Summary
Symptomatic infection by Neisseria gonorrhoeae (Gc) produces a potent inflammatory response, resulting in a neutrophil-rich exudate. A population of Gc can survive the killing activities of neutrophils for reasons not completely understood. Unlike other Gram-negative bacteria, Gc releases monomeric peptidoglycan (PG) extracellularly, dependent on two nonessential, nonredundant lytic transglycosylases (LTs), LtgA and LtgD. PG released by LtgA and LtgD can stimulate host immune responses. We report that ΔltgAΔltgD Gc were decreased in survival in the presence of primary human neutrophils but otherwise grew equally to wild-type Gc. Adding PG monomer failed to alter ΔltgAΔltgD Gc survival. Thus, LTs protect Gc from neutrophils independently of monomer release. We found two reasons to explain decreased survival of the double LT mutant. First, ΔltgAΔltgD Gc was more sensitive to the neutrophil antimicrobial proteins lysozyme and neutrophil elastase, but not others. Sensitivity to lysozyme correlated with decreased Gc envelope integrity. Second, exposure of neutrophils to ΔltgAΔltgD Gc increased the release of neutrophil granule contents extracellularly and into Gc phagosomes. We conclude that LtgA and LtgD protect Gc from neutrophils by contributing to envelope integrity and limiting bacterial exposure to select granule-localized antimicrobial proteins. These observations are the first to link bacterial degradation by lysozyme to increased neutrophil activation.
Keywords: Neisseria gonorrhoeae, neutrophil, lysozyme, peptidoglycan, lytic transglycosylase, antimicrobial
Introduction
The Gram-negative, obligate human pathogen Neisseria gonorrhoeae, or the gonococcus (Gc), is the causative agent of the sexually transmitted infection gonorrhea. With over 106 million cases estimated worldwide per year, gonorrhea is a major global health burden (World Health Organization, 2012). The number of cases in the United States is also on the rise, and rates of antibiotic resistance have accelerated, prompting the CDC to list antibiotic-resistant Gc as an urgent threat to public health (Centers for Disease Control and Prevention, 2013; Centers for Disease Control and Prevention, 2015). Thus, understanding the pathogenesis of Gc is both a timely endeavor and important platform to inform the development of new therapeutics.
Gc colonizes the mucosal epithelium of the urethra, cervix, pharynx, and rectum. Host recognition of pathogen-associated molecular patterns (PAMPs) released from Gc stimulates a potent inflammatory response, and one of the PAMPs implicated in mediating this inflammatory response is peptidoglycan (PG) (Fichorova et al., 2002; Kaparakis et al., 2010; Mavrogiorgos et al., 2014). In Gram-negative bacteria, long, crosslinked polymeric strands of PG are sandwiched between the inner and outer membranes (Turner et al., 2014). Due to the outer membrane barrier, the PG within the periplasm is protected from immune surveillance. However, Gc abundantly releases monomeric PG into the extracellular space upon cell wall processing and turnover (Cloud-Hansen et al., 2008; Garcia et al., 2008; Hebeler et al., 1976; Rosenthal, 1979; Sinha et al., 1980). This observation is unusual since related Gram-negatives efficiently internalize turned over monomer into the cytosol for recycling (Goodell et al., 1985; Greenway et al., 1985; Rosenthal et al., 1987; Woodhams et al., 2013). Importantly, the PG monomers released by Gc are recognized by the human pattern recognition receptor NOD1, to activate NF-κB and drive downstream inflammatory responses in cervical epithelial cells (Mavrogiorgos et al., 2014). These released PG monomers have also been implicated in inducing epithelial cytotoxicity (Melly et al., 1984).
The epithelial inflammatory response culminates in the recruitment of neutrophils, and the hallmark of gonorrheal disease is a purulent exudate containing an abundance of neutrophils and associated Gc. The role of PG surveillance in neutrophils, however, is not well understood. Some reports suggest that NOD1 is expressed at low levels in human neutrophils, and, consequently, neutrophils are largely unresponsive to NOD1 PG agonist (Ekman et al., 2010). Meanwhile, other reports indicate that incubation of human neutrophils with NOD1 agonist is sufficient to enhance the ability of neutrophils to kill Streptococcus pneumoniae (Clarke et al., 2010). Neutrophils have a variety of mechanisms to kill microbes, including the generation of reactive oxygen species (ROS), the production of neutrophil extracellular traps (NETs), and the delivery of preformed antimicrobials from within cytoplasmic granules (Nauseef et al., 2014). Despite this antimicrobial arsenal, a population of Gc can resist neutrophil killing in vivo and, also, ex vivo upon Gc exposure to adherent primary human neutrophils (Criss et al., 2009; Johnson et al., 2013b; Simons et al., 2005; Wiesner et al., 1980). Gc possesses mechanisms to thwart killing by neutrophils, including the presence of a nuclease that clears NETs, as well as a metalloprotease and a lipooligosaccharide (LOS)-modifying enzyme that defend Gc from cationic antimicrobial attack (Handing et al., 2015; Juneau et al., 2015; Stohl et al., 2013). Whether PG monomer released by Gc can influence survival from neutrophils is unknown.
To investigate the contribution of PG monomer release on Gc survival from human neutrophils, we used a Gc mutant lacking the lytic transglycosylases (LTs) LtgA and LtgD that are important for PG monomer release (Cloud-Hansen et al., 2008). Gc encode up to seven LTs, and most function to cleave polymeric PG into monomeric PG during PG turnover (Chan et al., 2012; Cloud-Hansen et al., 2008). Only LtgA and LtgD, however, are important for the extracellular release of PG monomer, and mutations in both genes are required for complete ablation of extracellular PG monomer release (Cloud-Hansen et al., 2008). Using an ex vivo model of IL-8-treated, adherent primary human neutrophils, we found that ΔltgAΔltgD double mutant Gc survival was significantly reduced in the presence of neutrophils. The addition of exogenous PG monomer, however, failed to rescue the survival defect of ΔltgAΔltgD Gc. This observation points to an alternative mechanism, independent of PG monomer release, for LtgA- and LtgD-mediated protection from neutrophils. ΔltgAΔltgD bacteria were found to be more sensitive to killing by the neutrophil antimicrobial proteins lysozyme and neutrophil elastase, but sensitivity to other antimicrobial proteins remained unaltered. The sensitivity of the ΔltgAΔltgD mutant to the PG hydrolase lysozyme was due to increased membrane permeability rather than due to increased intrinsic sensitivity of PG to lysozyme digestion. This supports a role for LtgA and LtgD in maintenance of envelope integrity in Gc. In addition, we found that neutrophils exposed to ΔltgAΔltgD Gc exhibited enhanced granule exocytosis and increased fusion of granules to the Gc-containing phagosome, suggesting that the mutant is exposed to a higher concentration of antimicrobial components, including lysozyme, during infection.
Results
LtgA and LtgD lytic transglycosylase activity is important for Gc survival from primary human neutrophils
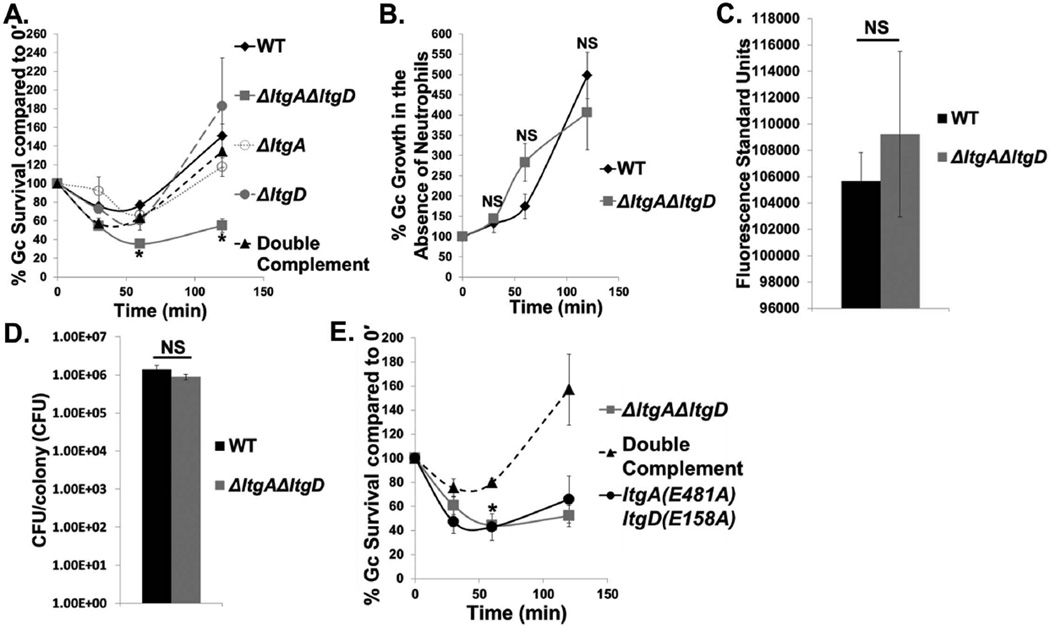
To examine the role of LtgA and LtgD in Gc survival from neutrophils, we performed an ex vivo survival assay with IL-8-treated and adherent primary human neutrophils, a model that mimics the state of neutrophils that are exposed to chemokines produced in acute gonorrhea and that have migrated to the site of infection (Criss et al., 2008). WT Gc survival was initially decreased in the presence of neutrophils, but survival recovered over time (Fig. 1A). In contrast, ΔltgAΔltgD Gc survival was significantly decreased in the presence of neutrophils and showed poor recovery at later time points. Neither single mutant, ΔltgA nor ΔltgD, was able to reproduce the survival defect of ΔltgAΔltgD Gc in the presence of neutrophils (Fig. 1A). Double complementation with ltgA+ and ltgD+ in the ΔltgAΔltgD background restored both PG monomer release (Fig. S1A) and survival to WT levels, indicating that reduced survival is specifically linked to genomic deletion of ltgA and ltgD (Fig. 1A).
Figure 1. LtgA and LtgD lytic transglycosylase activity is important for Gc survival from primary human neutrophils.
A. WT, ΔltgAΔltgD double mutant, ΔltgA single mutant, ΔltgD single mutant, and ltgA+ ltgD+ double complement Gc were exposed to adherent, IL-8-treated primary human neutrophils. Percent Gc survival was calculated by enumerating CFU from neutrophil lysates at 30, 60, and 120 min divided by CFU at 0 min. *P<0.05 for ΔltgAΔltgD compared to WT, ΔltgA, ΔltgD, and double complement; two-tailed t-test, n = 3 to 14 independent experiments.
B. WT and ΔltgAΔltgD Gc used in Fig. 1A were grown in media in the absence of neutrophils. Percent Gc growth was calculated by enumerating CFU at 30, 60, and 120 min divided by CFU at 0 min. n = 11 independent experiments.
