Abstract
Although haemoglobin SC (HbSC) accounts for 30% of sickle cell disease (SCD) in the United States and United Kingdom, evidence-based guidelines for genotype specific management are lacking. The unique pathology of HbSC disease is complex, characterized by erythrocyte dehydration, intracellular sickling and increased blood viscosity. The evaluation and treatment of patients with HbSC is largely inferred from studies of SCD consisting mostly of haemoglobin SS (HbSS) patients. These studies are underpowered to allow definitive conclusions about HbSC. We review the pathophysiology of HbSC disease, including known and potential differences between HbSS and HbSC, and highlight knowledge gaps in HbSC disease management. Clinical and translational research is needed to develop targeted treatments and to validate management recommendations for efficacy, safety and impact on quality of life for people with HbSC.
Keywords: Haemoglobin SC, Haemoglobin Sickle C, sickle cell disease, sickle cell anaemia
Background
Co-inheritance of the haemoglobin S (HbS) and haemoglobin C (HbC) beta globin gene (HBB) mutations causes haemoglobin SC (HbSC) disease. Valine replaces glutamic acid at position 6 (Glu6Val) in HbS, while lysine is substituted for glutamic acid (Glu6Lys) in HbC. Haemoglobin AC and haemoglobin AS (HbC and HbS trait, respectively) are largely asymptomatic carrier states, whereas HbSC has a unique pathological phenotype (Nagel et al, 2003; Steinberg & Nagel, 2009). Although considered a milder sickle cell disease (SCD) variant, HbSC is associated with potentially severe morbidities that warrant surveillance and intervention (Nagel et al, 2003; Steinberg & Nagel, 2009).
In 2014, the National Heart, Lung and Blood Institute (NHLBI) of the US National Institutes of Health released treatment guidelines, Evidence-Based Management of Sickle Cell Disease (Yawn et al, 2014), based on available scientific evidence and expert consensus. This report provides recommendations for clinical management of all patients with either SCD or sickle cell anaemia (SCA). The largest multi-centre study of HbSC, the 1,000+ HbSC cohort enrolled in the Cooperative Study of Sickle Cell Disease (CSSCD), is nearly 40 years old, and like most subsequent studies of patients with HbSC, this rich dataset was non-interventional and non-randomized. The lack of high quality disease-specific evidence precludes publication of evidence-based guidelines for optimal management of HbSC disease (Yawn et al, 2014). Here, we review the epidemiology, pathophysiology, clinical complications and treatment of HbSC, highlighting important knowledge gaps and research opportunities.
DISTRIBUTION & PATHOPHYSIOLOGY
Epidemiology
In the UK and US, HbSC accounts for nearly 30% of SCD. In the UK, 1:2000 babies are born with SCD and 1:7174 has HbSC (Streetly et al, 2008). Similarly, in the US, 1:941 babies are born with SCD, while 1:6173 newborns has HbSC (Therrell et al, 2015). In parts of West Africa, where HbC originated, HbSC accounts for more than 50% of SCD (Saraf et al, 2014). The allelic frequency of HbC is a consequence of the survival advantage against severe malaria conferred through inheritance of one HBB (beta globin gene) mutation (Modiano et al, 2001; Piel et al, 2013). Emigration from West Africa, forced and voluntary, spread HbC to Europe and the Americas. Worldwide, at least 55,000 children are born with HbSC annually (Weatherall, 2010).
Survival of patients with HbSC is superior to SCA, probably reflecting delayed onset of irreversible organ injury (Powars et al, 2002; Hassell, 2010; Steinberg & Nagel, 2009). In the decades-old CSSCD, median survival of patients with HbSC was 60 years for men (n = 417) and 68 years for women (n=427) (Platt et al, 1994). A recent single-centre study reported median survival of patients with HbSC and HbSβ+ patients (n = 93) to be 66 years (Elmariah et al, 2014). In these data sets, US patients received care at designated sickle cell centres. Older longitudinal studies from the US report median survival of 37 years (n = 248) (Powars et al, 2002) and 50 years (n =106) (Koduri et al, 2001). Life expectancy in HbSC, as for all SCD, remains context-dependent, with patients in high-income countries benefiting from access to newborn screening and to preventive, emergency and intensive care (Streetly et al, 2008; Chatuverdi & DeBaun, 2016).
