, yes | 36.6 | 48.7 | 0.005 |
| Race/Ethnicity | |||
| - African American (n = 242) | 43.1 | 41.1 | <0.001 |
| - European American (n = 228) | 44.1 | 32.0 | |
| - Other (n = 113) | 16.8 | 33.5 | |
| High School Diploma or Equivalent | 85.5 | 78.1 | 0.02 |
| 25-OH D < 30 ng/ml | 23.8 | 21.8 | 0.60 |
| 25-OH D < 20 ng/ml | 59.1 | 59.4 | 0.95 |
| 25-OH D < 12 ng/ml | 85.3 | 82.2 | 0.34 |
| Season of blood draw, high UV | 52.1 | 50.8 | 0.75 |
|
Vitamin D Supplement Use, yes (n = 655) |
15.3 | 12.2 | 0.31 |
|
Married (n = 653) |
59.8 | 62.2 | 0.57 |
| Obesityd | 31.8 | 32.5 | 0.87 |
| Alcohol-use, ever | 88.2 | 83.1 | 0.09 |
| Tobacco-use, ever | 54.4 | 60.0 | 0.20 |
| 5-ARI use | 5.4 | 17.3 | < 0.001 |
Wilcoxon rank-sum testing;
Chi-square analysis;
BMI information was missing for 1 patient in this cohort,
Obesity was defined as a BMI greater or equal to 30 ng/mL
Abbreviation: PSA = prostate specific antigen, 25-OH D = serum 25 hydroxyvitamin D, DRE = digital rectal examination, 5-ARI = 5 alpha reductase inhibitor, LUTS = lower urinary tract symptoms.
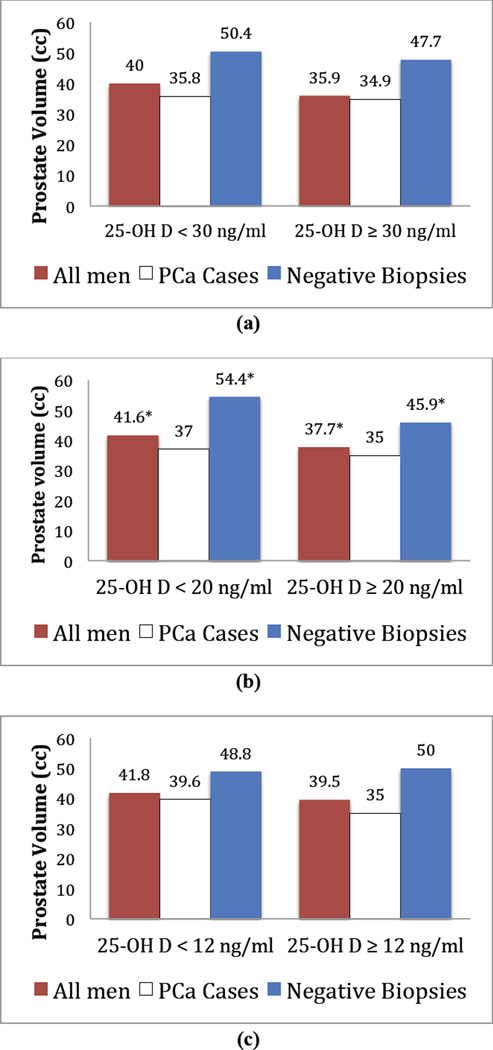
Figure 2 demonstrates prostate volume stratified by vitamin D cut points for the entire cohort. The median prostate volume was higher among men with vitamin D levels less than 20 ng/mL at 41.6 cm3 (IQR 30.4–59.3) compared to those with levels greater than 20 ng/mL at 37.7 cm3 (IQR 29.4–52.4), and was statistically significant (p = 0.02). The cut point of 20 ng/ml was also associated with significant difference in serum PSA (7.1 [IQR 4.8–11.9] vs. 5.4 [IQR 4.0–8.0] ng/ml, p < 0.001). Additionally, there was a statistically significant difference in serum PSA at 25 OH-D < 30 ng/ml (6.3 [IQR 4.4–9.9] vs. 5.3 [IQR 3.8–7.9] ng/ml, p = 0.002), and 25 OH-D < 12 ng/ml (7.5 [IQR 5.2–11.0] vs. 5.8 [IQR 4.2–9.1] ng/ml, p < 0.001). Prostate volume was higher for patients with vitamin D levels less than 30 and 12 ng/mL, but the difference was not statistically significant. Stratified analyses by ethnicity and season did not result in any significant differences in prostate volume.
