Summary
Cryptococcus neoformas infection of the central nervous system (CNS) continues to be an important cause of mortality and morbidity, and a major contributing factor is our incomplete knowledge of the pathogenesis of this disease. Here we provide the first direct evidence that C. neoformans exploits host cysteinyl leukotrienes (LTs), formed via LT biosynthetic pathways involving cytosolic phospholipase A2α (cPLA2α) and 5-lipoxygenase (5-LO) and acting via cysteinyl leukotriene type 1 receptor (CysLT1), for penetration of the blood-brain barrier. Gene deletion of cPLA2α and 5-LO as well as pharmacological inhibition of cPLA2α, 5-LO and CysLT1 were effective in preventing C. neoformans penetration of the blood-brain barrier in vitro and in vivo. A CysLT1 antagonist enhanced the efficacy of an anti-fungal agent in therapy of C. neoformans CNS infection in mice. These findings demonstrate that host cysteinyl LTs, dependent on the actions of cPLA2α and 5-LO, promote C. neoformans penetration of the blood-brain barrier and represent novel targets for elucidating the pathogenesis and therapeutic development of C. neoformans CNS infection.
Introduction
C. neoformans is responsible for life-threatening CNS infection in immunocompromised patients, especially those infected with HIV-1 (Day et al., 2013; Eisenman et al., 2007; Hakim et al., 2000; Jarvis et al., 2013; Mitchell et al., 1995; Mwaba et al., 2001; Park et al., 2009; Perfect et al., 2002; Powderly et al., 1993; Saag et al., 2000; Warkentien et al., 2010). One million cases of cryptococcal meningoencephalitis are estimated to occur globally per year, with >60% mortality within 3 months of infection (Day et al., 2013; Mwaba et al., 2001; Park et al., 2009). Even when treatment with amphotericin B and flucytosine is available, mortality ranges between 15–30% and is much higher (41–70%) in low-income countries where such antifungal regimens are not readily accessible (Day et al., 2013; Jarvis et al., 2013; Mwaba et al., 2001; Park et al., 2009; Saag et al., 2000). A recent study reported that cerebrospinal fluid (CSF) fungal burden predicts acute mortality in HIV-associated cryptococcal meningoencephalitis (Jarvis et al., 2013). These findings illustrate the need for a novel strategy for decreasing fungal burden in improving outcomes in HIV-infected patients.
C. neoformans CNS infection is a leading contributor to mortality in HIV-infected individuals with CD4+ counts <100 cells/μl, the threshold at risk for cryptococcal CNS infection, and blood cryptococcal antigen (CRAG) screen and early institution of preemptive antifungal therapy is shown to be efficacious in improving survival (Boulware et al., 2014; Jarvis et al., 2009; Meya et al., 2010). A 6-month survival in asymptomatic CRAG+ persons with CD4 <100/μl, however, is approximately 75% with the currently recommended WHO therapy, fluconazole (Boulware et al., 2014; Jarvis et al., 2009; Meya et al., 2010). The timing of mortality, occurring several weeks later, suggests that better antifungal therapy may be able to improve outcomes. These findings indicate that new approaches are needed to investigate the pathogenesis, prevention and therapy of C. neoformans CNS infection. Development of such a strategy, however, has been hampered by our incomplete knowledge on how C. neoformans penetrates the blood-brain barrier (Kim, 2008), the essential step required for the development of CNS infection.
C. neoformans is commonly acquired by inhalation. Extrapulmonary dissemination to the bloodstream leads to infection of distant organs, resulting in meningoencephalitis (Day et al., 2013; Eisenman et al., 2007; Hakim et al., 2000; Jarvis et al., 2013; Mitchell et al., 1995; Mwaba et al., 2001; Park et al., 2009; Perfect et al., 2002; Powderly et al., 1993; Saag et al., 2000; Warkentien et al., 2010). Several lines of evidence from human cases and experimental animal models of C. neoformans meningoencephalitis indicate that C. neoformans penetration into the brain follows fungemia, and cerebral capillaries are the portal of entry into the brain (Chang et al., 2004; Charlier et al., 2005; Chretien et al., 2002; Lee et al., 1996; Neuville et al., 2002; Olszewski et al., 2004; Shi et al., 2010). Since the entry of C. neoformans into the brain occurred in the cerebral microvasculature, we developed an in vitro blood-brain barrier model with human brain microvascular endothelial cells (HBMEC) (Chang et al., 2004; Kim, 2008, Kim et al.; 2004; Maruvada et al., 2012; Rüffer et al., 2004; Stins et al., 1997) for investigating C. neoformans penetration of the blood-brain barrier.
C. neoformans penetration into the brain has been shown to involve mainly transcellular and Trojan-horse penetrations of the blood-brain barrier and has been examined in animal models of intravenous, intranasal and intratracheal inoculations (Chang et al., 2004; Charlier et al., 2005; Chretien et al., 2002; Dromer et al., 2011; Kim, 2008; Neuville et al., 2002; Olszewski et al., 2004; Shi et al., 2010). Transcellular and Trojan-horse penetrations may not be mutually exclusive and can function in parallel in these animal models (Dromer et al., 2011). We showed that C. neoformans strains exhibit the ability to traverse the HBMEC monolayer without affecting HBMEC integrity, and HBMEC traversal is correlated with C. neoformans penetration into the brain (Chang et al., 2004; Kim, 2008; Maruvada et al., 2012; Shi et al., 2010). The underlying mechanisms involved in C. neoformans penetration of the blood-brain barrier, however, remain incompletely understood.
Previous studies have identified several cryptococcal and host factors contributing to penetration into the brain, which include a cryptococcal metalloprotease as well as cps1-CD44 and plb1-Rac1 interactions (Vu et al, 2014; Chang et al, 2006; Jong et al, 2012; Maruvada et al, 2012). We identified the CysLT1 antagonist, montelukast from our chemical screen inhibiting C. neoformans traversal of HBMEC monolayer. CysLT1 has been previously shown to contribute to invasion of HBMEC monolayer and penetration into the brain by bacteria causing meningitis such as E. coli and group B Streptococcus (Zhu et al, 2010; Maruvada et al, 2012). Here we elucidated the mechanisms by which cysteinyl leukotrienes are generated and contribute to C. neoformans penetration of the blood-brain barrier in vitro and in vivo.
Results and Discussion
Since C. neoformans traversal of the HBMEC monolayer is correlated with penetration into the brain, we used C. neoformans traversal of HBMEC monolayer as a relevant biological assay to screen a chemical library for discovery of targets affecting C. neoformans traversal of the blood-brain barrier. Our chemical screen identified montelukast as an inhibitor of C. neoformans traversal of HBMEC monolayer, yet montelukast did not affect the growth of C. neoformans strains B-3501A and H99 and also did not affect the HBMEC viability, as assessed by live/dead stain (Molecular probes) (Stins et al., 2001). Montelukast is a selective antagonist of cysteinyl leukotriene type 1 receptor (CysLT1) and inhibits cysteinyl leukotriene (CysLT) binding to CysLT1 (Peters-Golden and Henderson, 2007). Since host CysLTs are formed via LT biosynthetic pathways involving cytosolic phospholipase A2α (cPLA2α) and 5-lipoxygenase (5-LO), we hypothesize that host cPLA2α and 5-LO contribute to C. neoformans penetration of the blood-brain barrier.
