CAPSULE SUMMARY
Human milk oligosaccharides (HMOs) provide a main substrate to help shape the infant's gut microbiota and affect the maturation of the intestinal mucosal immune system. In our cohort, infants that received human milk with low Lacto-N-fucopentaose (LNFP) III concentrations (< 60μM) were more likely to become affected with cow's milk allergy when compared to high LNFP III-containing milk (odds ratio 6.7, 95% CI 2.0-22).
Keywords: Breastfeeding, breast milk, human milk, cow's milk, infants, allergy, gut mucosa, oligosaccharide, tolerance, microbiome
To the Editor,
Human milk oligosaccharides (HMO) provide a main substrate for infant's gut microbiota, not only to bifidobacteria but also some Bacteroides1. HMO are complex glycans and the third largest solid component in human milk. HMO composition varies between women, which partially depends on genetics. For example, HMO fucosylation is mediated by the two fucosyltransferases FUT2 (secretor gene) and FUT3 (Lewis gene), which also determine the mother's Secretor and Lewis blood group status. Non-secretor mothers, who lack the functional FUT2 enzyme, also lack most alpha1-2-fucosylated oligosaccharides like 2’fucosyllactose (2’FL) and lacto-N-fucopentaose (LNFP I). Infants fed by Non-secretor mothers are delayed in establishment of bifidobacteria-laden microbiota.2 Previous studies have linked individual HMO with reduced risk of mother-to-child HIV transmission3 and FUT2-dependent HMO with lower risk to manifest IgE-associated eczema in infants born via c-section4. Because there is emerging evidence in humans to support the concept that the infant gut microbiome plays a role in food sensitization/allergy,5 we sought to compare the HMO composition in breast milk received by infants who develop cow's milk allergy (CMA) with that in non-CMA infants.
We utilized stored human milk, foremilk collected in the morning, from a prospective birth cohort designed to assess immunologic factors in human milk, development of CMA within the first 18 months of life and oversampled for newborns at high risk for food allergies. The results for human milk cytokines in this cohort have been previously published.6 Clinical characteristics are shown in Table EI. The earliest available milk sample was assessed from each mother; at median 1.0 months in 41 mothers of non-CMA infants and at median 1.4 in 39 mothers of CMA infants. CMA was verified by oral food challenges at median 6 months of age. HMO composition was measured by HPLC after 2-aminobenzamide labeling (Table EII). Raffinose was added to milk samples as internal standard to allow for absolute quantification (see the supplementary text in this article's Online Repository at www.jacionline.org for details of statistical analysis). Freezing does not impact HMO levels, which are very stable in term milk day-to-day and diurnally.7 The study was approved by the institutional review boards of the Helsinki University Central Hospital, the City of Helsinki, and the University of Rochester Medical Center, Rochester, NY.
In our cohort, FUT2 Secretor status did not significantly correlate with CMA (p=0.38, Fisher's test). Duration of lactation in months (i.e. age of the infant) significantly correlated with levels of several HMO (Fig E1), whereas maternal atopy only marginally associated with one HMO (disialyllacto-N-tetraose (DSLNT), p=0.046) and maternal age did not significantly correlate with any HMO levels. After adjusting for babies’ age and maternal covariates, including atopic diseases, duration of lactation, and Secretor status, milk of mothers with an infant with CMA contained lower levels of 6’-sialyllactose (6'SL, p=0.019), DSLNT (p=0.028), LNFP I (p=0.021) and LNFP III (p=0.00036) than milk of mothers with a non-milk allergic infant, and there was a trend for lower levels of LS-tetrasaccharide c (LSTc, p=0.068) (Fig 1). After correction for multiple comparisons, the level of LNFP III remained significantly lower in the mothers with a CMA infant (29 μM vs. 57 μM (95% CI 11-43), adjusted p=0.0069). Infants who received low (< 60 μM) LNFP III-containing milk were more likely to become affected with CMA, when compared to high LNFP III-containing milk (odds ratio 6.7, 95% CI 2.0-22). When further classifying infants type of CMA, all mothers with an infant with delayed-onset CMA were Secretors (active FUT2, milk containing 2’FL and LNFP I), whereas those with an infant with immediate-type (IgE-mediated) CMA were not; otherwise HMO were comparable between these groups (Fig E2). Levels of LSTc, DSLNT and 6'SL (p=0.019, p=0.028 and p=0.044, respectively) were lower in the milk of mothers with an infant with atopic dermatitis (AD) when compared to those with an infant with no AD.
Figure 1.
Human milk oligosaccharide levels in mothers who have an infant with cow's milk allergy (CMA) and in those with a child without CMA. Non-parametric p-values (Wilcoxon) for the difference in distribution between CMA and non-CMA samples are indicated in each panel. For HMO abbreviations, see Supplementary Table E2.