C. WT and ΔltgAΔltgD Gc were diluted to equivalent optical densities and incubated with alamarBlue for 1 hr at 37°C with 5% CO2. n = 3 biological replicates.
D. WT and ΔltgAΔltgD Gc were grown on solid medium for 20 hr. Five colonies each were absorbed on filter paper and suspended in medium. CFU were enumerated after 24 hr incubation and CFU/colony calculated. n = 6 biological replicates.
E. ΔltgAΔltgD double mutant, ltgA+ ltgD+ double complement, and ltgA(E481A)ltgD(E158A) double point mutant Gc were exposed to neutrophils as in Fig. 1A. *P<0.05 for ltgA(E481A)ltgD(E158A) compared to double complement; two-tailed t-test, n = 3 independent experiments.
In the absence of neutrophils, WT and ΔltgAΔltgD Gc grew with similar kinetics, in agreement with previous reports (Fig. 1B) (Cloud-Hansen et al., 2008). Further, WT and ΔltgAΔltgD Gc displayed similar levels of metabolic activity as measured with alamarBlue, and the colonies of WT and ΔltgAΔltgD Gc yielded similar CFU (Fig. 1C, D). Deletion of ltgA and ltgD did not result in gross differences in the outer membrane protein profile (Fig. S2A). Moreover, the PG composition of the intact cell wall was similar between WT and ΔltgAΔltgD mutant Gc (Fig. S2B and Table S2). Taken together, these data indicate that loss of LtgA and LtgD does not have a major impact on bacterial growth, outer membrane protein composition, or cell wall composition. Instead, LtgA and LtgD are specifically important for Gc survival after exposure to neutrophils.
To test a role for the enzymatic activity of LtgA and LtgD in survival from neutrophils, we used an enzymatically inactive double point mutant, ltgA(E481)ltgD(E158A), that fails to release extracellular PG monomer (Fig. S1B). The ltgA(E481)ltgD(E158A) double point mutant displayed a similar survival defect as ΔltgAΔltgD bacteria in the presence of neutrophils (Fig. 1E). We conclude that LtgA and LtgD LT enzymatic activity is important for Gc survival from human neutrophils.
Since neutrophils are more efficient at killing intracellular than extracellular Gc (Criss et al., 2009; Handing et al., 2015; Johnson et al., 2015; Johnson et al., 2013b), we examined whether loss of ltgA and ltgD affected association of Gc with neutrophils. ΔltgAΔltgD Gc associated with neutrophils to the same degree as WT and double complement bacteria (Fig. S3A) but was more readily internalized by the neutrophils (Fig. S3B). To test whether this increase in internalization accounted for the survival defect of ΔltgAΔltgD Gc, double mutant and double complement bacteria were opsonized with normal human serum, which normalized their internalization by neutrophils (Fig. S3C). Opsonization did not affect the respective survival curves of ΔltgAΔltgD and the double complement in the presence of neutrophils, implicating other mechanisms in the survival defect of the double mutant (Fig. S3D).
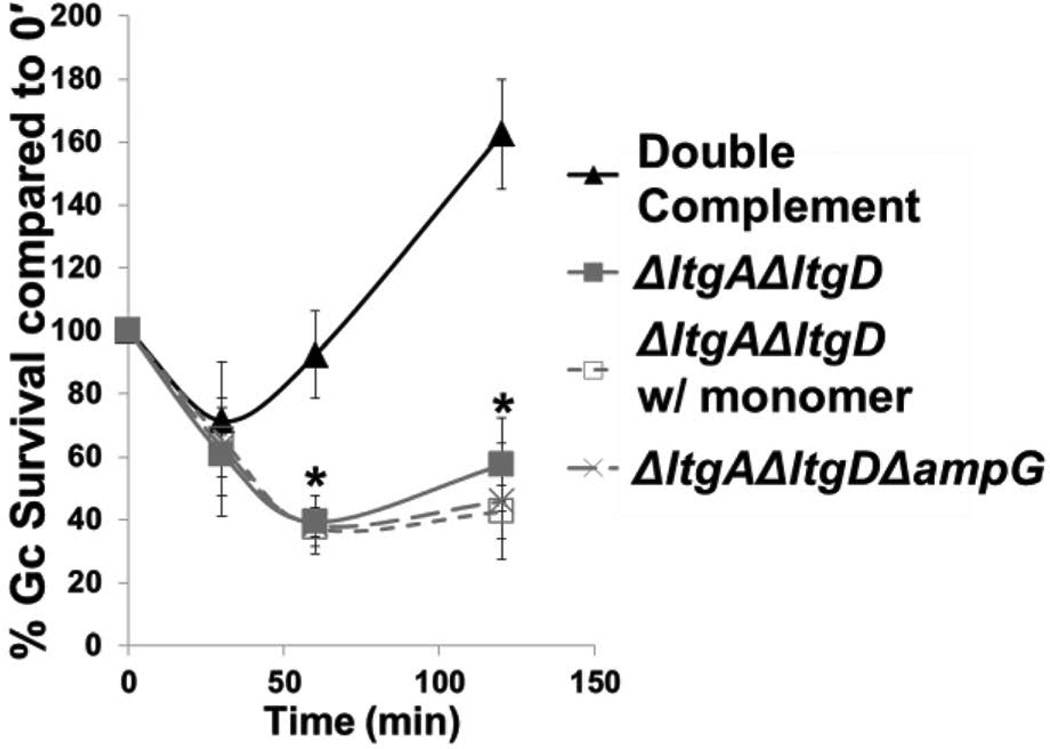
ΔltgAΔltgD Gc survival in primary human neutrophils is not affected by the presence of extracellular peptidoglycan monomers
Since PG monomers released from WT Gc have immunomodulatory potential and could be recognized by the proinflammatory NOD1 receptor (Mavrogiorgos et al., 2014; Melly et al., 1984), we examined whether the survival defect of ΔltgAΔltgD Gc was connected to the absence of monomer production in the double mutant. Addition of monomer to the infection medium failed to affect the survival profile of ΔltgAΔltgD Gc after exposure to neutrophils (Fig. 2). Moreover, mutation of the ampG permease in ΔltgAΔltgD (Garcia et al., 2008; Woodhams et al., 2013), which restored PG monomer release in the double mutant (Fig. S1B), failed to rescue the neutrophil survival defect conferred by loss of ltgA and ltgD (Fig. 2). These results indicate that monomer release is not responsible for the increased sensitivity of ΔltgAΔltgD Gc to killing by neutrophils.
Figure 2. ΔltgAΔltgD Gc survival in primary human neutrophils is not affected by the presence of peptidoglycan monomers.
ltgA+ ltgD+ double complement, ΔltgAΔltgD double mutant, and ΔltgAΔltgDΔampG triple mutant Gc were exposed to adherent, IL-8-treated neutrophils. For ΔltgAΔltgD treated with monomer, Gc infected neutrophils were exposed to 1 µg/mL monomer for the duration of the infection. *P<0.05 for ΔltgAΔltgD with monomer compared to double complement and for ΔltgAΔltgDΔampG compared to double complement; two-tailed t-test, n = 3 independent experiments.
LtgA and LtgD are important for Gc survival from the neutrophil antimicrobials lysozyme and neutrophil elastase
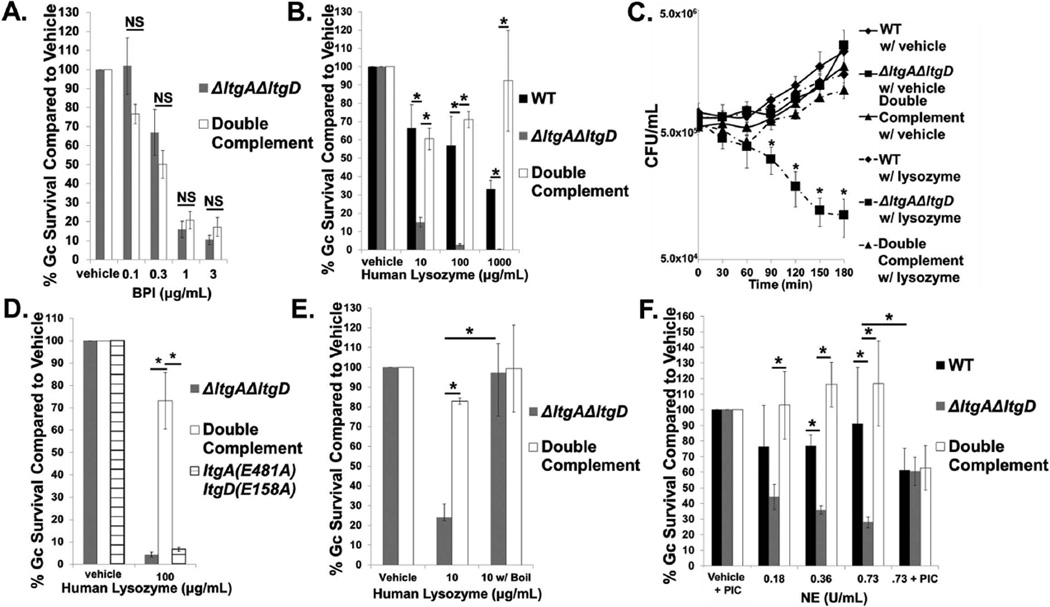
Since LtgA and LtgD are important for Gc survival in a mechanism independent of PG monomer release, we next examined whether LtgA and LtgD were important for Gc resistance to the antimicrobial proteins stored within neutrophils. To this end, ΔltgAΔltgD and double complement Gc were exposed in vitro to increasing concentrations of a variety of neutrophil antimicrobials. While changes to opacity protein expression in Gc can render bacteria more intrinsically sensitive to bactericidal permeability-increasing protein (BPI) (Johnson et al., 2015), we found that ΔltgAΔltgD Gc were unaffected in survival compared to the double complement in the presence of BPI (Fig. 3A). In addition, ΔltgAΔltgD Gc displayed equivalent survival to the neutrophil antimicrobials azurocidin, human neutrophil peptide-1 (HNP-1), LL-37, and cathepsin G compared to the double complement (Fig. S4A–D). We also found that ΔltgAΔltgD Gc was not more sensitive to the cationic antimicrobial polymyxin B (Fig. S4E).
Figure 3. LtgA and LtgD are important for survival from the neutrophil antimicrobials lysozyme and neutrophil elastase.
A. ΔltgAΔltgD double mutant and ltgA+ltgD+ double complement Gc were exposed to increasing concentrations of bactericidal permeability-increasing protein (BPI) or vehicle control for 45 min. Gc survival at each concentration was determined by first dividing CFU/mL at 45 min by CFU/mL at 0 hr and is expressed in relation to survival of vehicle control. n = 4 to 7 biological replicates.
B. WT, ΔltgAΔltgD double mutant, and ltgA+ltgD+ double complement Gc were exposed to increasing concentrations of human lysozyme or vehicle control for 3 hr. Gc survival is expressed as in Fig. 3A. n = 3 to 6 biological replicates.