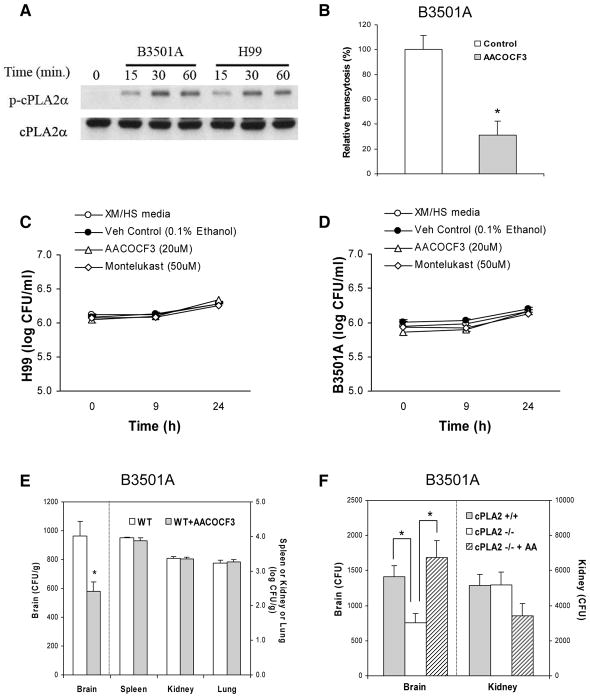
We showed that C. neoformans strains exploit host cPLA2α for their traversal of HBMEC monolayer, as shown by the time-dependent cPLA2α phosphorylation in response to C. neoformans strains B-3501A and H99 (Fig. 1A) and the ability of arachidonoyl trifluoromethylketone (AACOCF3, cPLA2α inhibitor) at 20 μM to significantly inhibit C. neoformans traversal of HBMEC monolayer (Fig. 1B). AACOCF3 at 20 μM was efficient in preventing cPLA2α phosphorylation in response to C. neoformans strains H99 (serotype A) and B-3501A (serotype D), but did not affect the growth of C. neoformans strains (Fig 1. C & D) or the integrity of the HBMEC monolayer, as assessed by live/dead staining (Molecular probes).
Figure 1. Host cPLA2α is involved in C. neoformans traversal of the HBMEC monolayer and penetration into the brain.
(A) Serine phosphorylation of cPLA2α occurs in response to C. neoformans strains B-3501A and H99 in HBMEC in a time-dependent manner. The lysates of HBMEC incubated with C. neoformans were examined for phospho-cPLA2α and cPLA2α in Western blot analysis using specific antibodies.
(B) AACOCF3 significantly inhibited penetration of C. neoformans strain B-3501A across the HBMEC monolayer. Penetration of B-3501A across the HBMEC monolayer was examined in Transwell filters (8 μm pore size). HBMECs were pretreated with 20 μM AACOCF3 for 60 min and then 20 μM AACOCF3 solution were added to every 2 hr (0, 2, 4, 6, and 8 hr, respectively) after addition of 1×106 CFU of strain B-3501A to the upper chamber. Our previous studies revealed that traversal of C. neoformans across the HBMEC monolayer was evident over a 9 hr incubation period (Chang et al., 2004). At 9 hr of incubation at 37 °C, sample was taken from the lower chamber and plated for determinations of CFUs. Colonies were counted after 2 days of incubation at 30° C. Transcytosis frequency (%) was determined by (total CFUs recovered from the lower chamber/total number of cryptococcal cells added to the upper chamber) × 100, and expressed as relative frequency compared to transcytosis frequency with vehicle control (0.125% ethanol). Transcytosis frequency of C. neoformans strain B-3501A across HBMEC monolayer at 9 hr of incubation at 37 °C ranged between 1.4 and 8.9 %. Data shown are means ± SEM of triplicates. * P<0.05, unpaired t-test, compared to vehicle control.
(C and D) AACOCF3, a cPLA2α inhibitor, and Montelukast, a CysLT1 receptor antagonist, show no significant effects on growth of C. neoformans strains H99 (C) or B3501A (D).
C. neoformans strains H99 (C) or B3501A (D) were growing in Hams-F12/M199 (1: 1, v/v), 5 % heat inactivated fetal bovine serum (FBS) (experimental medium, XM) and 5 % fresh human serum (HS) at 37°C, 5% CO2 incubator. Cryptococcus counts were performed at 0, 9, and 24h incubation time. Data shown are mean ± SEM. Each experiment was performed in triplicate.
(E) AACOCF3 decreases C. neoformans B-3501A penetration into the brain of BALB/c mice (WT). 4 mM AACOCF3 in 50μl PBS was injected through tail vein 30 min before, 3 hr and 6 hr after tail vein injection of C. neoformans B-3501A (1×105 cells in 100μl PBS). Control mice were injected with 7% ethanol in100 μl PBS. Cryptococcus counts were determined 24 hr after injection of B-3501A. Data shown are mean ± SEM. *P<0.05, Student’s t-test between WT (n=5), and WT + AACOCF3 (n=5).
(F) C. neoformans penetration into the brain of cPLA2α−/− and their wild type mice. Cryptococcus counts were determined 24 hr after injection of B-3501A (1×105 cells) in 100 μl PBS through tail vein. 40 μM arachidonic acid (AA) in 100 μl PBS (1.2 μg/mouse) was injected through tail vein 30 min before C. neoformans injection. Data shown are mean ± SEM. * P<0.05, Student’s t-test between cPLA2α +/+ (n=5), cPLA2α−/− (n=5), and cPLA2α −/− + AA (n=3).
We next examined whether pharmacologic inhibition and gene deletion of cPLA2α affects C. neoformans penetration into the brain in BALB/c mice. Each animal received 1 × 105 colony forming units (CFUs) of C. neoformans strain B-3501A via the tail vein. 24 hours later, blood specimens were obtained for determination of CFUs, and the animals perfused with sterile Ringer’s solution until the perfused solution became colorless. The brains, spleens, kidneys and lungs were removed, weighed, homogenized and cultured for determinations of CFUs, as described previously (Chang et al., 2004; Maruvada et al., 2012). C. neoformans strain B-3501A was completely cleared from the blood at 24 hours after intravenous injection and no viable yeasts were recovered from the blood. The CFUs in the brains were, therefore, most likely to represent the yeast cells that had penetrated into and survived in the brain.
For pharmacological inhibition, AACOCF3 (4 mM in 50 μl PBS, a dose which inhibits cPLA2α activity in mice, Kalyvas et al., 2004) was administered intravenously 30 mins before C. neoformans injection, and this resulted in significantly decreased penetration of C. neoformans into the brain of BALB/c mice (Fig. 1E). In contrast, AACOCF3 did not affect C. neoformans penetration into the spleen, kidney and lung, as shown by similar numbers of CFUs recovered from the animals receiving AACOCF3 or vehicle control (Fig. 1E).