Pathophysiology
HbSC red blood cells (RBCs) contain roughly equal amounts of HbC and HbS and low (1-3%) levels of HbF (Nagel et al, 2003; Gualandro et al, 2015). HbSC disease pathogenesis is modulated by interactions between HbS and HbC as well as RBC dehydration from altered membrane transporter function. When RBCs are dehydrated, the HbS concentration increases (Mozzarelli et al, 1987). At moderate to high intra-erythrocyte concentrations, HbS subunits polymerize, forming long, rigid molecules that persist as RBCs enter and obstruct the microcirculation (Nagel et al, 2003). At high concentrations of oxygenated HbC, tetragon crystals form, but in the deoxygenated state they dissolve rapidly (Nagel et al, 2003). HbF impedes HbC crystallization and HbS polymerization (Nagel et al, 2003). Increasing HbF is of therapeutic interest in HbSC, as it is for haemoglobin SS (HbSS) disease. Other potential sites of therapeutic intervention include the three pathways that cause RBC dehydration and cation loss: (1) deoxy-dependent, nonselective P-sickle pathway calcium ion permeability (Gallagher, 2015) (2) calcium-activated Gardos potassium channels (Gallagher, 2015); and (3) increased K-Cl cotransport that leads to potassium, chloride and water loss (Nagel et al, 2003; Rees et al, 2015). Erythrocyte water and solute transport is incompletely understood and has not yet been successfully therapeutically exploited (Gallagher, 2015). Initial trials of magnesium, a modulator of K-Cl cotransport in HbSS and HbSC, were disappointing (Rees et al, 2015; Nottage et al, 2016; Wang et al, 2011).
Alpha thalassaemia trait
Inheritance of alpha thalassaemia gene deletions decrease HbS polymerization (Steinberg & Embury, 1986) and modulate HbSC disease severity (Embury et al, 1982, 1984; Powars et al, 2002,). In a seminal observational cohort study of 248 patients with HbSC, Powars et al (2002) reported that patients with two HBA1/2 (alpha globin gene) deletions had less (17% vs. 32%) and later (48 vs. 33 years) osteonecrosis, later onset gallbladder disease (55 vs. 34 years), less retinopathy (26% vs. 41%) and fewer painful crises (48% vs. 80%). These patients also had favourable clinical outcomes measured by composite scores of major organ failure (Powars et al, 2002). Iron deficiency mimics alpha thalassaemia trait by decreasing alpha globin production (Bunn, 1994). This is one rationale for therapeutic phlebotomy in HbSC (Lionnet et al, 2016).
Laboratory findings in HbSC
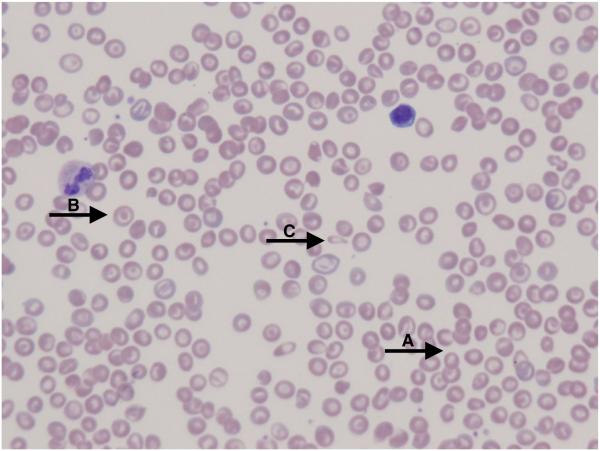
People with HbSC have a higher mean Hb and lower absolute reticulocyte count (ARC) than people with HbSS disease (Table I). Erythrocyte lifespan is twice that of HbSS (29 vs. 15 days) (McCurdy & Sherman, 1978; McCurdy et al, 1975). Over 70% of people with HbSC have anaemia, but only 10% have Hb lower than 100 g/l. Mean corpuscular volume (MCV) is low, and inheritance of alpha thalassaemia trait further reduces MCV (Embury et al, 1984). Leucocytosis is less pronounced or absent in HbSC compared to HbSS. Platelet counts may be normal, but mild to moderate thrombocytopenia occurs in patients with splenomegaly and hypersplenism. The abundance of target cells on the peripheral blood smear results from the relatively increased surface area secondary to RBC dehydration (Figure 1A). Distorted tri-concave or elongated erythrocytes containing Hb crystals occur infrequently. Irreversibly sickled cells are rare. Blood viscosity is increased even compared to HbSS (Nagel et al, 2003).
Table I.