C. CFU/mL was enumerated for WT, ΔltgAΔltgD double mutant, and ltgA+ltgD+ double complement Gc exposed to 100 µg/mL of human lysozyme or vehicle control. n = 3 biological replicates.
D. ΔltgAΔltgD double mutant, ltgA+ltgD+ double complement, and ltgA(E481A)ltgD(E158A) double point mutant Gc were exposed to 100 µg/mL human lysozyme or vehicle control for 3 hr. Gc survival is expressed as in Fig. 3A. n = 3 biological replicates.
E. ΔltgAΔltgD double mutant and ltgA+ltgD+ double complement Gc were exposed for 3 hr to lysozyme that had been boiled for 1 hr to eliminate PG hydrolase activity. Gc survival is expressed as in Fig. 3A. n = 3 biological replicates.
F. WT, ΔltgAΔltgD double mutant, and ltgA+ltgD+ double complement Gc were exposed to increasing concentrations of neutrophil elastase (NE) or vehicle control for 3 hr. In some experiments, 0.73 U/mL NE and vehicle were incubated with a protease inhibitor cocktail (PIC) for 30 min prior to addition of bacteria and for the duration of bacterial exposure. Gc survival is expressed as in Fig. 3A. n = 3 to 6 biological replicates.
In all cases, *P<0.05; two-tailed t-test.
In contrast, and to our surprise, ΔltgAΔltgD Gc was significantly decreased in survival upon exposure to human lysozyme compared to WT and the double complement (Fig. 3B). Survival of ΔltgAΔltgD Gc was significantly reduced as early as 90 minutes after exposure to human lysozyme (Fig. 3C). LT activity is important for Gc survival from human lysozyme since ltgA(E481)ltgD(E158A) displayed a similar, significant decrease in survival compared to ΔltgAΔltgD upon exposure to human lysozyme (Fig. 3D). Lysozyme can kill bacteria through its enzymatic PG hydrolase activity or through its cationic activity (Herbert et al., 2007; Nash et al., 2006). To test whether cationicity is important in ΔltgAΔltgD sensitivity to lysozyme, we exposed Gc to lysozyme that had been boiled for 1 hour, which has been shown to eliminate PG hydrolase activity while maintaining cationic antimicrobial activity (Herbert et al., 2007). We confirmed that boiled lysozyme no longer had activity against Micrococcus luteus, which is highly sensitive to PG hydrolase activity (Fig. S5). Exposure of ΔltgAΔltgD Gc to boiled lysozyme rescued its survival to double complement levels, indicating that the increased sensitivity of the mutant is due to lysozyme hydrolase activity (Fig. 3E). ΔltgAΔltgD Gc was also significantly decreased in survival in the presence of neutrophil elastase (NE) (Fig. 3F). NE is a serine protease and is also cationic (Korkmaz et al., 2007). Inhibiting NE protease activity significantly increased the survival of ΔltgAΔltgD Gc to levels similar to WT and double complement Gc, indicating that ΔltgAΔltgD Gc is specifically more sensitive to the protease activity of NE (Fig. 3F). These findings show that LtgA and LtgD are important for survival from two important neutrophil antimicrobials, NE and lysozyme. We subsequently focused on the lysozyme sensitivity of the ΔltgAΔltgD mutant.
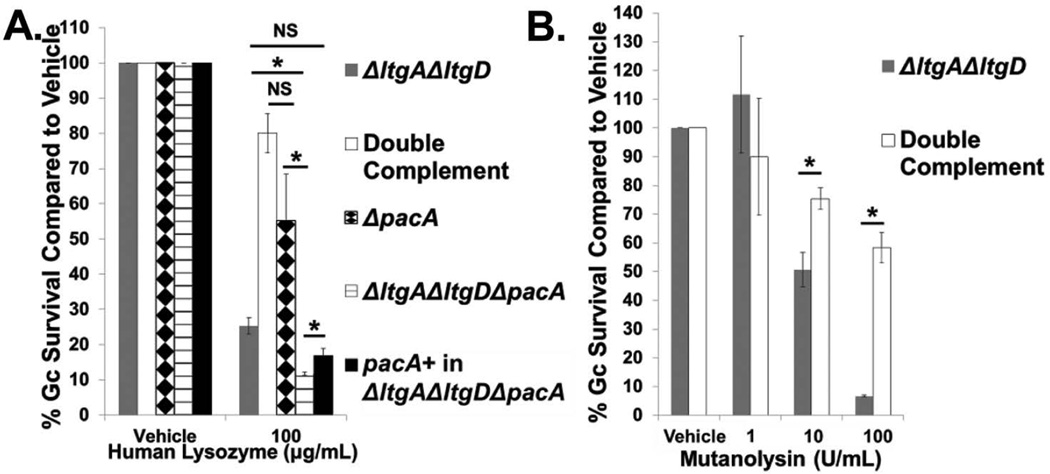
Lysozyme is not generally considered antibacterial to Gram-negative bacteria because it is too large (14 kDa) to pass through the outer membrane to access PG (Nikaido, 2003). Thus, we sought to define the mechanism underlying the increased sensitivity of ΔltgAΔltgD Gc to lysozyme. We first tested the hypothesis that the PG of ΔltgAΔltgD Gc is more sensitive to lysozyme digestion. Lysozyme kills bacteria via hydrolysis of polymeric PG into monomeric PG, consequently destabilizing the cell wall, but some bacteria can sterically inhibit lysozyme degradation by acetylating the PG. The ΔltgAΔltgD mutant exhibited a slight decrease in acetylation compared to wild type Gc. In the sacculus of the mutant, GlcNAc-O-acetyl-MurNAc-tripeptide decreased by 17%, and GlcNAc-O-acetyl-MurNAc-tetrapeptide decreased by 34% (Fig S2B and Table S2). The acetylation of PG in Gc requires the cooperative action of PacA and PacB; the PG purified from ΔpacA mutant Gc is significantly more susceptible to lysozyme degradation (Dillard et al., 2005; Moynihan et al., 2014). To test the contribution of acetylated PG in Gc resistance to lysozyme, we deleted pacA from WT and ΔltgAΔltgD Gc. Loss of pacA had no effect on the sensitivity of intact WT Gc to lysozyme (Fig. 4A). In contrast, pacA deletion significantly decreased the survival of ΔltgAΔltgD Gc after incubation with lysozyme, which was rescued by complementation with an intact copy of pacA (Fig. 4A). Further, deletion of pacA in the WT background did not affect the survival profile of WT Gc exposed to neutrophils (Fig. S6). These observations suggest that the PG of ΔltgAΔltgD Gc is not intrinsically more sensitive to digestion by lysozyme.
Figure 4. ΔltgAΔltgD sensitivity to PG digestion occurs independently of PG acetylation.
A. ΔltgAΔltgD double mutant, ltgA+ltgD+ double complement, ΔpacA, ΔltgAΔltgDΔpacA, and pacA+ complement in ΔltgAΔltgDΔpacA were exposed to 100 µg/mL human lysozyme for 90 min. Gc survival is expressed as in Fig. 3A. *P<0.05; two-tailed t-test, n = 3 to 6 biological replicates.
B. ΔltgAΔltgD double mutant and ltgA+ltgD+ double complement Gc were exposed to increasing concentrations of mutanolysin for 3 hr. Gc survival is expressed as in Fig. 3A. *P<0.05; two-tailed t-test, n = 3 biological replicates.
As a complementary approach, we tested the sensitivity of ΔltgAΔltgD Gc to mutanolysin, which cleaves PG similarly to lysozyme but is uninhibited by acetylation. ΔltgAΔltgD Gc was still significantly reduced in survival in the presence of mutanolysin compared to the double complement (Fig. 4B). The similar sensitivity of ΔltgAΔltgD bacteria to mutanolysin or lysozyme directly demonstrates that the composition of the PG in the mutant does not explain its lysozyme sensitivity. Taken together, these results suggest that PG acetylation is not responsible for the increased sensitivity of ΔltgAΔltgD Gc to PG hydrolases.
Our second hypothesis to explain the increased sensitivity of ΔltgAΔltgD Gc to human lysozyme was that the mutant has decreased envelope integrity, allowing lysozyme to access the normally inaccessible PG. In support of this hypothesis, we found that ΔltgAΔltgD Gc exhibited a lower minimum inhibitory concentration (MIC) to vancomycin, which is too large to cross the Gram-negative outer membrane, compared to WT or the double complement (Table 1). The mutant was unchanged in its susceptibility to streptomycin, which is not excluded by the outer membrane of Gram-negative bacteria (Table 1). Sensitivity to vancomycin also involved LT enzymatic activity since ltgA(E481)ltgD(E158A) and ΔltgAΔltgD bacteria had similar MICs (Table 1). We also found that a significantly greater percentage of ΔltgAΔltgD Gc were positive for the membrane impermeant dye, propidium iodide, compared to WT bacteria (Fig. 5A). In addition to measuring the ability of extracellular molecules to gain access to Gc, we also measured the leakage of bacterial molecules into the extracellular space as surrogate measures of envelope permeability. ΔltgAΔltgD Gc had increased amounts of extracellular ATP as well as increased extracellular adenylate kinase activity, compared with WT or double complement bacteria; ATP and adenylate kinase are normally cytosolic (Fig. 5B–C) (Schwechheimer et al., 2013). Since EDTA sequesters cations important for membrane stabilization, we used EDTA to artificially induce membrane permeability across all strains (Alakomi et al., 2003; Vaara, 1992). In the presence of both EDTA and lysozyme, ΔltgAΔltgD Gc survived as poorly as WT and the double complement (Fig. 5D). These results suggest that LtgA and LtgD contribute to the integrity of the membrane barrier in Gc, specifically helping protect PG from lysozyme.
Table 1. Minimum inhibitory concentration (MIC) of WT, ΔltgAΔltgD double mutant, ltgA+ ltgD+ double complement, and ltgA(E481A)ltgD(E158A) double point mutant Gc to vancomycin and streptomycin.