The effect of gene deletion of host cPLA2α in C. neoformans penetration into the brain was assessed in cPLA2α−/− mice compared to their littermate control cPLA2α+/+ mice (Sapirstein et al., 2005; Zhu et al, 2010). The yeast counts recovered from the brains (CFUs/gm) of cPLA2α−/− mice were significantly less than those of cPLA2α+/+ animals (Fig. 1F), while the yeast counts from the kidneys did not differ between cPLA2α−/− and cPLA2α+/+ mice. These findings support that host cPLA2α contributes to C. neoformans penetration into the brain in vivo.
cPLA2α mediates arachidonic acid release from membrane phospholipids (Ghosh et al., 2006), and we examined whether exogenous arachidonic acid affects C. neoformans penetration into the brain of cPLA2α−/− mice. Intravenous administration of arachidonic acid (1.2 μg/mouse in 50 μl PBS, a dose which restores cPLA2α-dependent vascular responses in cPLA2α−/− mice, Ichinose et al., 2002; Zhu et al, 2010) 30 min before C. neoformans injection significantly enhanced C. neoformans penetration into the brain to the level observed in the wild type mice, while arachidonic acid failed to enhance C. neoformans penetration into the kidney (Fig. 1F). The enhancement of C. neoformans penetration into the brain by exogenous arachidonic acid in cPLA2α−/− mice was not accompanied by any changes in the blood-brain barrier permeability, as shown by a lack of significantly increased extravasation of intravenously administered Evans blue dye into the brain of infected animals receiving arachidonic acid compared to those without arachidonic acid administration. These findings are consistent with those of our previous studies, where C. neoformans penetration of the blood-brain barrier was not accompanied by any changes in the blood-brain barrier integrity (Chang et al., 2004). These in vitro and in vivo findings using pharmacological inhibition and gene deletion demonstrate that C. neoformans exploits host cPLA2α for traversal of the blood-brain barrier and penetration into the brain, while host cPLA2α does not affect C. neoformans penetration into non-brain organs.
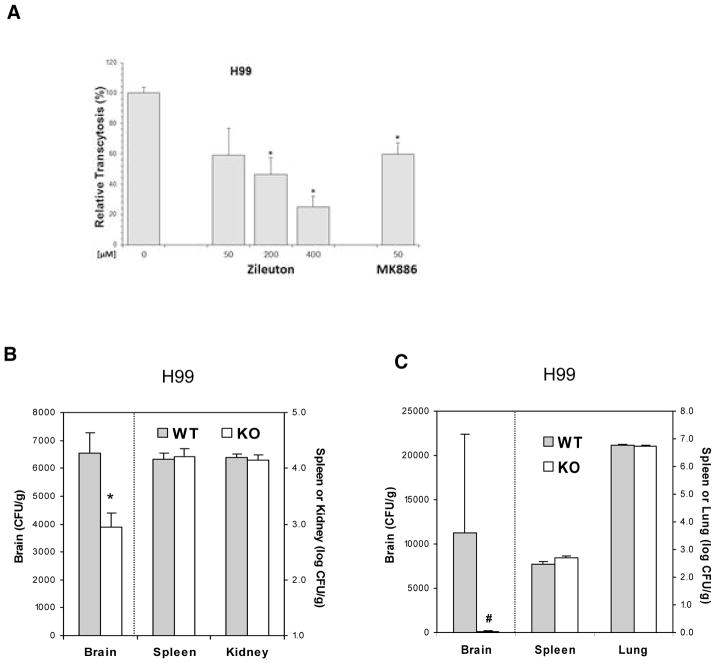
Leukotrienes (LTs) are synthesized from arachidonate by 5-lipoxygenase (5-LO) and 5-LO activating protein (FLAP) (Peters-Golden and Henderson, 2007), and we next determined whether C. neoformans exploitation of host cPLA2α for penetration of the blood-brain barrier depends on 5-LO products of arachidonic acid. This issue was examined initially using zileuton (a selective inhibitor of 5-LO) and MK886 (an inhibitor of FLAP) for assessing their effect on C. neoformans traversal of HBMEC monolayer. Zileuton exhibited a dose-dependent inhibition of C. neoformans strains H99 and B-3501A traversal of HBMEC, and MK886 at 50 μM inhibited strain H99 traversal of HBMEC (Fig. 2A). Zileuton at 400 μM and MK886 at 50 μM did not affect the growth of C. neoformans strains and also did not affect the integrity of HBMEC monolayer, as assessed by transendothelial electrical resistance (TEER) before and after traversal assays. The inhibition of HBMEC traversal by pharmacological inhibition of 5-LO and FLAP indicate that endogenous LT biosynthesis via 5-LO and FLAP is likely to play an important role in C. neoformans penetration of the blood-brain barrier.
Figure 2. 5-LO is involved in C. neoformans traversal across the HBMEC monolayer and penetration into the brain.
(A) Zileuton (5-LO inhibitor) and MK886 (FLAP inhibitor) inhibited C. neoformans strain H99 traversal of the HBMEC monolayer in a dose-dependent manner. Data shown are means ± SEM of triplicates. * P<0.05, unpaired t-test, compared to vehicle control.
(B) C. neoformans penetration into the brain following intravenous inoculation is significantly decreased in 5-LO−/− mice compared to their wild type animals. The yeast counts from the brain, kidney, and spleen were determined 24 hr after injection of H99 (1×105 cells) in 100μl PBS through tail vein. Data shown are mean ± SEM. * P<0.05 by Student’s t-test between wild type (WT, n=4) and 5-LO−/− mice (KO, n=4).
(C) C. neoformans penetration into the brain following intratracheal inoculation is significantly decreased in 5-LO−/− mice compared to wild type mice. The yeast counts from the brain, spleen, and lung were determined 7d after intratracheal inoculation of H99 (1×105 cells). Data shown are mean ± SEM. # P<0.05 by Wilcoxon Rank Sum test between wild type (WT, n=6) and 5-LO−/− mice (KO, n=6).
The role of 5-LO in C. neoformans penetration into the brain was examined using 5-LO−/− mice as compared to their strain-matched wild type mice (Serezani et al., 2005; Zhu et al., 2010). In these animal studies, C. neoformans strain H99 was administered via intravenous inoculation as described above, as well as via intratracheal inoculation to mimic the natural route of infection (acquisition via inhalation). The intravenous inoculation model measures the ability of C. neoformans to penetrate into the brain from systemic dissemination, while the intratracheal inoculation model measures the ability of extrapulmonary dissemination to the brain from the lungs, and both models have been used for assessing transcellular and Trojan-horse penetrations of the blood-brain barrier (Chang et al., 2004; Charlier et al., 2005; Chretien et al., 2002; Dromer et al., 2011; Neuville et al., 2002; Olszewski et al., 2004; Shi et al., 2010). C. neoformans strain H99 penetration into the brain following intravenous as well as intratracheal inoculations was significantly less in 5-LO−/− mice than in their wild type animals, as shown by significantly less yeast counts recovered from the brains of 5-LO −/− mice compared to those of their wild type animals (Fig. 2, B & C). In contrast, the yeast counts recovered from the non-brain sites (e.g., spleen, kidney and lung) were similar between 5-LO−/− and wild type mice (Fig. 2, B &C). These findings demonstrate that C. neoformans exploits host 5-LO for penetration into the brain following intravenous and intratracheal administrations.