Laboratory Results at Baseline and After 12 Months of Hydroxycarbamide Therapy in Patients with HbSC Disease
| Hb (g/l) |
MCV (fl) |
ARC (×109/l) |
WBC count (×109/l) |
Platelet count (×109/l) |
ANC (×109/l) |
%HbA | %HbS | %HbC | %HbF | %HbA2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HbSC1 | 108 ± 12 | 75.7 ± 7 | 161 ± 97 | 9.6 ± 3.8 | 329 ± 155 | 4.88 ± 2.72 | 0 | 50 | 45 | 2.8 ± 2.9 | <3.5 |
|
HbSC
treated with HC1 |
111 ± 12 | 87 ± 12 | 129 ± 88 | 7.5 ± 2.8 | 273 ± 145 | 3.58 ± 2.08 | NR | NR | NR | 5.7 ± 4.7 | NR |
Values taken from Luchtman-Jones et al (2016)
ANC, absolute neutrophil count; ARC, absolute reticulocyte count; Hb, haemoglobin; HbSC, haemoglobin SC; HC, hydroxycarbamide; MCV, mean corpuscular volume; NR, not reported; WBC, white blood cell.
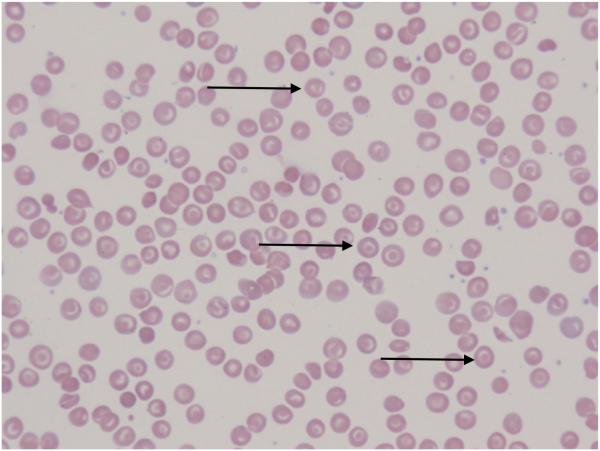
FIGURE 1.
The peripheral blood smear from a patient with HbSC (A) shows abundant red blood cell (RBC) polychromasia (A) as well as target cells with relatively increased surface area secondary to RBC dehydration (B). Distorted tri-concave or elongated erythrocytes containing haemoglobin crystals occur infrequently (C). Irreversibly sickled cells are rare. With hydroxycarbamide exposure, the peripheral blood smear (B) demonstrates more uniformity in the RBC target cell population (arrows) with an increase in mean corpuscular volume and less polychromasia.
Diagnosis
In newborn screening programmes for SCD in the US and the UK, screening and confirmatory testing is performed using high-performance liquid chromatography and isoelectric focusing. Newborn screening reduces SCD morbidity and mortality by facilitating implementation of early preventive medical care (Streetly et al, 2008). Because many people with HbSC emigrate from countries without newborn screening, patients may only be diagnosed when a disease-related complication arises. Primary care and emergency department clinicians should consider undiagnosed HbSC disease in children or adult patients presenting with complications such as priapism, hypersplenism and painful crises.
Identifying couples at risk for having a child with HbSC enables pre-conception counselling and diagnostic strategies, including pre-implantation genetic testing, and post-conception chorionic villus sampling or amniocentesis (Mason et al, 2016; Kuliev et al, 2011). Non-invasive analysis of cell-free fetal DNA extracted from maternal blood samples is an emerging technology for prenatal SCD diagnosis (Perlado et al, 2016; Barrett et al, 2012).
Penicillin Prophylaxis and Vaccination
Reducing the risk of life threatening complications, such as infection, provides one rationale for newborn screening for SCD. Unfortunately, the efficacy and timing of penicillin prophylaxis in HbSC are debated because of a lack of evidence (Lane et al, 1995; Yawn et al, 2014). In the CSSCD, bacterial sepsis in HbSC children (n=177, 339 patient years) occurred mostly before age 2 years (Zarkowsky et al, 1986); however, in the Dallas Newborn Cohort, the rate of bacterial sepsis in non-prophylaxed children with HbSC (n=242, 842 patient years) was similar to that of age-matched controls (Rogers & Buchanan, 1995), perhaps because of a later loss of splenic function in HbSC (Buchanan et al, 1983). Faced with uncertainty about the timing and extent of splenic function loss in HbSC and the efficacy of prophylactic measures, we prescribe penicillin prophylaxis to children with HbSC as for all with SCD. Our patients also receive multi-valent vaccination against encapsulated organisms (meningococcus, pneumococcus and Haemophilus influenza) and immunizations against hepatitis A and B (Yawn et al, 2014; Gaston 1986). We urgently evaluate and empirically treat febrile patients, regardless of vaccination status (Yawn et al, 2014).