Figure 2.
Plot of prostate volume versus 25-OH D cutpoints (a: < 30 ng/ml, b: < 20 ng/ml, c: < 12 ng/ml) stratified by prostate biopsy result. * denotes p < 0.05.
On multivariable linear regression analysis adjusting for age, serum PSA, 5-ARI use, obesity, and PCa diagnosis, prostate volume demonstrated a significant association between serum 25 OH-D level (continuous) β −0.22 (95 % CI, −0.42, −0.03, p = 0.03, see Table 2) and serum 25 OH-D < 20 ng/ml β 4.44 (95 % CI, 0.33, 8.54, p = 0.03, see Table 3). Serum vitamin D < 20 ng/ml was chosen as a categorical variable for our final model based on the result of our univariate tests, which was utilized as a sensitivity analysis. The same adjusted model was restricted to men with negative biopsies, and showed a non-significant inverse relationship between prostate volume and serum vitamin D as a continuous variable, β −0.12 (95 % CI, −0.32, 0.09, p = 0.27), and 25 OH-D < 20 ng/ml with β 6.50 (95 % CI, −1.40, 14.39, p = 0.11). Similarly, adjusted models for men with PCa diagnosis, showed a non-significant inverse association between vitamin D as a continuous variable, β −0.12 (95 % CI, −0.32, 0.09, p = 0.27), and vitamin D < 20 ng/ml, β 2.96 (95 % CI, −1.13, 7.05, p = 0.16). Ethnicity, season of blood draw, and study site were excluded from all final models for p-value > 0.10.
Table 2.
Multivariable linear regression analysis of prostate volume controlling for serum 25-OH D and covariates of prostate volumea
| Characteristic | All Men (n = 571) |
Negative Biopsies (N = 197) |
Prostate Cancer (N = 374) |
|||
|---|---|---|---|---|---|---|
| β Coeff. (95 % CI) | p-value | β Coeff. (95 % CI) | p-value | β Coeff. (95 % CI) | p-value | |
| Age at enrollment, years | 0.38 (0.08, 0.66) | 0.01 | 0.83 (0.27, 1.39) | 0.004 | 0.31 (−0.01, 0.62) | 0.06 |
| Serum 25-OH D, ng/ml | −0.22 (−0.42, -0.03) | 0.03 | −0.33 (−0.70, 0.04) | 0.08 | −0.12 (−0.33, 0.09) | 0.27 |
| PSA at diagnosis, ng/ml | 0.34 (0.08, 0.61) | 0.01 | 0.68 (0.12, 1.24) | 0.02 | 0.30 (0.03, 0.57) | 0.03 |
| 5-ARI use, yes | 18.8 (11.6, 26.0) | < 0.001 | 11.5 (0.07, 22.9) | 0.049 | 13.6 (3.87, 23.3) | 0.006 |
| Obesityb | 5.61 (1.19, 10.0) | 0.01 | 7.92 (−1.04, 16.9) | 0.08 | 4.58 (−0.03, 9.20) | 0.051 |
Model controlling for all variables above; covariates removed from model if p > 0.10;
Defined as a BMI greater than or equal to 30 kg/m2;
Abbreviation: 5-ARI = 5-alpha reductase inhibitor, PSA = prostate specific antigen
Table 3.