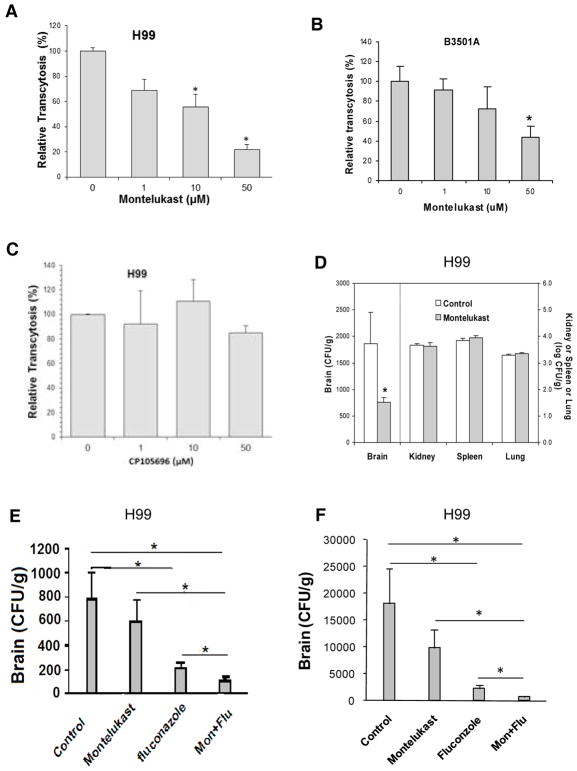
Arachidonic acid is metabolized to LTB4 or cysteinyl leukotrienes (LTC4, LTD4 and LTE4) and these terminal LTs exhibit their biological actions via interaction with their respective G-protein coupled receptors, BLT1 and CysLT1 (Murphy et al., 2007; Peters-Golden et al., 2007). The role of individual classes of LTs in C. neoformans penetration of the blood-brain barrier was next determined by examining the effects of the CysLT1 antagonist (montelukast) and the BLT1 antagonist (CP105696) (Peters-Golden and Henderson, 2007) in cryptococcal traversal of the HBMEC monolayer. Pretreatment of HBMEC with montelukast significantly inhibited C. neoformans traversal in a dose-dependent manner (Fig. 3, A & B), while CP105696 did not exhibit any inhibition (Fig. 3C). Montelukast at 50 μM did not affect the growth of C. neoformans strains and also did not affect the integrity of HBMEC monolayer, as assessed by TEER before and after traversal assays.
Figure 3. Cysteinyl LTs are involved in C. neoformans traversal across the HBMEC monolayer and penetration into the brain.
(A and B) Montelukast (CysLT1 antagonist), but (C) not CP105696 (BLT1 antagonist) inhibited traversal of C. neoformans strains H99 (A and C) and B-3501A (B) across the HBMEC monolayer. Transcytosis of H99 or B-3501A was examined in Transwell filters (8 μm pore size). HBMECs were pretreated with montelukast at indicated concentrations (1, 10, 50 μM) for 60 min and then montelukast solution were added every 4 hr (0, 4, and 8 hr, respectively) after addition of 1×106 CFUs of strain H99 or B-3501A to the upper chamber. Data shown are mean ± SEM of triplicates. * P<0.05 by unpaired t-test, compared to vehicle control (0.1% ethanol).
(D) Montelukast decreases C. neoformans H99 penetration into the brain of BALB/c mice. 1.6 mM montelukast in 100μl PBS was injected through tail vein 15 min before, and 6 hr after tail vein injection of H99 (1×105 cells in 100μl PBS). Control mice received 4% DMSO in100 μl PBS. The yeast counts were determined after 24 hr injection of H99 (n=6 for each group). Data shown are mean ± SEM. * P<0.05 by two-tailed Wilcoxon Rank-Sum test.
(E and F) Effects of montelukast and fluoconzole on C. neoformans penetration into the brain of BALB/c mice (E) 7 and (F) 14 d after intratracheal inoculation of H99. On the day of intratracheal inoculation of C. neoformans, montelukast (Mon) was given at dose of 5 mg/kg in 100 μl PBS via intraperitoneal (i.p.) injection 30 min before and 6 hr after inoculation of H99, followed by 5 mg/kg i.p. daily for 6 and 13 d. After intratracheal inoculation of C. neoformans, fluconazole (Flu) was suspended in sterile water and was given at dose of 15 mg/kg twice daily in a volume about 100 μl by gavage for 6 and 13 d. Control animals received 3.3% DMSO i.p. and sterile water via oral gavage. The yeast counts from the brain were determined 7 and 14 d after intratracheal inoculation of H99 (1×105 CFUs). Data represent mean ± SEM (n=10). * P<0.05 by Student’s t-test.
We next examined the effect of montelukast in C. neoformans penetration into the brain following intravenous inoculation. Administration of montelukast (1.6 mM in 100 μl PBS, a dose which exhibits CysLT1 antagonist activity in mice, Genovese et al., 2008) intravenously 15 mins before and 6 hours after C. neoformans injection significantly decreased C. neoformans penetration of the brain of BALB/c mice, but did not affect C. neoformans penetration into the spleen, kidney and lung (Fig. 3D). These in vitro and in vivo findings with montelukast demonstrate for the first time that cysteinyl LTs contribute to C. neoformans penetration of the blood-brain barrier.
CysLT1 antagonists have been developed for allergic airway disease and shown to be safe and well tolerated in clinical trials (Barnes et al., 1997; Capra et al., 2007; Montuschi et al., 2007). Since CysLT1 antagonist (montelukast) was effective in preventing C. neoformans penetration of the blood-brain barrier, we next examined its efficacy in the treatment of C. neoformans CNS infection, alone and in combination with anti-fungal drug (fluconazole). The animals received C. neoformans via intratracheal inoculation, and then montelukast via intraperitonal administration, followed by daily administration of montelukast and fluconazole, alone or in combination, for 6 and 13 days to mimic a likely application of these drugs for therapy of C. neoformans CNS infection. As expected, administration of fluconazole was effective in significantly inhibiting C. neoformans CFUs recovered from the brain compared to vehicle control (Fig. 3,E&F). The combination of montelukast and fluconazole, however, was significantly more effective than individual drugs alone in reducing C. neoformans CFUs recovered from the brain (Fig. 3, E & F).
In addition, our immunofluorescence studies revealed that the expression of CysLT1 was evident in the brain capillaries of animals with C. neoformans penetration into the brain following intratracheal inoculation, while CysLT1 expression was not discernible in the brain capillaries of uninfected animals (Fig 4), suggesting that the CysLT1 expression in the brain capillaries is likely to be up-regulated with C. neoformans infection of the brain.
Figure 4. Immnofluorescence demonstration of CysLT1 in the mouse brain microvessels following intratracheal inoculation of GFP-tagged Cryptococcus neoformans (H99-GFP).
CysLT1 expression was not evident in the brain microvessels of uninfected mice (Sham), while CysLT1 expression was demonstrated in the brain microvessels (shown with PECAM-1) of mice infected with GFP-tagged Cryptococcus neoformans (H99-GFP). A and D, CysLT1 expression; B and E, microvessels shown with PECAM-1; C and F, merge of CysLT1 and PECAM-1 staining. Yellow arrows indicate H99-GFP, and white arrows indicate microvessels. Scale bar= 50 μm.
Taken together, these findings suggest that counteracting a host factor involved in C. neoformans penetration of the blood-brain barrier (e.g., cysteinyl LTs) is beneficial in prevention and therapy of C. neoformans CNS infection, and also suggest that a combination of montelukast and fluconazole may represent an attractive preemptive therapeutic regimen for asymptomatic CRAG+ persons with CD4<100/μl.