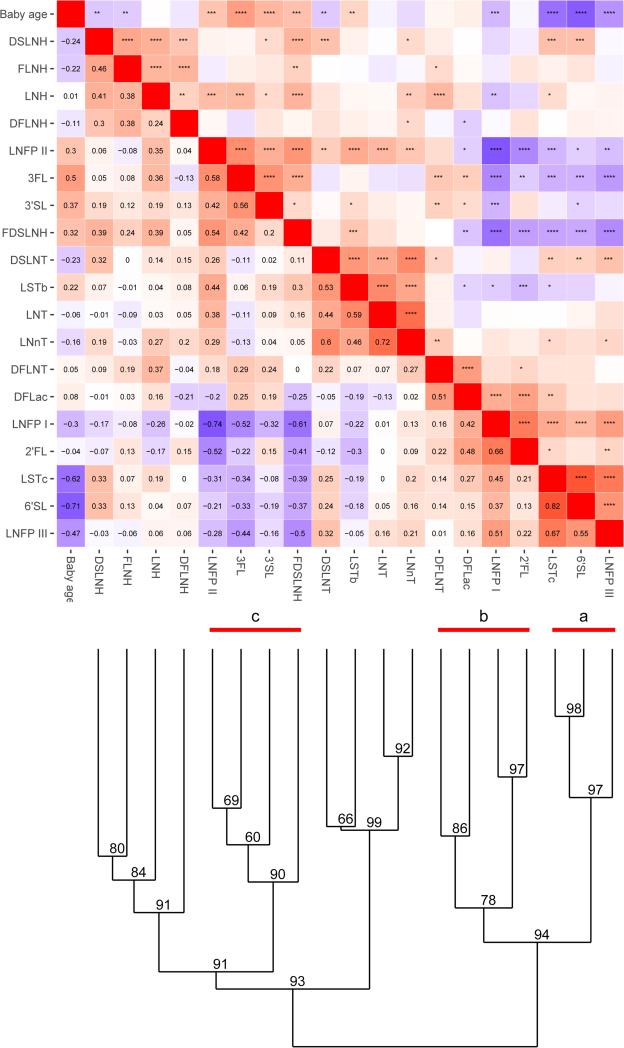
Cluster analysis that groups HMO based on similarity of expression patterns and baby's age (Fig 2) shows that HMOs that directly depend on FUT2 expression (2’FL and LNFP I, group b) were co-expressed, and correlate with FUT2 status, as expected. Also, noteworthy that three seemingly unrelated HMOs, 6'SL, LSTc and LNFP III (group a) formed a co-expressed cluster, which together significantly correlated with CMA status (MANOVA, p=0.015). It was not immediately obvious from the cluster analysis what regulates this expression pattern, as these three HMO do not share a known biosynthetic pathway that would easily explain this pattern. It is noteworthy, however, that a third group c with FDSLNH, LNFP II, 3FL and 3'SL negatively correlated with groups a and b, suggesting a common regulatory mechanism that diverts HMO composition between two different “glycotypes”.
Figure 2.
A correlation heatmap and cluster dendrogram for expression of HMOs. AU (approximately unbiased) p-value for each cluster is shown in the dendrogram. Significant clusters (AU > 95) are labeled (a, b and c). Heatmap of the distance matrix is shown with Spearman correlation values on the lower triangle and the corresponding p-value in the upper triangle. ****≤ 0.001, ***≤0.01, **≤0.05, *≤0.1.
One previous study assesses association of HMO with infant allergic status,4 indicating that infants at a high hereditary risk for allergic diseases born by C-section might have a lower risk to manifest IgE-associated eczema at 2 years, when fed breast milk with FUT2-dependent HMO. Food allergy or individual HMO components were not separately assessed. While we have no data on C-section births in our cohort, our data suggests that it is the Lewis X antigen that is present in LNFP III, and not the FUT2 (Secretor status) that is associated with protection against CMA. Lewis X antigen is recognized by some C-type lectins. In fact, HMO have recently been suggested to bind dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), mediated by Lewis X structure.8 It remains to be seen whether Lewis X or other HMO structures in breast milk could compete with binding of glycoproteins to DC-SIGN or other C-type lectins in the gastrointestinal tract, thereby modulating infant's tolerance to foods.
We cannot exclude the possibility that other substances, such as cytokines, antibodies or exosomes, in breast milk could have contributed to the development of CMA. Additionally, while our data suggests that higher LNFP III concentrations are associated with the lack of development of CMA, they are not required to prevent CMA. Therefore, other mechanisms must be in play. Findings of our small cohort need to be validated in a larger sample.
Supplementary Material
Acknowledgments
Funding: The project described was supported by Grant Number K08 AI091655 (K.M. Järvinen) from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We thank Mayte Suarez-Farinas, PhD for her help and critical review of the statistical methods.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yu ZT, Chen C, Kling DE, et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. 2013;23(2):169–177. doi: 10.1093/glycob/cws138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis ZT, Totten SM, Smilowitz JT, et al. Microbiome. Vol. 3. 13-015-0071-z: 2015. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode L, Kuhn L, Kim HY, et al. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr. 2012;96(4):831–839. doi: 10.3945/ajcn.112.039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprenger N, Odenwald H, Kukkonen AK, Kuitunen M, Savilahti E, Kunz C. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr. 2016 Feb 24; doi: 10.1007/s00394-016-1180-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Azad MB, Konya T, Guttman DS, et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin Exp Allergy. 2015;45(3):632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 6.Jarvinen KM, Suarez-Farinas M, Savilahti E, Sampson HA, Berin MC. Immune factors in breast milk related to infant milk allergy are independent of maternal atopy. J Allergy Clin Immunol. 2015;135(5):1390–3. e1–6. doi: 10.1016/j.jaci.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurl S, Munzert M, Henker J, et al. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010 Nov;104(9):1261–71. doi: 10.1017/S0007114510002072. [DOI] [PubMed] [Google Scholar]
- 8.Naarding MA, Ludwig IS, Groot F, et al. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest. 2005;115(11):3256–3264. doi: 10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.