Multivariable linear regression analysis of prostate volume controlling for serum 25-OH D and covariates of prostate volumea
| Characteristic | All Men (n = 571) |
Negative Biopsies (N = 197) |
Prostate Cancer (N = 374) |
|||
|---|---|---|---|---|---|---|
| β Coeff. (95 % CI) | p-value | β Coeff. (95 % CI) | p-value | β Coeff. (95 % CI) | p-value | |
| Age at enrollment, years | 0.37 (0.08, 0.66) | 0.01 | 0.84 (0.28, 1.40) | 0.003 | 0.31 (−0.01, 0.622) | 0.057 |
| Serum 25-OH D < 20 ng/ml | 4.40 0.13, 8.65) | 0.04 | 7.71 (−0.68, 16.1) | 0.07 | 2.80 (−1.66, 7.26) | 0.22 |
| PSA at diagnosis, ng/ml | 0.34 (0.07, 0.60) | 0.01 | 0.67 (0.11, 1.23) | 0.02 | 0.29 (0.01, 0.56) | 0.04 |
| 5-ARI use, yes | 18.7 (11.5, 26.0) | < 0.001 | 11.4 (−0.03, 22.8) | 0.051 | 13.4 (3.72, 23.1) | 0.007 |
| Obesityb | 5.91 (1.51, 10.3) | 0.009 | 9.10 (0.20, 18.0) | 0.045 | 4.58 (−0.03, 9.18) | 0.052 |
Model controlling for all variables above; covariates removed from model if p > 0.10;
Defined as a BMI greater than or equal to 30 kg/m2;
Abbreviation: 5-ARI = 5-alpha reductase inhibitor, PSA = prostate specific antigen
Adjusted models were also constructed by logistic regression to assess predictors of a prostate volume greater than 40 gm (Table 4 and 5). Serum vitamin D levels (continuous) were inversely associated with prostate volume with a odds ratio 0.97 (95% CI 0.96, 0.99, p = 0.001) and a 25-OH D level < 30 ng/ml was associated with increased risk of prostate volume greater than 40 gm, odds ratio 1.60 (95% CI 1.06, 2.41, p = 0.02). The model was adjusted for season of blood draw (p < 0.10), and ethnicity, study site, and PSA at diagnosis were excluded from the model for p > 0.10.
Table 4.
Multivariable logistic regression analysis of prostate volume (≥ 40 gm) controlling for serum 25-OH D and covariates of prostate volumea
| Characteristic | All Men (n = 571) |
Negative Biopsies (N = 197) |
Prostate Cancer (N = 374) |
|||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | |
| Age at enrollment, years | 1.06 (1.03, 1.09) | < 0.001 | 1.12 (1.07, 1.18) | < 0.001 | 1.05 (1.02, 1.08) | 0.002 |
| Serum 25-OH D, ng/ml | 0.97 (0.96, 0.99) | 0.001 | 0.97 (0.94, 0.99) | 0.03 | 0.97 (0.95, 0.99) | 0.02 |
| 5-ARI use, yes | 2.91 (1.53, 5.54) | 0.001 | 2.31 (0.86, 6.16) | 0.95 | 1.97 (0.77, 5.03) | 0.15 |
Model controlling for all variables above and season of blood draw; covariates removed from model if p > 0.10;
Abbreviation: 5-ARI = 5-alpha reductase inhibitor, PSA = prostate specific antigen
Table 5.
Multivariable logistic regression analysis of prostate volume (≥ 40 gm) controlling for serum 25-OH D and covariates of prostate volumea
| Characteristic | All Men (n = 571) |
Negative Biopsies (N = 197) |
Prostate Cancer (N = 374) |
|||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | |
| Age at enrollment, years | 1.06 (1.03, 1.08) | < 0.001 | 1.12 (1.07, 1.18) | < 0.001 | 1.05 (1.02, 1.08) | 0.003 |
| Serum 25-OH D < 30 ng/ml | 1.60 (1.06, 2.41) | 0.02 | 1.72 (0.79, 3.74) | 0.17 | 1.59 (0.96, 2.65) | 0.07 |
| 5-ARI use, yes | 2.98 (1.57, 5.65) | 0.001 | 2.40 (0.90, 6.41) | 0.08 | 2.05 (0.81, 5.20) | 0.13 |
Model controlling for all variables above and season of blood draw; covariates removed from model if p > 0.10;
Abbreviation: 5-ARI = 5-alpha reductase inhibitor, PSA = prostate specific antigen
DISCUSSION
Serum 25-OH level below 20 ng/mL was associated with an increased prostate volume on univariate analysis. This finding was also noted to remain significant in adjusted model controlling for known covariates of prostate volume including age and 5-ARI use, and stratified by cancer status. In fact, our findings demonstrated a change in prostate volume of 0.22 cm3 per unit change of serum 25-OH D. Moreover, although modest, there was a difference of 4.40 cm3 in median prostate volume among all patients in the cohort (p = 0.04) comparing men with a 25 OH-D level cutpoint of 20 ng/ml. However, among men with negative biopsies, the median difference was even larger at 7.71 cm3 (p = 0.07). Similarly, on multivariable logistic regression model, 25-OH D less than 30 ng/ml was associated with an increased risk of prostate volume greater than 40 grams, which is a clinical significant cutpoint based on the result of the prospective Medical Theraphy of Prostatic Symptoms (12). Overall, our findings are consistent with a smaller series from The Osteoperotoic Fracture in Men from Sweden, which demonstrated an inverse relationship between prostate size and vitamin D (7). However, men with PCa diagnosis on pathologic evaluation of prostate biopsy cores did not display a statistically significant difference in prostate volume by differing 25-OH D cutpoints. This association may be difficult to find due to PCa heterogeneity in the contribution of the tumor to overall prostate volume.