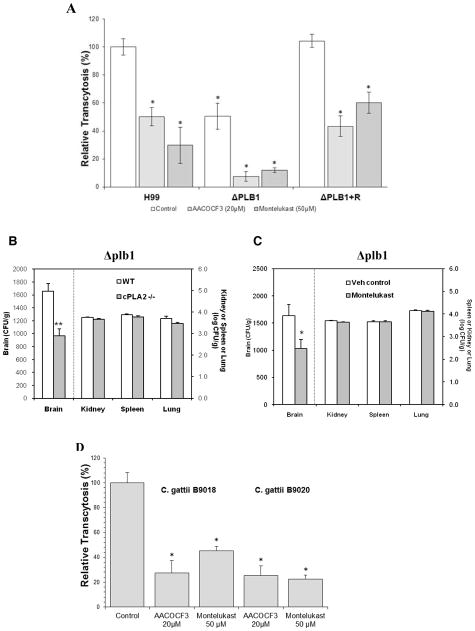
It has been documented that eicosanoids can be produced not only by the host, but also by Cryptococcus phospholipase (Noverr et al., 2003; Noverr et al., 2001). We next examined whether prevention of C. neoformans penetration into the brain by the cPLA2α inhibitor and the CysLT1 antagonist was the result of their inhibitory effects on cysteinyl LTs arising from arachidonate liberated by Cryptococcus or host phospholipase. This issue was examined by using the phospholipase B1 (plb1) mutant derived from C. neoformans strain H99 compared to its reconstituted strain along with wild type strain H99 (Noverr et al., 2003). As shown previously (Maruvada et al, 2012), the penetration of the plb1 mutant across the HBMEC monolayer was significantly decreased compared to that of the reconstituted strain and strain H99 (Fig 5A). AACOCF3 and montelukast were effective in significant inhibition of HBMEC traversal by the plb1 mutant, the reconstituted and wild type strains (Fig 5A). More importantly, the penetration of the plb1 mutant into the brain was significantly defective in cPLA2α−/− mice compared to wild type mice (Fig. 5B). In contrast, the yeast counts recovered from non-brain sites (kidney, spleen and lung) did not differ between cPLA2α−/− and wild type mice. We also showed that montelukast significantly inhibited the plb1 mutant’s penetration into the brain compared to vehicle control (Fig. 5C). Our findings with pharmacological inhibition of cPLA2α and CysLT1 as well as genetic deletion of cPLA2α are, therefore, likely to stem from their effect on host cPLA2α and CysLT1, not on fungal plb1, supporting that host cPLA2α and cysteinyl LTs contribute to C. neoformans traversal of HBMEC and penetration into the brain, independent of Cryptococcus phospholipase. These findings are also consistent with those of pharmacological inhibition studies (Fig 3D, E & F), where C. neoformans penetration into the brain was prevented by administration of montelukast alone and in combination with fluconazole, indicating that host-derived, rather than cryptococcal-derived, eicosanoid production is responsible for C. neoformans penetration of the blood-brain barrier. In addition, we showed that C. gattii strains exhibited the ability to traverse the HBMEC monolayer and their traversal frequency (1.3 % to 2.4 %) was similar to that of C. neoformans. Of interest, C. gattii traversal was inhibited by AACOCF3 and montelukast (Fig 5D). Taken together, the above findings demonstrate that host cPLA2α, 5-LO and cysteinyl LTs are exploited by C. neoformas var. neoformans (strain 3501A), C. neofromans var. grubii (strain H99) as well as C. gattii strains for penetration of the blood-brain barrier.
Figure 5. Roles of cryptococccal Plb1 in C. neoformans penetration of the blood-brain barrier.
(A). The plb1 mutant (ΔPLB1) was significantly defective in traversal of the HBMEC monolayer compared to its parent strain H99 and reconstituted strain (ΔPLB1 + R). AACOCF3, a cPLA2α inhibitor, and montelukast, the CysLT1 antagonist, significantly inhibited the HBMEC traversal of the plb1 mutant, its parent strain and reconstituted strain. Traversal of the HBMEC monolayer was examined in Transwell filters (8 μm pore size). HBMEC were pretreated with 20 μM AACOCF3 for 60 min and then 20 μM AACOCF3 fresh solution were added to the transwell every 2 hr (0, 2, 4, 6, and 8 hr, respectively) or HBMECs were pretreated with 50 μM montelukast for 60 min and then montelukast solution were added every 4 hr (0, 4, and 8 hr, respectively) after addition of 1×106 CFUs of cryptococcal strains to the upper chamber. Data shown are means ± SEM of triplicates. * P<0.05, Student’s t-test compared to vehicle control (n=3).
(B). The penetration of the plb1 mutant into the brain is significantly decreased in cPLA2α−/− mice compared to their wild type animals (WT). The yeast counts in the brain, kidney, spleen and lung were determined 24 hr after injection of the plb1 mutant (1×105 cells) in 100 μl PBS through the tail vein. Data shown are mean ± SEM. **P<0.001, Student’s t-test between WT and cPLA2 −/− mice (n=7).
(C). Montelukast (the CysLT1 antagonist) significantly decreases the penetration of the plb1 mutant into the brain of BALB/cJ mice. Montelukast 5 mg/kg in 100μl PBS was injected through tail vein 15 min before and 6 hr after tail vein injection of the plb1 mutant (1×105 cells in 100μl PBS). Control mice received 3.3% ethanol in 100μl PBS. The yeast counts were determined after 24 hr injection of the plb1 mutant (n=6). Data shown are mean ± SEM. * P<0.05, Wilcoxon Rank-Sum test.
(D) C. gattii strains traversal of the HBMEC monolayer was inhibited by AACOCF3 and montelukast. Data shown are means ± SEM of triplicates. * P<0.05, Student’s t-test compared to vehicle control (n=3).
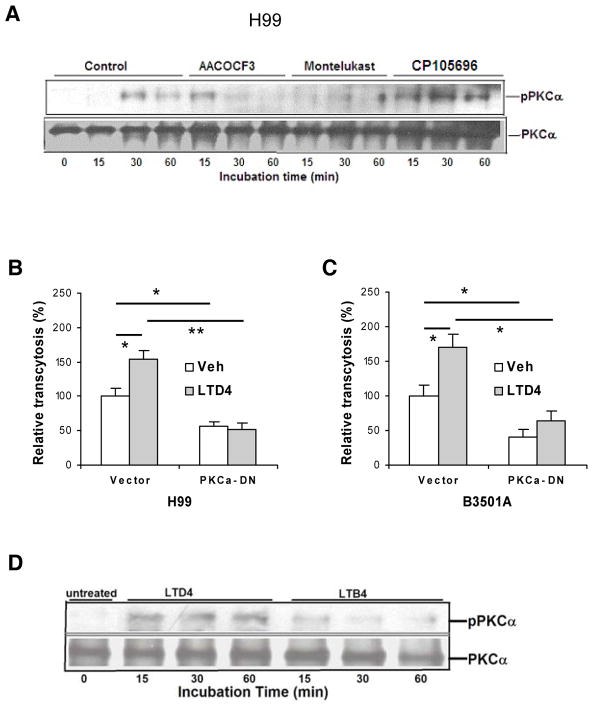
Protein kinase C (PKC) is a family of at least 10 serine/threonine kinases that transduce multiple signals in the regulation of a variety of cellular functions, which include cytoskeleton rearrangements (Hryciw et al., 2005; Larsson et al., 2006). We have previously shown that C. neoformans exploits host cell cytoskeleton rearrangements for penetration of HBMEC monolayer, as documented by microvilli formation at the entry sites of HBMEC (Chang et al., 2004). We, therefore, hypothesized that C. neoformans exploits PKCα for traversal of HBMEC monolayer. The role of PKCα in C. neoformans traversal of HBMEC monolayer was shown by our demonstrations that PKCα activation occurs in response to C. neoformans in a time-dependent manner in HBMEC (Fig. 6A), and that C. neoformans traversal was significantly decreased in HBMEC expressing dominant-negative PKCα compared to the control vector-transfected HBMEC (Fig. 6, B & C).