Lawns of WT, ΔltgAΔltgD double mutant, ltgA+ ltgD+ double complement, and ltgA(E481A)ltgD(E158A) double point mutant Gc on solid medium were exposed to vancomycin or streptomycin Etests to determine MIC after 24 hr incubation at 37°C with 5% CO2. MIC is represented as a range from 3 biological replicates.
| Antibiotic | WT | ΔltgAΔltgD | Double Complement |
ltgA(E481A) ltgD(E158A) |
|---|---|---|---|---|
| Vancomycin | 16–24 µg/mL | 6–8 µg/mL | 16 µg/mL | 8 µg/mL |
| Streptomycin | >1.024 mg/mL | >1.024 mg/mL | >1.024 mg/mL | >1.024 mg/mL |
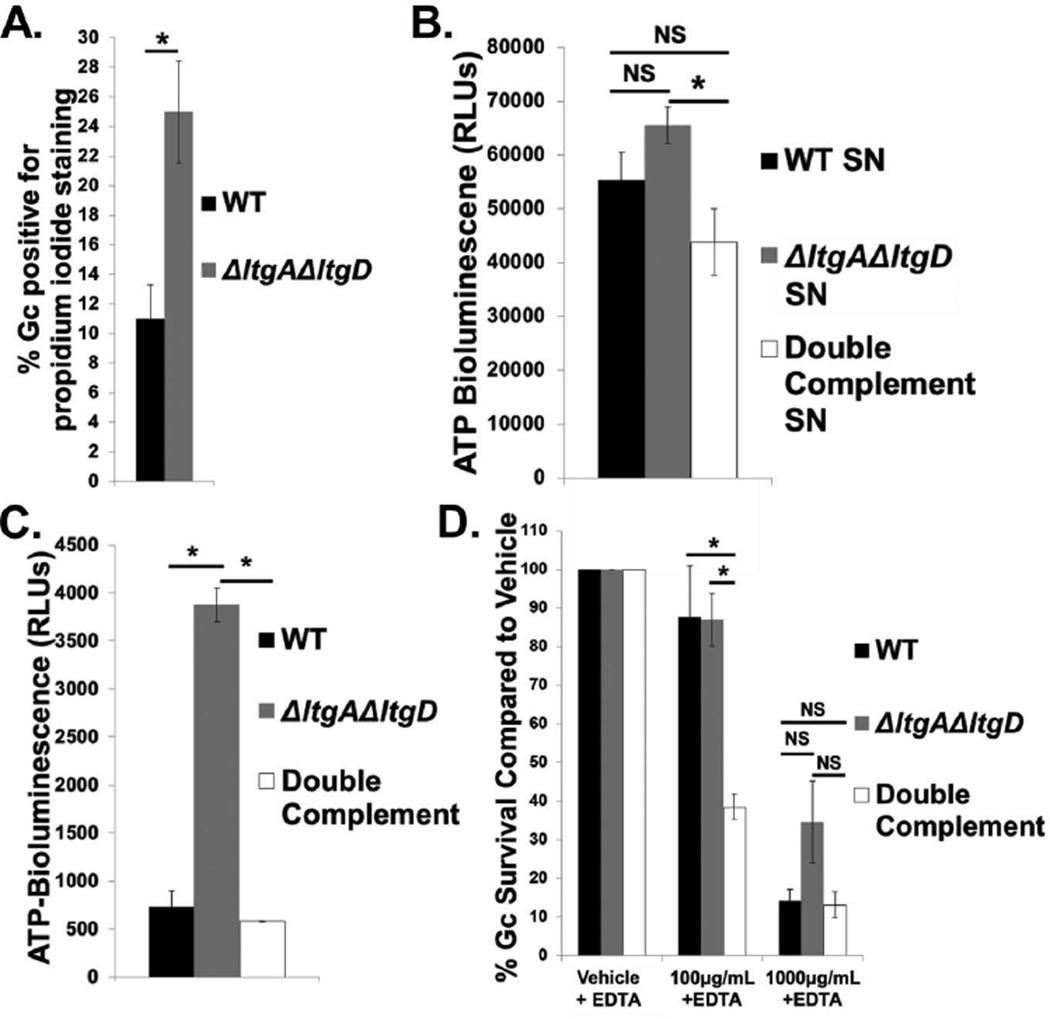
Figure 5. LtgA and LtgD contribute to Gc membrane integrity.
A. WT and ΔltgAΔltgD double mutant Gc were treated with propidium iodide to label membrane-permeant Gc and Syto9 to label total Gc. Percent Gc positive for propidium iodide staining was calculated by dividing total propidium iodide positive Gc by total Gc. n = 5 biological replicates.
B. Supernatant (SN) was harvested from WT, ΔltgAΔltgD double mutant, and ltgA+ltgD+ double complement Gc at an equivalent optical density and incubated with luciferin and luciferase from an ATP determination kit. Bioluminescence was measured with a luminometer as relative light units (RLUs). n = 3 biological replicates.
C. WT, ΔltgAΔltgD double mutant, and ltgA+ltgD+ double complement Gc at an equivalent optical density were incubated with Toxilight reagent containing ADP, luciferin, and luciferase for 5 min at room temperature. Positive extracellular adenylate kinase activity produced bioluminescence, reported in RLUs. n = 3 biological replicates.
D. WT, ΔltgAΔltgD double mutant, and ltgA+ltgD+ double complement Gc were exposed to increasing concentrations of human lysozyme or vehicle control in the presence of 1mM EDTA for all conditions, for 30 min. Gc survival is expressed as in Fig. 3A. n = 6 to 9 biological replicates.
In all cases, *P<0.05; two-tailed t-test.
Because ΔltgAΔltgD Gc showed increased permeability in vitro, we next asked whether Gc permeability was altered in the presence of neutrophils ex vivo. To this end, we used a fluorescence-based assay to examine the permeability of cell-associated Gc to propidium iodide while discriminating between intracellular and extracellular bacteria (Johnson et al., 2013a). We found that ΔltgAΔltgD Gc was significantly increased in permeability to propidium iodide both intracellularly and extracellularly, compared with WT or the double complement (Fig. 6A,B). While bacteria with more permeant membranes are positively stained for propidium iodide, positive staining also correlates with bacterial viability. In this assay, we cannot distinguish between Gc that have a more permeable membrane or Gc that are nonviable. However, since ΔltgAΔltgD Gc are decreased in survival as measured by CFU (Fig. 1A), some propidium iodide-positive ΔltgAΔltgD likely represent nonviable bacteria.
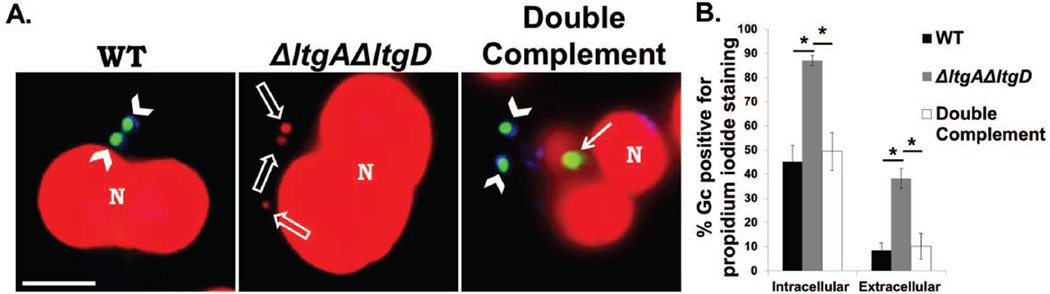
Figure 6. LtgA and LtgD are important for Gc integrity both in the intracellular and extracellular compartments of primary human neutrophils.
A. WT, ΔltgAΔltgD double mutant, and ltgA+ ltgD+ double complement Gc were exposed to adherent, IL-8-treated primary human neutrophils for 1 hr. Extracellular Gc were stained with Alexa Fluor 647 conjugated soybean lectin (blue). Impermeant Gc were stained with Syto9 (green) while permeant Gc were stained with propidium iodide (red). Arrowheads indicate extracellular, impermeant Gc. Solid arrows indicate intracellular, impermeant Gc. Open arrows indicate intracellular, permeant Gc. Neutrophil nuclei were also stained with propidium iodide (N). Scale bar, 5 µm. Quantitiation of the percent of propidium iodide-positive intracellular and extracellular Gc is presented in B.
*P<0.05; two-tailed t-test, n = 6 independent experiments.
We conclude that LtgA and LtgD are essential for Gc resistance to lysozyme and NE but dispensable for Gc defense from cationic antimicrobials. Our results show that LtgA and LtgD contribute to envelope stability, which may aid in Gc resistance to lysozyme and to neutrophil killing within the intracellular and extracellular compartments.
Increased secondary granule exocytosis and secondary granule fusion to the phagosome upon exposure of neutrophils to ΔltgAΔltgD Gc
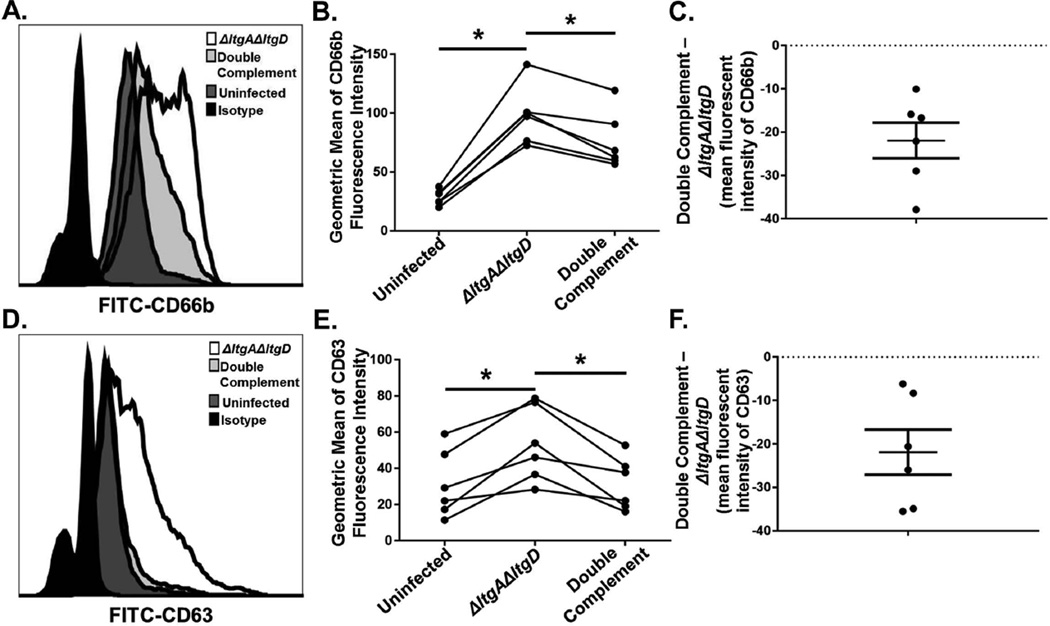
In neutrophils, lysozyme is stored within both primary and secondary granules, while NE is stored within primary granules (Baggiolini et al., 1969; Cramer et al., 1987). These granule subsets can be exocytosed to the cell surface and to phagosomes. We found that neutrophils infected with ΔltgAΔltgD Gc displayed significantly increased surface expression of the secondary granule protein CD66b compared to double complement-infected neutrophils, indicative of enhanced secondary granule exocytosis (Fig. 7A–C). ΔltgAΔltgD-infected neutrophils also exhibited increased CD63 surface expression, a marker for primary granule exocytosis, compared to double complement infected neutrophils (Fig. 7D–F).