Observational studies have shown that the intake of both dietary and supplemental vitamin are inversely associated with BPH prevalence (13). In fact elocalcitol, a vitamin D analogue, has an inhibitory effect on the in vitro proliferation of patient-derived benign prostatic stromal cells and PCa epithelial cells (14). More studies have shown that vitamin D is involved in cellular proliferation (6,15), differentiation (5,15), and apoptosis (4,16). Vitamin D has been demonstrated to be an effective anti-proliferative agent in prostatic epithelium and stromal fibroblasts (17), through its inhibition of the cell-cycle and promotion of cellular apoptosis (18,19). Specifically, vitamin D exerts inhibitory effects on the RhoA/Rho kinase pathways (14), COX-2 expression and prostaglandin expression in prostate stromal cells (13,20).
Clinically, prostate gland size does not always correlate with the severity of obstructive urinary symptoms (21). However, treatment with the vitamin D BXL628 for 12 weeks correlated with smaller prostate glands, which accompanied small decreases in urinary symptom score (13,22). Interestingly, our findings suggest that any effect that vitamin D may have on prostate volume is modest in men with PCa, which suggests that higher levels of 25-OH D may preferentially favor pathways for differentiation, anti-angiogenesis and anti-proliferation PCa cells or variability in tumor growth and contributions to overall size makes this association difficult to find. This may be reflected by the higher PSA levels in men with cancer observed in this cohort among deficient men with PCa diagnoses compared to those with negative biopsies. This phenomenon also likely explains the clinical observation of less aggressive PCa phenotypes in men with normal serum vitamin D levels in our prior studies (3,10). Additionally, evidence from the colon and rectal literature suggests that inflammation regulates vitamin D receptor expression (23). Consequently, the pro-inflammatory state of PCa may lead to lower expression of the vitamin D receptor and deficiency would further promote proliferation in men with PCa.
Studies have suggested that large prostatic glands are associated with less aggressive form of PCa (24). Although, purely speculative at this time, the relationship seen between prostate size and disease aggression may reflect the biologic effect that 25-OH D appears to play in prostate epithelial proliferation and differentiation. This relationship requires further investigation via genetic and histologic evaluation of clinical patients in the future.
The limitations of our study include the cross-sectional study design, which inherently holds a selection bias that may impede the ability to control for unmeasured confounders and assess causality, though we measured many of the known risk factors for BPH, PCa and vitamin D deficiency. We did not have access to diabetes status, which has been associated with prostate volume and weakly associated with serum vitamin D status (25,26). Also, this study is limited by the fact that a one-time serum vitamin D level may not be representative of long term vitamin D, which is theoretically important for long-term prostate growth. Additionally, the men with negative prostate biopsies may have indolent microscopic cancer. Although the men in the negative biopsy group were followed for a minimum of 2 years, we recognize that some men categorized as having bening disease may be subsequently diagnosed with prostate cancer. We also note, that our association was not observed at a vitamin D level less than 12 ng/ml; however, the number of patients with serum levels less than 12 ng/ml was small, which likely limited our statistical power to assess for observed differences in prostate volume seen in Figure 2. Also, men undergoing prostate biopsy may not represent the general population of men without PCa. Lastly, a review of the literature also demonstrates that elevated serum 25 OH D is associated with an increased risk of prostate cancer (2,27). Elevated serum levels are uncommon in our patient population likely as a result of the lower sunlight exposure at the higher latitude in Chicago, Illinois. As a result, we were not able to assess the relationship between elevated serum vitamin D levels and prostate volume.