Figure 6. cPLA2α and cysteinyl LTs contribute to C. neoformans traversal of the HBMEC monolayer via PKCα.
(A) HBMEC incubated with C. neoformans strain H99 at 37°C for various times in the presence of inhibitors/antagonists or vehicle control were immunoprecipitated with PKCα antibody, and then assessed for phospho-PKC by Western blotting with phospho-PKC antibody. The HBMEC lysates were examined for the total amounts of PKC.
(B and C) Effect of LTD4 on penetration of C. neoformans strain H99 (B) or B-3501A (C) across monolayer of HBMEC transfected with dominant-negative PKCα construct. Subconfluent HBMEC in transwell insert were infected with adenovirus Ad5CA dominant negative-PKC-α (DN) or vector control (Vec) at MOI of 50. HBMEC were pretreated with 1 μM LTD4 for 30 min followed by transcytosis assays. 0.5% ethanol was used as vehicle control (Veh). Data shown are mean ± SEM. Each experiment was performed in triplicate. *P <0.05; **P <0.01, Student’s t test.
(D) LTD4 is involved in PKCα activation in HBMEC. HBMEC incubated with LTD4 (1 μM) or LTB4 (1 μM) at 37°C for various times (min) were immunoprecipitated with PKCα antibody, and then assessed for phospho-PKC by Western blotting with phospho-PKC antibody. The HBMEC lysates were examined for the total amounts of PKC.
More importantly, PKCα activation in response to C. neoformans was inhibited by cPLA2α inhibitor (AACOCF3) and CysLT1 antagonist (montelukast), but not by BLT1 antagonist (CP105696) (Fig. 6A). These findings indicate that host cPLA2α, 5-LO and cysteinyl LTs, but not LTB4, contribute to C. neoformans traversal of HBMEC monolayer, most likely via PKCα activation. This concept was further supported by our demonstration that exogenous cysteinyl LT (LTD4, 1 μM) significantly enhanced C. neoformans traversal of control vector-transfected HBMEC compared to vehicle control (0.5% ethanol), while it failed to exhibit such an enhancement in HBMEC expressing dominant-negative PKCα (Fig. 6, B & C). In addition, PKCα activation was shown to occur in response to LTD4, but not to LTB4, in HBMEC (Fig 6D). Taken together, these findings demonstrate for the first time that PKCα is downstream of cPLA2α, 5-LO and cysteinyl LTs in C. neoformans penetration of the blood-brain barrier.
cPLA2α has been shown to be involved in the development of arthritis, bone resorption and pulmonary fibrosis (Hegen et al., 2003; Miyaura et al., 2003; Nagase et al., 2002), while LTs have been involved in respiratory diseases, allergic diseases and cardiovascular diseases (Evans et al., 2008; Funk et al., 2005; Montuschi et al., 2007; Peters-Golden and Henderson, 2007), but the roles of cPLA2α, 5-LO and LTs in C. neoformans penetration of the blood-brain barrier have not been explored. Our findings reported here demonstrate for the first time that (a) C. neoformans exploits host cPLA2α and 5-LO for the generation of cysteinyl LTs responsible for penetration of the blood-brain barrier in vitro and in vivo, (b) the actions of cysteinyl LTs occur via CysLT1 and PKCα, and (c) the contribution of host cPLA2α and CysLT1 to C. neoformans penetration of the blood-brain barrier was independent of cryptococcal plb1. Our findings also demonstrate that inhibition of host molecules exploited by C. neoformans and C. gattii for penetration of the blood-brain barrier, as shown here with cPLA2α inhibitor and CysLT1 antagonist, is likely to provide a novel approach for prevention of C. neoformans and C. gattii penetration into the brain, the essential step required for development of meningoencephalitis. We also show that the CysLT1 antagonist (montelukast) in combination with anti-fungal drug (fluconazole) was significantly more effective than single agents alone in therapy of C. neoformans CNS infection. These findings suggest that pharmacologic inhibition of host factors involved in C. neoformans penetration of the blood-brain barrier is a useful adjunct to anti-fungal drugs in prevention and therapy of C. neoformans meningoencephalitis.
Experimental procedures
Reagents
Arachidonic acid (AA) was purchased from Cayman Chemical Company (Ann Arbor, MI). Evans Blue was purchased from Sigma (St Louis, NO). Arachidonyltrifluoromethyl ketone (AACOCF3; cPLA2 inhibitor) was purchased from Biomol Laboratories. (Plymouth Meeting, PA). Leukotriene D4 (LTD4), Leukotriene B4 (LTB4), and montelukast (cysteinyl-leukotriene type 1 receptor antagonist) were purchased from Cayman Chemical Company (Ann Arbor, MI). CP105696 (LTB4 receptor antagonist) was a gift from Pfizer. cPLA2, phospho-cPLA2α and phospho-PKCα antibodies were purchased from Cell Signaling Technologies (Danvers, MA), and PKC antibodies, PECAM-1 antibodies, and cysteinyl-leukotriene type 1 receptor (CysLT1) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA)
Mice
cPLA2α−/− and littermate control cPLA2α+/+, either male or female, between 10~13w and 18~26g, that had been backcrossed on the BALB/C strain for >10 generations (Sapirstein et al., 2005; Zhu et al, 2010), and female 5-LO−/− (129-Alox5tm1Fun/J) and strain-matched wild-type mice (129SvEv), 9~12w and 18~26g (Serezani et al., 2005; Zhu et al, 2010), were used. All procedures and handling techniques were approved by The Johns Hopkins Animal Care and Use Committee and Fujian Medical University Animal Care and Use Committee, Fuzhou, China.
Yeast strains
C. neoformans strains H99 and B-3501A represent encapsulated serotype A and D strains, respectively, that have been used for genomic sequencing (Loftus et al., 2003). plb1 isogenic strain of H99 was generated as previously described (Cox et al., 2001). GFP-tagged H99 (H99-GFP) was provided by Robin C. May, University of Birmingham, UK (Voelz et al., 2010). C. gattii strains were provided by S. Zhang, Johns Hopkins Hopsital Microbiology Laboratory. Yeast cells were grown aerobically at 37 °C in 1 % yeast extract, 2 % peptone and 2 % glucose (YPD) broth. Cells were harvested at early exponential phase, washed with PBS and resuspended in Hams-F12/M199 (1: 1, v/v), 5 % fresh human serum (experimental medium). The numbers of Cryptococcus cells were determined by direct counting from a haemocytometer, which was verified by determinations of colony forming units (CFUs) on YPD agar after 2 days of incubation at 30° C.