Figure 7. Increased secondary and primary granule exocytosis in primary human neutrophils exposed to ΔltgAΔltgD double mutant Gc.
IL-8-treated, adherent human neutrophils were left untreated, exposed to ΔltgAΔltgD double mutant, or exposed to ltgA+ltgD+ double complement Gc for 1 hr. Neutrophils were subsequently stained with FITC-CD66b as a marker for secondary granule exocytosis (A) or FITC-CD63 as a marker for primary granule exocytosis (D) and analyzed by flow cytometry. The geometric mean fluorescence intensity for CD66b (B) and CD63 (E) was calculated from the granulocyte population. The differences of mean fluorescence intensity of ΔltgAΔltgD mutant Gc from double complement Gc were determined for CD66b (C) and CD63 (F); data are shown as mean ± SEM. *P < 0.05; paired, two-tailed t-test, n = 6 independent experiments.
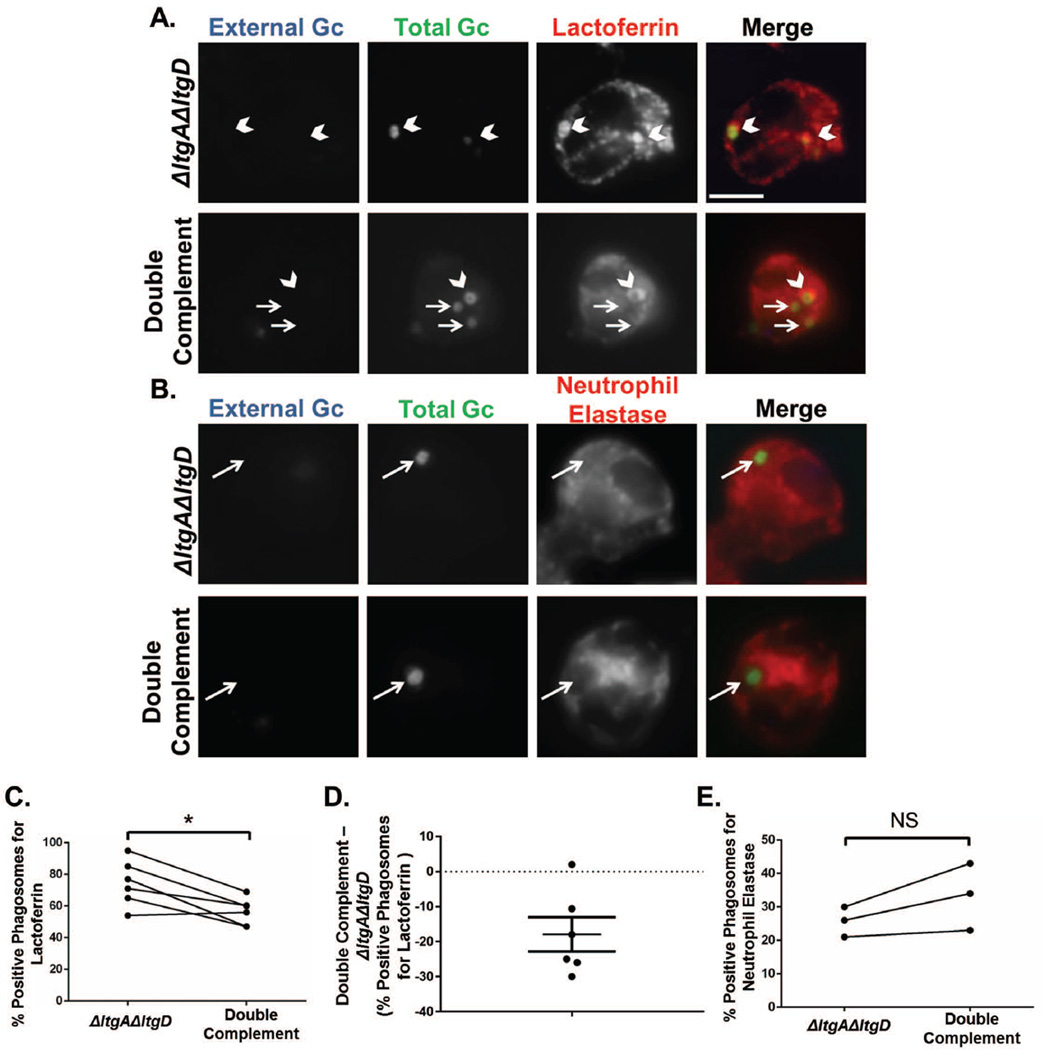
Since phagosomal maturation impacts the survival of Gc inside neutrophils (Johnson et al., 2013b), we also tested whether granule fusion to the Gc-containing phagosome was altered between ΔltgAΔltgD and double complement bacteria. Phagosomes containing ΔltgAΔltgD Gc showed a significant increase in colocalization with the secondary granule protein lactoferrin compared to phagosomes with double complement Gc (Fig. 8A,C,D). In contrast, ΔltgAΔltgD and double complement Gc phagosomes were similarly enriched for the primary granule protein NE (Fig. 8B,E). Together, these data suggest that neutrophils infected with ΔltgAΔltgD Gc, but not double complement Gc, have greater secondary and primary granule exocytosis and secondary granule fusion to the phagosome. Consequently, ΔltgAΔltgD Gc may have increased exposure to lysozyme and NE contained within primary and secondary granules during infection.
Figure 8. Increased delivery of secondary granules to neutrophil phagosomes containing ΔltgAΔltgD double mutant Gc.
Neutrophils were exposed to CFSE-labelled ΔltgAΔltgD double mutant or ltgA+ltgD+ double complement Gc (green) for 1 hr. Extracellular bacteria were labelled with an anti-Gc antibody (blue). Phagosomes were labelled with an anti-lactoferrin antibody to identify secondary granule-positive phagosomes (A) or an anti-neutrophil elastase antibody to identify primary granule-positive phagosomes (B). Phagosomes were considered positive when greater than 50% of the ring around the bacterium is positively stained for the antibody. Scale bar, 5 µm. Percent phagosomes positive for lactoferrin (C) and neutrophil elastase (E) were quantified by dividing positive phagosomes by total phagosomes. D depicts the difference in lactoferrin-positive phagosomes between ΔltgAΔltgD double mutant and double complement Gc as mean ± SEM. *P<0.05 for ΔltgAΔltgD compared to double complement; two-tailed t-test, n= 3 to 6 independent experiments.
Discussion
In this work, we found that the LTs LtgA and LtgD contribute to Gc survival both intracellularly and extracellularly in the presence of primary human neutrophils. Although LtgA and LtgD are required for the extracellular release of PG monomers recognized by NOD1 (Cloud-Hansen et al., 2008; Mavrogiorgos et al., 2014), we did not find a role for extracellular monomer in mediating bacterial survival in neutrophils. Instead, LtgA and LtgD help maintain Gc envelope integrity and thereby exclude lysozyme from its periplasmic target. To our knowledge, this is the first mechanism described for resistance of intact Gc bacteria to lysozyme. LtgA and LtgD also contribute to Gc resistance to NE. Furthermore, when exposed to Gc expressing LtgA and LtgD, neutrophils deliver less lysozyme to Gc phagosomes and less lysozyme and NE to the extracellular space. Thus, these two LTs not only provide intrinsic resistance to lysozyme and NE, but also contribute to limiting Gc exposure to these antimicrobial compounds when challenged with neutrophils.
LtgA and LtgD contribute to Gc envelope integrity, yet membrane perturbations do not render Gc universally sensitive to all antimicrobials. LTs are upregulated upon exposure to host cells for some pathogens, and their deletion results in decreased virulence (Bartoleschi et al., 2002; Boch et al., 2002); however, it is still unclear how these nonessential proteins contribute to pathogenicity. Interestingly, membrane-bound LTs, but not soluble LTs, were found to be important for envelope integrity in Pseudomonas aeruginosa (Lamers et al., 2015). Both LtgA and LtgD are predicted to be anchored in the outer membrane of Gc based on their primary sequence (Chan et al., 2012) (R.E. Schaub, Y.A. Chan, D Hesek, M Lee, S Mobashery, JP Dillard, under review). Similarly, deletion of six LTs in E. coli resulted in decreased envelope integrity (Heidrich et al., 2002). Even though cationic antimicrobials typically target the cell envelope (Hancock et al., 2000; Wiesner et al., 2010), reduced envelope integrity failed to alter Gc sensitivity to the cationic activities of lysozyme and NE, as well as other cationic neutrophil-derived antimicrobial proteins and polymyxin B. Instead, reduced envelope integrity was linked to Gc sensitivity to the enzymatic activities of lysozyme and NE, suggesting that membrane perturbations can reveal otherwise inaccessible targets to some, but not all, antimicrobials.
LtgA and LtgD have overlapping functions in Gc. Both ltgA and ltgD are required to fully eliminate extracellular PG monomer release (Cloud-Hansen et al., 2008; Cloud et al., 2004), and, here, we found both mutations were required to produce a survival defect in neutrophils. This suggests that LtgA and LtgD may have cooperative, non-overlapping functions in Gc. LtgA and LtgD were recently found to reside in spatially distinct locations in the Gc cell (R.E. Schaub, Y.A. Chan, D Hesek, M Lee, S Mobashery, JP Dillard, under review). LTs may function as structural components in larger complexes, rendering the complex dysfunctional upon deletion. However, we found that LtgA and LtgD contribute to survival from neutrophils and lysozyme dependent on their LT enzymatic activity, and this activity was also important for membrane permeability.
As for other Gram-negative bacteria, the primary defense for Gc against lysozyme-mediated killing is the presence of an outer membrane. Recent in vivo evidence suggests that lysozyme cationic activity, not enzymatic activity, is more important for killing of the Gram-negative bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae (Nash et al., 2006). There are few methods to inhibit lysozyme, but we found boiling was sufficient to inhibit enzymatic activity, which also inhibited killing of ΔltgAΔltgD Gc. Thus, enzymatic activity is important for sensitivity of ΔltgAΔltgD bacteria to lysozyme. Gc acetylates its PG via the cooperative action of PacA and PacB to inhibit lysozyme digestion of purified PG (Dillard et al., 2005; Moynihan et al., 2014). Inactivating the acetylation machinery in other Gram-negative bacteria, such as Helicobacter pylori, increases their sensitivity to lysozyme (Wang et al., 2012; Wang et al., 2010). However, we did not find a role for acetylation in Gc in lysozyme resistance of intact bacteria nor in survival in the presence of neutrophils, except in the context of loss of LtgA and LtgD. This suggests that acetylated PG only provides resistance to lysozyme hydrolysis if the enzyme can access the periplasm.