The strengths of this study include the diversity in age and race of the participants, its large sample size as well as the follow up of negative biopsy patients to confirm their benign disease status. Our results contribute to epidemiologic evidence for a role for vitamin D in benign and malignant prostatic disease. Further longitudinal studies are needed to assess the impact of daily vitamin D supplementation on the development of lower urinary tract symptoms/benign prostatic hyperplasia and PCa. Additionally, there is a need for more randomized control trials among various populations with prevalent vitamin D deficiency [i.e. AAs, men living in low UV climates (28)] who can contribute tissue for molecular characterization (i.e. men on active surveillance, pre-radical prostatectomy, pre-prostate biopsy). BPH-focused randomized control trials could also be performed using vitamin D and conventional therapies to measure American Urologic Association International Prostate Symptom Scores, prostate volume and surgical intervention rates.
CONCLUSION
Serum 25-OH D levels are inversely correlated with prostate volume, a relationship most markedly appreciated in men without PCa. Future studies are required to prospectively investigate vitamin D in a clinical trial to better assess the temporal effect of 25-OH D on prostate growth rates and disease progression in benign and malignant prostatic disease. Given that we have shown associations of low serum vitamin D with aggressive prostate cancer and now find associations of low vitamin D with prostate volume, it may be prudent to measure vitamin D levels in men with BPH/LUTS, elevated PSA and prostate cancer. Supplementation of vitamin D3 is largely non-toxic and the bone health benefits are significant in elderly men. Future studies will have to clarify the impact of supplementation on prostate disease outcomes.
Acknowledgments
Funding: This work was supported by the following grants: 1R01MD007105-01 (R. Kittles); IK2CX000926-01 (A.B. Murphy), W81XWH-10-1-0532 pd22E (A.B. Murphy) and P50 CA090386-10S1 (W.J. Catalona)
None
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: Results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014 Aug;23(8):1484–1493. doi: 10.1158/1055-9965.EPI-13-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, et al. Plasma vitamin D and prostate cancer risk: Results from the selenium and vitamin E cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014 Aug;23(8):1494–1504. doi: 10.1158/1055-9965.EPI-14-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyame YA, Murphy AB, Bowen DK, Jordan G, Batai K, Dixon M, et al. Associations between serum vitamin D and adverse pathology in men undergoing radical prostatectomy. J Clin Oncol. 2016 Feb;22 doi: 10.1200/JCO.2015.65.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in lncap cells is blocked by overexpression of bcl-2. Endocrinology. 2000 Jan;141(1):10–17. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 5.Zhao XY, Ly LH, Peehl DM, Feldman D. Induction of androgen receptor by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in lncap human prostate cancer cells. Endocrinology. 1999 Mar;140(3):1205–1212. doi: 10.1210/endo.140.3.6561. [DOI] [PubMed] [Google Scholar]
- 6.Blutt SE, Allegretto EA, Pike JW, Weigel NL. 1,25-dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of lncap prostate cells and cause accumulation of cells in G1. Endocrinology. 1997 Apr;138(4):1491–1497. doi: 10.1210/endo.138.4.5063. [DOI] [PubMed] [Google Scholar]
- 7.Haghsheno M-A, Mellström D, Behre C-J, Damber J-E, Johansson H, Karlsson M, et al. Low 25-OH vitamin D is associated with benign prostatic hyperplasia. J Urol. 2013 Aug;190(2):608–614. doi: 10.1016/j.juro.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 8.Del Valle HB, Yaktine AL, Taylor CL, Ross AC. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. [PubMed] [Google Scholar]