Characterization and culture of HBMEC
HBMEC were isolated and characterized as described previously (Stins et al., 1997). Briefly, brain specimens were cut into small pieces and homogenized in DMEM containing 2 % FBS (DMEM-S) using a Dounce homogenizer with a loose fitting. The homogenate was centrifuged in 15 % dextran in DMEM-S for 10 min at 10 000 g. The pellet containing crude microvessels was further digested in a solution containing 1 mg/ml collagenase/dispase in DMEM-S for 1 h at 37 °C. Microvascular capillaries were isolated by adsorption to a column of glass beads (0.25–0.3 mm) and washing off from the beads. HBMEC were plated on rat tail collagen/fibronectin-coated dishes or glass coverslips and cultured in RPMI 1640-based medium with growth factors, 10 % heat-inactivated FBS, 10 % NuSerum, 5 U heparin ml-1, 2 mM L-glutamine, 1 mM sodium pyruvate, non-essential amino acids, vitamins and 100 U penicillin and streptomycin ml-1. Viability of HBMEC was assessed by examining morphology and by trypan blue exclusion. HBMEC were positive for factor VIII-Rag, took up fluorescently labeled acetylated low-density lipoprotein and expressed γ-glutamyl transpeptidase. HBMEC were maintained in RPMI-based medium, including 10 % FBS and 10 % NuSerum (BD Biosciences), at 37 °C in a humid atmosphere of 5 % CO2.
Identification of montelukast affecting C. neoformans traversal of primary HBMEC monolayer
We used C. neoformans traversal of the primary HBMEC monolayer as a biologically relevant assay for screen of the Johns Hopkins Drug Library (JHDL) (Chong et al., 2006) for identification of targets affecting C. neoformans traversal of the blood-brain barrier, as follows. Primary HBMEC grown in 96-well Transwell inserts (with a pore size of 8 μm) were incubated with the JHDL (at a final concentration of 10 μM) for 60 min at room temperature, and then examined for C. neoformans traversal, as previously described (Chang et al., 2004; Maruvada et al., 2012). This screening assay included strain H99 in vehicle (DMSO)-treated HBMEC as a positive control for transcytosis, while the wells without HBMEC were used as control for any inhibitory effect of the drugs on growth of C. neoformans. Since this JHDL contains antifungal drugs, those wells exposed to antifungals were used as a positive control for determination of drugs that affect C. neoformans growth. The assay was highly reproducible, and the coefficient of correlation from at least two separate experiments was r = 0.98 (P<0.0001). From this screen, we identified montelukast, an antagonist of cysteinyl leukotriene type 1 receptor (CysLT1) (Peters-Golden et al., 2007), inhibited C. neoformans traversal of HBMEC, without affecting HBMEC integrity, as assessed by live/dead stain (Molecular Probes) and C. neoformans growth. CysLT1 has not been previously appreciated for its involvement in C. neoformans penetration of the blood-brain barrier.
Infection of HBMEC with adenovirus
HBMEC (~70% confluency) were infected (at MOI of 50) with dialyzed adenovirus of Ad5CA dominant negative-PKC-α and vector control, as described previously (Gorshkova et al., 2008)
Traversal of C. neoformans across the HBMEC monolayer
HBMEC were cultured on Transwell polycarbonate tissue-culture inserts with a pore diameter of 8 μm (Corning Costar) for 5 days (Chang et al., 2004). On the morning of the assay, HBMEC monolayer was washed with experimental medium and 107 C. neoformans cells were added to the upper chamber. At 9 h of incubation at 37 °C, sample was taken from the lower chamber and plated for determinations of CFUs. Colonies were counted after 2 days of incubation at 30° C. The integrity of the HBMEC monolayer was assessed by measurements of the transendothelial electrical resistance (TEER) before and after assays.
Experimental hematogenous C. neoformans CNS infection in mice
Mice were anesthetized with pentobarbital sodium given subcutaneously at 50 mg/kg. Montelukast 5 mg/kg in 100μl PBS was injected through tail vein 15 min before and 6h after tail vein injection of C. neoformans (1×105 cells in 100 μl PBS). Control mice received 3.3% ethanol in 100 μl PBS. 24h after C. neoformans injection, mouse chest was cut open, and blood from right ventricle was collected and plated for bacterial counts (CFUs). Then mouse was perfused with a mammalian Ringer’s solution by transcardiac perfusion through a 23-gauge needle inserted into the left ventricle of the heart under the perfusion pressure of about 100 mmHg. The perfusate exited through a cut in the right atrium. The composition of the mammalian Ringer solution was: (in mM) 132 NaCl, 4.6 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5.0 NaHCO3, and 20 HEPES and Na-HEPES, containing 10 mg/ml BSA; pH of the Ringer solution was maintained at 7.40–7.45 by adjustment of the ratio of Na-HEPES to HEPES. Ringer solution used in this study has been shown not to change the microvessel permeability (Zhu et al, 2004). 30 min after perfusion of Ringer solution, mice were decapitated. The brains were removed, weighed and homogenized in 2ml RPMI followed by plating for brain Cryptococcus counts in YPD agar plates. Kidneys, lungs and spleens were also dissected out for determinations of bacterial counts (CFUs/gm). The brains were removed, weighed and homogenized in 2ml RPMI followed by plating for brain Cryptococcus counts in YPD agar plates.
Intratracheal inoculation
Mice were anesthetized with pentobarbital sodium given subcutaneously at 50 mg/kg. A small incision was made in the skin over the trachea. A 30-gauge needle (Becton Dickinson, Franklin Lakes, NJ) was attached to a tuberculin syringe (Becton Dickinson). The needle was bent and inserted into the trachea, and (containing 105 CFU Cryptococcus) was delivered. For control (sham) group mice, a 30-μl PBS was delivered. The skin was sutured with a cyanoacrylate adhesive, and the mice recovered with no visible trauma (Noverr et al., 2003).
Immunofluorescence
After 7 days of intratracheal inoculation with GFP-tagged C. neoformans (H99-GFP), mouse chest was cut open, and mouse was perfused with a mammalian Ringer’s solution by transcardiac perfusion through a 23-gauge needle inserted into the left ventricle of the heart under the perfusion pressure of about 100 mmHg for 20 min. The brains were removed and put in liquid nitrogen. Serial cryosections (10 μm) were incubated overnight with a monoclonal rat anti-PECAM-1 primary antibody (Santa Cruz, CA) and a polyclonal rabbit anti-CysLT1primary antibody (Santa Cruz, CA). Afterward, sections were incubated with Dylight 488 goat Anti-Rat IgG secondary antibody (EarthOx Life Sciences, Millbrae, CA) and Cy3 goat anti-Rabbit IgG secondary antibody (Beyotime Biotechnology, Shanghai, China). Slides were imaged through fluorescence microscopy with a Nikon Eclipse Ti-S and DS-Ri2 digital camera (Nikon)
Immunoblotting and immunoprecipitation
The lysates of HBMEC incubated with C. neoformans were prepared for Western blotting and immunoprecipitation as described previously (Reddy et al., 2000).
Statistical Analysis
Data are expressed as mean ± SEM. Differences of Cryptococcus counts in the brain, spleen, kidney and lung (CFUs/gm of organs) between different groups of mice were determined by Wilcoxon rank sum test or Student’s t test. Differences of Cryptococcus traversal across HBMEC monolayer were determined by Student’s t test. P<0.05 was considered significant.
Acknowledgments
This work was supported in part by the NIH grants (AI84894 and NS94012) and by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc. (KSK), as well as in part by grant from the National Natural Science Foundation of China (No. 81171115). The opinions expressed in this paper are those of the authors and do not necessary represent those of Merck & Co., Inc. The authors thank V Natarajan for providing adenovirus constructs with dominant-negative PKCα or vector control, J Perfect for providing C. neoformans strain H99 and the plb1 mutant, R. C. May for providing C. neoformans strain GFP-H99, J. Kwon-Chung for providing C. neoformans strain B-3501A, and S. Zhang for providng C. gattii strains..