Bacterial sensitivity to lysozyme coincides with enhanced killing in neutrophils. Mutants of Yersinia pestis, Streptococcus suis, and Shigella flexneri can be rendered more sensitive to lysozyme in vitro at similar concentrations used in this study. This also translates to enhanced sensitivity to killing by neutrophils ex vivo, supporting our findings here (Derbise et al., 2012; Fittipaldi et al., 2008; Kaoukab-Raji et al., 2012). In light of our in vitro conditions, the concentration of lysozyme in the phagosome of neutrophils should be high enough to adversely affect bacterial survival, since only a small fraction of the neutrophil’s total lysozyme is needed to produce high concentrations in the femtoliter-sized phagosome (Hansen et al., 1973). Further, the amount of lysozyme at the mucosal surface correlates to the concentrations used in this study, suggesting that ΔltgAΔltgD Gc may also be decreased in survival at the site of infection (Hein et al., 2002). The link between lysozyme sensitivity and enhanced neutrophil killing is further enforced because the addition of NOD1-activating PG monomers failed to alter ΔltgAΔltgD survival in neutrophils. Thus, LtgA and LtgD protection is not mediated through the release of immunomodulatory PG. These data further suggest that neutrophil killing activities are not enhanced by the addition of NOD1 agonists, in agreement with another study (Ekman et al., 2010).
Lysozyme sensitivity and PG alterations affect activation of the innate compartment. Bacterial mutants that become sensitive to lysozyme in vitro are more pro-inflammatory in the presence of macrophages (Shimada et al., 2010; Wolf et al., 2011). Moreover, alterations to the PG backbone that enhance host-driven bacterial degradation influences macrophage activation (Muller et al., 2015). Here, we show that neutrophils exposed to lysozyme-sensitive ΔltgAΔltgD Gc display increased secondary and primary granule exocytosis and secondary granule fusion to the phagosome. This is the first evidence correlating enhanced activation of human neutrophils in response to lysozyme-sensitive bacteria. Based on our present knowledge, these data suggest that bacterial digestibility by lysozyme within neutrophils can alter neutrophil responses. Moreover, Gc may defend itself from neutrophil killing by reducing exposure to degradative antimicrobials and/or through the maintenance of envelope integrity.
Experimental Procedures
Bacterial Strains
All Gc used in this study are in the MS11 background and are listed in Table S1. WT Gc is the VD300 pilin variant of MS11, which has a point mutation in the G4 region upstream of pilE to abolish antigenic variation (Cahoon et al., 2009) and is RecA+. ΔltgAΔltgD Gc was used from previous work (Cloud-Hansen et al., 2008). To create the ltgA+ ltgD+ double complement, ΔltgAΔltgD was transformed with pKH96 to create an IPTG inducible LtgA at the lctP-aspC site. To make pKH96, ltgA was PCR amplified with primers LtgA-Xba-RBS (GTC TAG ACC CTG ACC GTT CAG GGT TCC G) and LtgA-Pst (CGC GCT GCA GCG TCA ATG CCG TTC GGG AT) and inserted into pKH37 (Kohler et al., 2007). The resulting strain was then transformed with pRS62 to create an anhydrotetracycline (AT)-regulated LtgD construct at the iga-trpB site. To create pRS62, ltgD was PCR amplified with primers LtgD-SD-SacI-F (5’-TTGAGCTCACTTACCCTATTTGTTCGGAATGTG-3’) and LtgD-SpeI-R (5’-TTACTAGTCCGAAACGTGGTTCAGACAGC-3’) and inserted into pMR68 (Ramsey et al., 2012) at SacI/SpeI sites.
To construct the ltgA(E481A)ltgD(E158A) double point mutant, pRS91 and pRS92 were used to make the ltgA and ltgD mutations, respectively (R.E. Schaub, Y.A. Chan, D Hesek, M Lee, S Mobashery, JP Dillard, under review). We confirmed that LtgA was expressed in ltgA(E481)ltgD(E158A) at the protein level in our experimental conditions (data not shown). To construct the ΔltgAΔltgDΔampG triple mutant, ΔltgAΔltgD Gc was transformed with pDG132 (Garcia et al., 2008).
The ΔpacA mutation was introduced into the ΔltgAΔltgD background with pKH40. pKH40 carries a copy of pacA cloned from the RD5 strain of Gc, which has a 26bp deletion in the coding sequence (Dillard et al., 2005). Linearized pKH40 was used to transform ΔltgAΔltgD Gc. Positive transformants were screened by PCR with pacA internal primers (5’-TTATTCCTCCTGCGTGTACC-3’ and 5’- ATTGGCAAAGAGGGCACTGA −3’) followed by digestion with restriction enzyme BsiEI. Sequencing of positive transformants confirmed the 26bp deletion of pacA in the chromosome. pacA+ was complemented into ΔltgAΔltgDΔpacA using pKH38 (Dillard et al., 2005). Positive transformants were selected on 6µg/mL chloramphenicol. Sequencing with internal pacA primers above and primers at the complementation site between lctP and aspC (5’-CTGAAGACATCCGCCAACAA-3’ and 5’-TTCCGGCTCGTATGTTGTGT-3’) confirmed complementation with MS11 WT pacA. The ΔpacA mutation was introduced into the WT strain as described above.
MS11 pacA msbB::aph-3, strain KH624, was used to purify PG fragments. This strain was made by transforming KH518 (Dillard et al., 2005) with DNA from 1291msbB::aph-3 (Post et al., 2002) to limit proinflammatory LOS. The KH518 strain carries a pacA mutation which aids in enhanced monomer yield.
Bacterial Growth Conditions
Piliated Gc were grown on Gonococcal Medium Base (GCB, Difco) plus Kellogg’s supplements (Kellogg et al., 1963) at 37°C with 5% CO2. Gc was grown in rich liquid medium (GCBL) for successive rounds of dilution until bacterial growth in mid-logarithmic phase was achieved, as previously described (Criss et al., 2008). All Gc lacked Opa expression as confirmed by immunoblotting with the 4B12 pan-Opa antibody (data not shown). To induce the double complement, 1mM IPTG and 10 ng/mL AT were added to growing Gc at least 2.5 hr prior to use. To induce the pacA complement, 1mM IPTG was added to growing Gc 20 hr prior to use. Where indicated, 1×108 CFU of Gc were labelled with 40 µg/mL of carboxyfluorecein diacetate succinimidyl ester (CFSE) for 20 min at 37°C. For serum opsonization, 1×108 CFU of Gc were incubated with 50% human autologous serum from healthy human donors for 20 min at 37°C.
Neutrophil Isolation
Venous blood was collected from healthy human donors with their informed consent in accordance with an approved protocol by the University of Virginia Institutional Review Board for Health Sciences Research. Neutrophils were isolated and stored as described (Stohl et al., 2005). Neutrophils less than two hours old were used for all experiments. In some cases, venous blood was also collected in the absence of heparin, incubated at 37°C to clot red blood cells, and spun down to harvest serum.
Gc Survival from IL-8-treated, Adherent Neutrophils
Synchronized Gc-neutrophil infections were carried out at an MOI of 1, and analysis of survival was performed as previously described (Stohl et al., 2005). In brief, adherent neutrophils were exposed to 10nM human IL-8 (R&D) in RPMI with 10% FBS at 37°C with 5% CO2 for at least 30 min prior to synchronous exposure to Gc. Nonadherent bacteria were removed, and Gc-exposed neutrophils were incubated in RPMI with 10% FBS at 37°C with 5% CO2. As a control, Gc were also incubated in RPMI with 10% FBS in the absence of neutrophils to verify each strain grows similarly under these experimental conditions. At given times, neutrophils were lysed in 1% saponin and serial dilutions of the lysates plated on GCB. CFU/mL was derived from enumerated colonies, and percent survival was calculated by dividing CFU/mL at each time point by CFU/mL at 0 min. For experiments involving the addition of Gc-derived peptidoglycan monomers, 1 µg/mL 1,6-anhydrotripeptide monomer and 1 µg/mL 1,6-anhydrotetrapeptide monomer were added to the medium throughout the infection. These are the predominant PG monomers released by WT Gc (Rosenthal, 1979; Sinha et al., 1980).
Characterization of Released Peptidoglycan Fragments
Released peptidoglycan fragments were characterized as described (Cloud et al., 2002) except that log-phase cultures of Gc were pulse-labeled with 10 µCi/mL [6-3H]glucosamine for 45 min.
Characterization of Outer Membrane Protein Composition
Outer membrane fractions were prepared as previously described with the following changes (Ramsey et al., 2014). Gc was cultivated from overnight lawns in 24 mL modified GCBL at a starting OD540 of 0.25 for 3 hr at 37°C. The bacteria were pelleted, washed in 1×PBS, and resuspended in 11.25 mL cold lithium acetate buffer followed by gentle rocking for 10 min. Membrane blebbing was induced by passage through a 22-gauge syringe-needle approximately 16 times, and cellular debris was pelleted at 10,000 rpm at 4°C for 10 min. Supernatants were passed through a 0.45 µm filter membrane, and the filtrate was ultracentrifuged at 80,000 rpm at 4°C for 2 hr with a Beckman TLA110 rotor. The outer membrane pellet was washed once with 0.01 M Tris-HCl, pH 7.0 and ultracentrifuged as before. The pellet was resuspended in H2O and analyzed for protein concentration by BCA assay. Silver staining was conducted according to the manufacturer (Pierce).
Characterization of Cell Wall PG by Mass Spectrometry
Three 250 mL cultures of WT and ΔltgAΔltgD mutant Gc were grown to late log phase in GCBL containing Kellogg’s supplements, and PG was isolated as described previously (Dillard et al., 2005). Purified sacculi were digested with a final concentration of 20 µg/mL mutanolysin (Sigma) for48 hrs. Insoluble peptidoglycan and proteins were removed using an Amico-Ultra 10 kDa filter. Soluble peptidoglycan fragments were separated by HPLC as described (Dougherty, 1985).
Fluorescence Assays
Extracellular and Intracellular Permeability of Gc in Neutrophils
Baclight viability dyes (Life Technologies) were used to stain membrane permeant bacteria (propidium iodide) and total bacteria (Syto9) and analyzed as previously described (Johnson et al., 2013a). Briefly, acid-washed glass coverslips were coated with 50% normal human serum at 37°C for 30 min prior to infection. Mid-log phase Gc were exposed to neutrophils for 1 hr at 37°C at an MOI of 1. The percent of Gc positive for propidium iodide was calculated relative to total bacteria (propidium iodide-positive and –negative) for the intracellular and extracellular compartments. From these experiments, percent internalization was also determined by dividing total intracellular bacteria by total bacteria, regardless of propidium iodide fluorescence. For experiments assessing serum opsonization and internalization, extracellular bacteria were stained with Alexa Fluor 647-coupled soybean lectin (ThermoFisher), and total bacteria were stained with 5 µM Syto9 (ThermoFisher) following neutrophil permeabilization; percent internalization was determined by dividing the number of intracellular bacteria by the number of cell-associated (internal and external) bacteria. At least 60 bacterial cells were assessed per strain for each independent experiment.