- 9.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (finland) Cancer Causes Control. 2000 Oct;11(9):847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 10.Murphy AB, Nyame Y, Martin IK, Catalona WJ, Hollowell CMP, Nadler RB, et al. Vitamin D deficiency predicts prostate biopsy outcomes. Clin Cancer Res. 2014 May 1;20(9):2289–2299. doi: 10.1158/1078-0432.CCR-13-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto M, Shimizu N, Sugimoto K, Hongoh S, Minami T, Nozawa M, et al. Efficacy of adding dutasteride to α-blocker therapy treated benign prostatic hyperplasia patients with small volume prostate (<30 ml) Low Urin Tract Symptoms. 2016 Mar;16 doi: 10.1111/luts.12127. [DOI] [PubMed] [Google Scholar]
- 12.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003 Dec 18;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa G, Esposito R, Kazzazi A, Djavan B. Vitamin D and benign prostatic hyperplasia -- a review. Can J Urol. 2013 Aug;20(4):6820–6825. [PubMed] [Google Scholar]
- 14.Penna G, Fibbi B, Amuchastegui S, Corsiero E, Laverny G, Silvestrini E, et al. The vitamin D receptor agonist elocalcitol inhibits il-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the rhoa/rho kinase and nf-kappab pathways. Prostate. 2009 Apr 1;69(5):480–493. doi: 10.1002/pros.20896. [DOI] [PubMed] [Google Scholar]
- 15.Freedman LP. Transcriptional targets of the vitamin D3 receptor-mediating cell cycle arrest and differentiation. J Nutr. 1999 Feb;129(2S Suppl):581S–586S. doi: 10.1093/jn/129.2.581S. [DOI] [PubMed] [Google Scholar]
- 16.Sintov AC, Yarmolinsky L, Dahan A, Ben-Shabat S. Pharmacological effects of vitamin D and its analogs: Recent developments. Drug Discov Today. 2014 Nov;19(11):1769–1774. doi: 10.1016/j.drudis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994 Feb 1;54(3):805–810. [PubMed] [Google Scholar]
- 18.Murthy S, Agoulnik IU, Weigel NL. Androgen receptor signaling and vitamin D receptor action in prostate cancer cells. Prostate. 2005 Sep 1;64(4):362–372. doi: 10.1002/pros.20251. [DOI] [PubMed] [Google Scholar]
- 19.Yee SW, Campbell MJ, Simons C. Inhibition of vitamin D3 metabolism enhances VDR signalling in androgen-independent prostate cancer cells. J Steroid Biochem Mol Biol. 2006 Mar;98(4–5):228–235. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Moreno J, Krishnan AV, Feldman D. Molecular mechanisms mediating the anti-proliferative effects of vitamin D in prostate cancer. J Steroid Biochem Mol Biol. 2005 Oct;97(1–2):31–36. doi: 10.1016/j.jsbmb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Terris MK, Afzal N, Kabalin JN. Correlation of transrectal ultrasound measurements of prostate and transition zone size with symptom score, bother score, urinary flow rate, and post-void residual volume. Urology. 1998 Sep;52(3):462–466. doi: 10.1016/s0090-4295(98)00310-0. [DOI] [PubMed] [Google Scholar]
- 22.Colli E, Rigatti P, Montorsi F, Artibani W, Petta S, Mondaini N, et al. BXL628, a novel vitamin D3 analog arrests prostate growth in patients with benign prostatic hyperplasia: A randomized clinical trial. Eur Urol. 2006 Jan;49(1):82–86. doi: 10.1016/j.eururo.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009 Nov;22(5):1021–1025. doi: 10.3892/or_00000530. [DOI] [PubMed] [Google Scholar]
- 24.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: A search database study. J Clin Oncol. 2005 Oct 20;23(30):7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen NO, Bjerregaard P, Rønn PF, Friis H, Andersen S, Melbye M, et al. Associations between vitamin D status and type 2 diabetes measures among inuit in greenland may be affected by other factors. PLoS One. 2016;11(4):e0152763. doi: 10.1371/journal.pone.0152763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006 Jul;91(7):2562–2568. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: New findings from an updated meta-analysis. J Cancer Res Clin Oncol. 2014 Sep;140(9):1465–1477. doi: 10.1007/s00432-014-1706-3. [DOI] [PubMed] [Google Scholar]
- 28.Murphy AB, Kelley B, Nyame YA, Martin IK, Smith DJ, Castaneda L, et al. Predictors of serum vitamin D levels in african american and european american men in chicago. Am J Mens Health. 2012 Sep;6(5):420–426. doi: 10.1177/1557988312437240. [DOI] [PMC free article] [PubMed] [Google Scholar]