Footnotes
Authors Contributions Statements. LZ and RM performed research. AS and MP-G provided animals, reagents and input into data interpretation. KSK conceived and designed research, and wrote the manuscript.
Competing Interests Statement
The authors declare that they have no competing financial interest.
References
- Barnes NC, de Jong B, Miyamoto T. Worldwide clinical experience with the first marked leukotriene receptor anagonist. Chest. 1997;111:52–60. doi: 10.1378/chest.111.2_supplement.52s. [DOI] [PubMed] [Google Scholar]
- Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- Chang YC, Jong A, Huang S, Zerfas P, Kwon-Chung KJ. CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans. Infect Immun. 2006;74:3930–8. doi: 10.1128/IAI.00089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Chrétien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol. 2005;166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ. A clinical drug library screen identified astemizole as an antimalarial agent. Nat Chem Biol. 2006;2:415–416. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- Chrétien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F, Levitz S. Cryptococcus, from human pathogen to model yeast. Chapter 34. American Society of Microbiology; 2011. [Google Scholar]
- Eisenman HC, Casadevall A, McClelland EE. New insights on the pathogenesis of invasive Cryptococcus neoformans infection. Curr Infect Dis Rep. 2007;9:457–464. doi: 10.1007/s11908-007-0070-8. [DOI] [PubMed] [Google Scholar]
- Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What’s all the FLAP about?:5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol Sci. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- Genovese T, Rossi A, Mazzon E, Di Paola R, Muià C, Caminiti R, et al. Effects of zileuton and montelukast in mouse experimental spinal cord injury. Br J Pharmacol. 2008;153:568–582. doi: 10.1038/sj.bjp.0707577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gorshkova I, He D, Berdyshev E, Usatuyk P, Burns M, Kalari S, et al. Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J Biol Chem. 2008;283:11794–11806. doi: 10.1074/jbc.M800250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–1407. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- Hegen M, Sun L, Uozumi N, Kume K, Goad ME, Nickerson-Nutter CL, et al. Cytosolic phospholipase A2alpha-deficient mice are resistant to collagen-induced arthritis. J Exp Med. 2003;197:1297–1302. doi: 10.1084/jem.20030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryciw DH, Pollock CA, Poronnik P. PKC-alpha-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am J Physiol Renal Physiol. 2005;288:F1227–1235. doi: 10.1152/ajprenal.00428.2003. [DOI] [PubMed] [Google Scholar]
- Ichinose F, Ullrich R, Sapirstein A, Jones RC, Bonventre JV, Serhan CN, et al. Cytosolic phospholipase A(2) in hypoxic pulmonary vasoconstriction. J Clin Invest. 2002;109:1493–1500. doi: 10.1172/JCI14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–862. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK, Cho WL, Huang SH. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem. 2012;287:15298–306. doi: 10.1074/jbc.M112.353375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyvas A, David S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol. 2008;6:625–634. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YV, Di Cello F, Hillaire CS, Kim KS. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brainmicrovascular endothelial cells. Am J Physiol Cell Physiol. 2004;286:C31–42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-saharan africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada R, Zhu L, Pearce D, Sapirstein A, Kim KS. Host cytosolic phospholipase A2α contributes to group B Streptococcus penetration of the blood-brain barrier. Infect Immun. 2011;79:4088–4093. doi: 10.1128/IAI.05506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada R, Zhu L, Pearce D, Zheng Y, Perfect J, Kwon-Chung KJ, Kim KS. Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood-brain barrier. Cell Microbiol. 2012;14:1544–1553. doi: 10.1111/j.1462-5822.2012.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV infected persons with a CD4+ cell count < or = 100 cells/mcl who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura C, Inada M, Matsumoto C, Ohshiba T, Uozumi N, Shimizu T, Ito A. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J Exp Med. 2003;197:1303–1310. doi: 10.1084/jem.20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Sala A, Dahlén SE, Folco G. Pharmacological modulation of the leukotriene pathway in allergic airway disease. Drug Discov Today. 2007;12:404–412. doi: 10.1016/j.drudis.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- Mwaba P, Mwansa J, Chintu C, Pobee J, Scarborough M, Portsmouth S, Zumla A. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–773. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Uozumi N, Ishii S, Kita Y, Yamamoto H, Ohga E, et al. Pivotal role of cytosolic phospholipase A(2) in bleomycin-induced pulmonary fibrosis. Nat Med. 2002;8:480–484. doi: 10.1038/nm0502-480. [DOI] [PubMed] [Google Scholar]
- Neuville S, Dromer F, Chrétien F, Gray F, Lortholary O. Physiopathology of meningoencephalitis caused by Cryptococcus neoformans. Ann Med Interne (Paris) 2002;153:323–328. [PubMed] [Google Scholar]
- Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol. 2004;164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Perfect JR, Casadevall A. Cryptococcosis. Infect Dis Clin North Am. 2002;16:837–874. doi: 10.1016/s0891-5520(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WR. Leukotrienes. N Eng J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Powderly WG. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993;17:837–842. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Prasadarao NV, Wass CA, Kim KS. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J Biol Chem. 2000;275:36769–36774. doi: 10.1074/jbc.M007382200. [DOI] [PubMed] [Google Scholar]
- Rüffer C, Strey A, Janning A, Kim KS, Gerke V. Cell-cell junctions of dermal microvascular endothelial cells contain tight and adherens junction proteins inspatial proximity. Biochemistry. 2004;43:5360–5369. doi: 10.1021/bi035517c. [DOI] [PubMed] [Google Scholar]
- Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- Sapirstein A, Saito H, Texel SJ, Samad TA, O’Leary E, Bonventre JV. Cytosolic phospholipase A2alpha regulates induction of brain cyclooxygenase-2 in a mouse model of inflammation. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1774–1782. doi: 10.1152/ajpregu.00815.2004. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stins MF, Badger JL, Kim KS. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathogenesis. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Voelz K, Johnston SA, Rutherford JC, May RC. Automated analysis of cryptococcal macrophage parasitism using GFP-tagged cryptococci. PLoS one. 2010;5:e15968. doi: 10.1371/journal.pone.0015968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K, Tham R, Uhrig JP, Thompson GR, 3rd, Na Pombejra S, Jamklang M, Bautos JM, Gelli A. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5(3):e01101–14. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentien T, Crum-Cianflone NF. An update on Cryptococcus among HIV-infected patients. Int J STD AIDS. 2010;21:679–684. doi: 10.1258/ijsa.2010.010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Maruvada R, Sapirstein A, Malik KU, Peters-Golden M, Kim KS. Arachidonic acid metabolism regulates Escherichia coli penetration of the blood-brain barrier. Infect Immun. 2010;78:4302–4310. doi: 10.1128/IAI.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Schwegler-Berry D, Castranova V, He P. Internalization of caveolin-1 scaffolding domain facilitated by Antennapedia homeodomain attenuates PAF-induced increase in microvessel permeability. Am J Physiol Heart Circ Physiol. 2004;286:H195–201. doi: 10.1152/ajpheart.00667.2003. [DOI] [PubMed] [Google Scholar]