Granule Fusion to the Gc-containing Phagosome
Granule fusion to the Gc-containing phagosome was measured as previously described (Handing et al., 2015). Bacterial phagosomes were considered positive for granule fusion when greater than 50% of the phagosome was enriched for staining. Percent phagosomes positive for granule fusion was calculated by dividing the number of positive phagosomes by the total number of phagosomes. Approximately 70 phagosomes were counted per strain for each independent experiment.
Measurement of Membrane Permeability with Propidium Iodide
Mid-log phase Gc were exposed to BacLight viability dyes propidium iodide and Syto9. Approximately 200 bacteria were counted per biological replicate. The percent of Gc positive for propidium iodide staining, indicating bacteria with permeable membranes, was calculated by dividing propidium iodide positive bacteria by total bacteria.
Image Acquisition
Images were obtained using a Nikon E800 with Hamamatsu Orca-ER digital camera using Openlab software and processed in Adobe Photoshop CS3.
Isolation of Peptidoglycan Monomer from Gc
Sacculi were purified from 40 agar plates of N. gonorrhoeae strain KH624 by the boiling SDS method essentially as described (Stohl et al., 2012). The preparation (0.5ml) was digested at 37°C with 80µl of purified NGO1686 (final concentration, 1.4µM) in 25mM sodium phosphate buffer, pH 6.0 containing 625 µM ZnSO4 (final reaction volume 800µl) overnight to cleave peptide crosslinks. Reaction was boiled for 10 min to inactivate NGO1686. 25µl of purified LtgA (final concentration, 0.5µM) was added and incubation was continued overnight at 37°C to cleave the peptidoglycan strands into small soluble fragments; LtgA or LtgD cleavage of PG results in a 1,6-anhydro monomer that reproduces the monomer phenotype released by WT Gc (Sinha et al., 1980). The solution was boiled for 10 min and centrifuged at 16,000 × g for 10 min to remove insoluble material. Additional large molecular weight molecules were removed by centrifugation of the solution through a 10,000 MW cutoff Centricon filter. Monomeric peptidoglycan fragments were separated from other soluble peptidoglycan fragments by reversed-phase HPLC, using a Waters Xselect CSH C18 preparative column (5µ, 10×250mm) and a 4–13% acetonitrile gradient over 30 min. Fractions containing PG monomers were pooled, lyophilized, and suspended in water. PG was quantified using the Fluoraldehyde OPA reagent (Thermo Scientific Pierce) based on reactivity with free amines.
Antimicrobial Protein Susceptibility
Mid-log phase Gc were suspended in 0.5× GCBL (where Kellogg’s supplements and NaHCO3 were also at 0.5×) and exposed to the following antimicrobial proteins at 37°C with 5% CO2. Percent survival is depicted as the percent survival of Gc during antimicrobial treatment divided by the percent survival of Gc during vehicle control treatment to normalize to vehicle-treated bacteria.
Lysozyme
Gc was incubated with indicated concentrations of human lysozyme (Sigma) reconstituted in water for 3 hr or otherwise indicated. To inactivate hydrolase activity, lysozyme was diluted in 50mM potassium phosphate buffer, pH 7.0 and boiled for 1 hr. For lysozyme treatment with EDTA, GC was incubated with the indicated concentrations of lysozyme in the presence of 1mM EDTA for 30 min.
Mutanolysin
Gc was incubated with indicated concentrations of mutanolysin (Sigma) reconstituted in 100mM potassium phosphate buffer, pH 6.2 for 3 hr.
Neutrophil Elastase (NE)
Gc was incubated with indicated concentrations of NE (Sigma) reconstituted in 0.5× GCBL for 3 hr. For treatment with a protease inhibitor cocktail, NE and vehicle control was incubated with 1× strength protease inhibitor cocktail set V (Millipore) for 30 min at 37°C prior to incubation with Gc.
Azurocidin
Gc was incubated with indicated concentrations of Azurocidin (Sigma) reconstituted in 0.5× GCBL for 45 min.
Human Neutrophil Peptide-1 (HNP-1)
Gc was incubated with indicated concentrations of the α-defensin HNP-1 (Sigma) reconstituted in 0.5× GCBL for 45 min.
LL-37
Gc was incubated with indicated concentrations of LL-37 (from William Shafer, Emory University) diluted in water for 45 min.
Bactericidal Permeability-Increasing Protein (BPI)
Gc was incubated with indicated concentrations of BPI (Novatein Biosciences) diluted in 0.01% acetic acid for 45 min.
Cathepsin G
Cathepsin G (MP Biomedicals) was prepared for use by overnight dialysis at 4°C in distilled water using SpectraPor dialysis membrane MW cutoff 3500 (Spectrum labs). Gc was incubated in increasing concentrations of dialyzed Cathepsin G diluted in water for 3 hr. Cathepsin G was treated with protease inhibitor cocktail as described above for NE.
Polymyxin B
Gc was incubated with indicated concentrations of polymyxin B (Alexis Biomedicals) diluted in water for 45 min.
Micrococcus luteus sensitivity to lysozyme enzymatic activity
Micrococcus luteus (ATCC 4698) was grown to stationary phase for 24 hr at 30°C in tryptic soy broth. For the assay, M. luteus was resuspended in 0.5× GCBL and exposed to human lysozyme. OD450 was measured over time with a Victor3 multilabel plate reader (Perkin-Elmer).
Measurement of Extracellular Adenylate Kinase Activity
Mid-log phase Gc of equivalent optical density was added to an equal volume of room temperature Toxilight Bioassay Kit reagent (Lonza) containing ADP, luciferin, and luciferase and incubated for 5 min at room temperature. Bioluminescence was measured with a Victor3 multilabel plate reader (Perkin-Elmer).
Measurement of Extracellular ATP
Mid-log phase Gc with equivalent optical density (OD) was pelleted at 10,000 × g for 3 min. Bacterial supernatants were diluted 1:10 with luciferin/luciferase standard reaction solution (Life Technologies) and incubated for 5 min at room temperature. Bioluminescence was measured as above.
Antibiotic Susceptibility
Gc was grown on GCB with 1mM IPTG and 10 ng/mL AT for 20 hr. Gc was suspended in GCBL at an OD600 of 0.5. A polystyrene swab (Puritan) was dipped into the culture and dabbed on the side of the tube prior to spreading on a fresh GCB plate with IPTG and AT. Etest strips with vancomycin or streptomycin (bioMérieux) were aseptically applied to the swabbed plate and incubated at 37°C with 5% CO2 for 24 hr. The minimum inhibitory concentration (MIC) was determined by location of the clearance endpoint along the Etest strip according to the manufacturer.
Flow Cytometry
Granule Exocytosis in Neutrophils
Exocytosis of neutrophil granules was measured by flow cytometry as adapted from Uriarte, et al (Uriarte et al., 2011). Mid-log phase Gc were exposed to neutrophils as for the fluorescence assays. Media was carefully aspirated, and 5mM EDTA was added to lift the cells for 10 min on ice. Lifted cells were washed twice with cold DPBS-0.1% dextrose. Cells were unstained, stained with FITC-CD63 (Ancel 215-040), stained with FITC-CD66b (Sanquin Pelicluster M1594), or stained with FITC isotype IgG1 control (BD 555748) for 30 min on ice. Cells were washed twice and fixed with 1% PFA. A FACSCalibur Benchtop Analyzer (BD) and FlowJo were used to acquire and analyze data. The geometric mean of FITC fluorescence intensity was derived from a gate that includes all granulocytes by SSC and FSC.
Imaging Flow Cytometry
Gc association to neutrophils was measured and analyzed by imaging flow cytometry using a spot count algorithm, as previously described (Smirnov et al., 2015).
Gc Metabolic Health as Measured by alamarBlue
Mid-log phase Gc with equivalent optical density (OD) were incubated 10:1 with alamarBlue cell viability reagent (Invitrogen) for 1 hr at 37°C with 5% CO2. A change in fluorescence after exposure to alamarBlue, indicative of the presence of metabolically active cells, was measured using a Victor 3 multilabel plate reader, using an excitation filter of 530/10 nm and emission filter of 590/10.
Gc CFU per Colony Assay
Gc were streaked on GCB agar for 24 hr at 37°C with 5% CO2. Five colonies from each strain from areas of the plate of equal colony density were absorbed on sterile filter paper. The filter paper was then vigorously vortexed in GCBL, bacteria were plated on GCB, and colonies were enumerated after 24 hr incubation at 37°C with 5% CO2.
Statistics
Experimental values are presented as the mean ± standard error of the mean (SEM) of at least three independent replicates. For assays involving neutrophils, each experiment comes from at least three different donors. A two-tailed Student’s t-test was used to determine significance unless otherwise noted. A p-value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We are grateful for Asya Smirnov’s expertise with the flow cytometry assays, Silvia Uriarte’s advice on the granule exocytosis assays, and Mary Gray’s assistance with the alamarBlue assay. We thank William Shafer for the generous gift of LL-37. We would also like to extend our gratitude to Bob Nakamoto and the members of the Criss laboratory, who have provided invaluable feedback and support. This work was supported by NIH R01 AI097157 to J.P. Dillard (subcontract to A.K. Criss) and R01 AI097312 to A.K. Criss. S.A. Ragland was supported in part by NIH T32 AI007046. Imagestream was supported by NIH SIG 1S10RR031633. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Author Contributions
SAR, JPD, and AKC designed and conceived this study. SAR and AKC designed the experiments. SAR performed and analyzed the experiments. RES constructed some of the strains used in this study and analyzed their PG release profiles and PG composition. KTH purified the PG fragments. SAR and AKC wrote the manuscript with assistance and advice from RES, KTH, and JPD.
References
- Alakomi HL, Saarela M, Helander IM. Effect of EDTA on Salmonella enterica serovar Typhimurium involves a component not assignable to lipopolysaccharide release. Microbiology. 2003;149:2015–2021. doi: 10.1099/mic.0.26312-0. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Hirsch JG, De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969;40:529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoleschi C, Pardini MC, Scaringi C, Martino MC, Pazzani C, Bernardini ML. Selection of Shigella flexneri candidate virulence genes specifically induced in bacteria resident in host cell cytoplasm. Cell Microbiol. 2002;4:613–626. doi: 10.1046/j.1462-5822.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Boch J, Joardar V, Gao L, Robertson TL, Lim M, Kunkel BN. Identification of Pseudomonas syringae pv. tomato genes induced during infection of Arabidopsis thaliana. Mol Microbiol. 2002;44:73–88. doi: 10.1046/j.1365-2958.2002.02877.x. [DOI] [PubMed] [Google Scholar]
- Cahoon LA, Seifert HS. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science. 2009;325:764–767. doi: 10.1126/science.1175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. US Department of Health and Human Services; 2013. [Google Scholar]
- Centers for Disease Control and Prevention. Reported STDs in the United States, 2014 National Data for Chlamydia, Gonorrhea, and Syphilis. United States Department of Health and Human Services; 2015. [Google Scholar]
- Chan YA, Hackett KT, Dillard JP. The lytic transglycosylases of Neisseria gonorrhoeae. Microb Drug Resist. 2012;18:271–279. doi: 10.1089/mdr.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud-Hansen KA, Hackett KT, Garcia DL, Dillard JP. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J Bacteriol. 2008;190:5989–5994. doi: 10.1128/JB.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud KA, Dillard JP. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect Immun. 2002;70:2752–2757. doi: 10.1128/IAI.70.6.2752-2757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud KA, Dillard JP. Mutation of a single lytic transglycosylase causes aberrant septation and inhibits cell separation of Neisseria gonorrhoeae. J Bacteriol. 2004;186:7811–7814. doi: 10.1128/JB.186.22.7811-7814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer EM, Breton-Gorius J. Ultrastructural localization of lysozyme in human neutrophils by immunogold. J Leukoc Biol. 1987;41:242–247. doi: 10.1002/jlb.41.3.242. [DOI] [PubMed] [Google Scholar]
- Criss AK, Katz BZ, Seifert HS. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 2009;11:1074–1087. doi: 10.1111/j.1462-5822.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss AK, Seifert HS. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell Microbiol. 2008;10:2257–2270. doi: 10.1111/j.1462-5822.2008.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbise A, Cerda Marin A, Ave P, Blisnick T, Huerre M, Carniel E, Demeure CE. An encapsulated Yersinia pseudotuberculosis is a highly efficient vaccine against pneumonic plague. PLoS Negl Trop Dis. 2012;6:e1528. doi: 10.1371/journal.pntd.0001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard JP, Hackett KT. Mutations affecting peptidoglycan acetylation in Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 2005;73:5697–5705. doi: 10.1128/IAI.73.9.5697-5705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty TJ. Analysis of Neisseria gonorrhoeae peptidoglycan by reverse-phase, high-pressure liquid chromatography. J Bacteriol. 1985;163:69–74. doi: 10.1128/jb.163.1.69-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman AK, Cardell LO. The expression and function of Nod-like receptors in neutrophils. Immunology. 2010;130:55–63. doi: 10.1111/j.1365-2567.2009.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–2432. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- Fittipaldi N, Sekizaki T, Takamatsu D, de la Cruz Dominguez-Punaro M, Harel J, Bui NK, et al. Significant contribution of the pgdA gene to the virulence of Streptococcus suis. Mol Microbiol. 2008;70:1120–1135. doi: 10.1111/j.1365-2958.2008.06463.x. [DOI] [PubMed] [Google Scholar]
- Garcia DL, Dillard JP. Mutations in ampG or ampD affect peptidoglycan fragment release from Neisseria gonorrhoeae. J Bacteriol. 2008;190:3799–3807. doi: 10.1128/JB.01194-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell EW, Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J Bacteriol. 1985;162:391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway DL, Perkins HR. Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J Gen Microbiol. 1985;131:253–263. doi: 10.1099/00221287-131-2-253. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- Handing JW, Criss AK. The lipooligosaccharide-modifying enzyme LptA enhances gonococcal defence against human neutrophils. Cell Microbiol. 2015;17:910–921. doi: 10.1111/cmi.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen NE, Andersen V. Lysozyme activity in human neutrophilic granulocytes. Br J Haematol. 1973;24:613–623. doi: 10.1111/j.1365-2141.1973.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Hebeler BH, Young FE. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976;126:1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich C, Ursinus A, Berger J, Schwarz H, Holtje JV. Effects of Multiple Deletions of Murein Hydrolases on Viability, Septum Cleavage, and Sensitivity to Large Toxic Molecules in Escherichia coli. J Bacteriol. 2002;184:6093–6099. doi: 10.1128/JB.184.22.6093-6099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- Herbert S, Bera A, Nerz C, Kraus D, Peschel A, Goerke C, et al. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 2007;3:e102. doi: 10.1371/journal.ppat.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Ball LM, Daily KP, Martin JN, Columbus L, Criss AK. Opa+ Neisseria gonorrhoeae exhibits reduced survival in human neutrophils via Src family kinase-mediated bacterial trafficking into mature phagolysosomes. Cell Microbiol. 2015;17:648–665. doi: 10.1111/cmi.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Criss AK. Fluorescence microscopy methods for determining the viability of bacteria in association with mammalian cells. J Vis Exp. 2013a;79 doi: 10.3791/50729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Criss AK. Neisseria gonorrhoeae phagosomes delay fusion with primary granules to enhance bacterial survival inside human neutrophils. Cell Microbiol. 2013b;15:1323–1340. doi: 10.1111/cmi.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau RA, Stevens JS, Apicella MA, Criss AK. A thermonuclease of Neisseria gonorrhoeae enhances bacterial escape from killing by neutrophil extracellular traps. J Infect Dis. 2015;212:316–324. doi: 10.1093/infdis/jiv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaoukab-Raji A, Biskri L, Bernardini ML, Allaoui A. Characterization of SfPgdA, a Shigella flexneri peptidoglycan deacetylase required for bacterial persistence within polymorphonuclear neutrophils. Microbes Infect. 2012;14:619–627. doi: 10.1016/j.micinf.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–385. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler PL, Hamilton HL, Cloud-Hansen K, Dillard JP. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J Bacteriol. 2007;189:5421–5428. doi: 10.1128/JB.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz B, Hajjar E, Kalupov T, Reuter N, Brillard-Bourdet M, Moreau T, et al. Influence of charge distribution at the active site surface on the substrate specificity of human neutrophil protease 3 and elastase. A kinetic and molecular modeling analysis. J Biol Chem. 2007;282:1989–1997. doi: 10.1074/jbc.M608700200. [DOI] [PubMed] [Google Scholar]
- Lamers RP, Nguyen UT, Nguyen Y, Buensuceso RN, Burrows LL. Loss of membrane-bound lytic transglycosylases increases outer membrane permeability and beta-lactam sensitivity in Pseudomonas aeruginosa. Microbiologyopen. 2015;6:879–895. doi: 10.1002/mbo3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogiorgos N, Mekasha S, Yang Y, Kelliher MA, Ingalls RR. Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response. Innate Immun. 2014;20:377–389. doi: 10.1177/1753425913493453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly MA, McGee ZA, Rosenthal RS. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984;149:378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Moynihan PJ, Clarke AJ. Substrate specificity and kinetic characterization of peptidoglycan O-acetyltransferase B from Neisseria gonorrhoeae. J Biol Chem. 2014;289:16748–16760. doi: 10.1074/jbc.M114.567388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Wolf AJ, Iliev ID, Berg BL, Underhill DM, Liu GY. Poorly Cross-Linked Peptidoglycan in MRSA Due to mecA Induction Activates the Inflammasome and Exacerbates Immunopathology. Cell Host Microbe. 2015;18:604–612. doi: 10.1016/j.chom.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JA, Ballard TN, Weaver TE, Akinbi HT. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J Immunol. 2006;177:519–526. doi: 10.4049/jimmunol.177.1.519. [DOI] [PubMed] [Google Scholar]
- Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DM, Phillips NJ, Shao JQ, Entz DD, Gibson BW, Apicella MA. Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect Immun. 2002;70:909–920. doi: 10.1128/iai.70.2.909-920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey ME, Hackett KT, Bender T, Kotha C, van der Does C, Dillard JP. TraK and TraB are conserved outer membrane proteins of the Neisseria gonorrhoeae Type IV secretion system and are expressed at low levels in wild-type cells. J Bacteriol. 2014;196:2954–2968. doi: 10.1128/JB.01825-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey ME, Hackett KT, Kotha C, Dillard JP. New complementation constructs for inducible and constitutive gene expression in Neisseria gonorrhoeae and Neisseria meningitidis. Appl Environ Microbiol. 2012;78:3068–3078. doi: 10.1128/AEM.07871-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RS. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979;24:869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RS, Nogami W, Cookson BT, Goldman WE, Folkening WJ. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect Immun. 1987;55:2117–2120. doi: 10.1128/iai.55.9.2117-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Kuehn MJ. Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli. J Bacteriol. 2013;195:4161–4173. doi: 10.1128/JB.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, et al. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe. 2010;7:38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun. 2005;73:1971–1977. doi: 10.1128/IAI.73.4.1971-1977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha RK, Rosenthal RS. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980;29:914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov A, Solga MD, Lannigan J, Criss AK. An improved method for differentiating cell-bound from internalized particles by imaging flow cytometry. J Immunol Methods. 2015;423:60–69. doi: 10.1016/j.jim.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl EA, Chan YA, Hackett KT, Kohler PL, Dillard JP, Seifert HS. Neisseria gonorrhoeae virulence factor NG1686 is a bifunctional M23B family metallopeptidase that influences resistance to hydrogen peroxide and colony morphology. J Biol Chem. 2012;287:11222–11233. doi: 10.1074/jbc.M111.338830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl EA, Criss AK, Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol. 2005;58:520–532. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl EA, Dale EM, Criss AK, Seifert HS. Neisseria gonorrhoeae metalloprotease NGO1686 is required for full piliation, and piliation is required for resistance to H2O2- and neutrophil-mediated killing. MBio. 2013;4:e00399–e00313. doi: 10.1128/mBio.00399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RD, Vollmer W, Foster SJ. Different walls for rods and balls: the diversity of peptidoglycan. Mol Microbiol. 2014;91:862–874. doi: 10.1111/mmi.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriarte SM, Rane MJ, Luerman GC, Barati MT, Ward RA, Nauseef WM, McLeish KR. Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J Immunol. 2011;187:391–400. doi: 10.4049/jimmunol.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Lo LF, Forsberg LS, Maier RJ. Helicobacter pylori peptidoglycan modifications confer lysozyme resistance and contribute to survival in the host. MBio. 2012;3:e00409–e00412. doi: 10.1128/mBio.00409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Maier SE, Lo LF, Maier G, Dosi S, Maier RJ. Peptidoglycan deacetylation in Helicobacter pylori contributes to bacterial survival by mitigating host immune responses. Infect Immun. 2010;78:4660–4666. doi: 10.1128/IAI.00307-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- Wiesner PJ, Thompson SE3rd. Gonococcal diseases. Dis Mon. 1980;26:1–44. doi: 10.1016/s0011-5029(80)80002-2. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Arruda A, Reyes CN, Kaplan AT, Shimada T, Shimada K, et al. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J Immunol. 2011;187:6002–6010. doi: 10.4049/jimmunol.1100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams KL, Chan JM, Lenz JD, Hackett KT, Dillard JP. Peptidoglycan fragment release from Neisseria meningitidis. Infect Immun. 2013;81:3490–3498. doi: 10.1128/IAI.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. Geneva, Switzerland: 2012. http://www.who.int/reproductivehealth/publications/rtis/stisestimates/en/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.