SYSTEMIC COMPLICATIONS
Ocular Manifestations
Proliferative sickle retinopathy, or retinitis proliferans (RP), is the most common complication of HbSC disease, occurring in 30-70% of patients with HbSC compared to 3% in HbSS (Lutty et al, 2001; Lemonne et al, 2014; Gualandro et al, 2015; Myint et al, 2015). RP is caused by excessive retinal blood vessel growth. It may occur late in the first decade of life, and incidence peaks in the third and fourth decades (Leveziel et al, 2012). The retinal neovascularization of RP is classically described as “black sea fan”, “black sunburst” and “salmon patch” (Welch & Goldberg 1966). One hypothesis explaining the higher incidence of RP is that in HbSC, peripheral retinal vessels are occluded but the minimal oxygen delivery needed for neovascularization is maintained, while in HbSS, frank retinal ischaemia inhibits neovascularization (Nagel et al, 2003). Co-inheritance of alpha thalassaemia trait is associated with decreased rates of retinopathy (Powars et al, 2002). RP may be more directly associated with hyperviscosity in HbSC, reflecting RBC rheology and Hbconcentration, rather than Hbor RBC density alone (Lemonne et al, 2014). Proliferative neovascularization causes vision loss by vitreous haemorrhage and tractional retinal detachment.
Non-proliferative damage occurs via occlusion of ocular structures (conjunctiva, anterior segment, choroid, retina or optic nerve) and typically presents as acute vision loss. Central retinal artery occlusion is rare in HbSC (Fine et al, 2000). Acute vision loss in SCD patients requires emergent evaluation to optimize preservation of function (Myint et al, 2015; Yawn et al, 2014).
Early detection and intervention are effective in preventing vision loss. NHLBI guidelines recommend annual ophthalmological screening for SCD patients beginning at age 10 years and prompt referral for expert management of complications. Given the higher incidence of RP in HbSC, we recommend annual ophthalmological examinations. Treatment options include diathermy, cryotherapy and transpupillary or transscleral diode laser photocoagulation. The preferred intervention is transpupillary laser photocoagulation. Other laser therapies merit ongoing investigation (Myint et al, 2015). High-grade evidence for treatment with simple or exchange transfusions is lacking.
Pain
Painful crises are a hallmark of HbSC disease. At least 50% of people with HbSC report a painful episode requiring a hospital visit (Powars et al, 2002) and about 5% of HbSC patients may have frequent debilitating painful events (Luchtman-Jones et al, 2016). Prevention and treatment strategies for painful crises in HbSC include hydration, analgesia and adequate oxygenation. Rehydration of HbSC erythrocytes in vitro normalizes the mean corpuscular haemoglobin concentration (MCHC), increases Hb oxygen affinity, lowers the viscosity of deoxygenated RBC suspensions, reduces the rate of sickling and improves the deoxygenation-induced potassium efflux (Nagel et al, 2003). Acute treatment recommendations include prompt and aggressive pain management, using non-steroidal anti-inflammatory drugs (NSAIDs) with the addition of opiates, depending upon renal function and pain severity. Mechanism-specific medications, like the K-Cl cotransporter inhibitor magnesium, have not demonstrated efficacy in modulating acute painful crises (Rees et al, 2015; Nottage et al, 2016).
Health-related quality of life (HRQL) measurements in SCD are not genotype-specific. Pain negatively impacts the quality of life in people with SCD. A systematic review of HRQL in SCD concluded that adults and children have significantly impaired HRQL similar or worse than in other chronic diseases (Panepinto & Bonner, 2012). Physical functioning domains are especially affected, probably due to pain. Other factors that modify HRQL are age, socioeconomic status, medical or neurobehavioural comorbidities, and disease-modifying therapies (Panepinto & Bonner, 2012). Future clinical trials for HbSC should include validated HRQL measures.
Splenic complications
Splenic acidosis and relative hypoxia can precipitate RBC sickling. Aging and damaged RBCs are removed by the spleen (Crary & Buchanan, 2009). Functional asplenia is reported in 45% of HbSC patients by age 12 years (Lane et al, 1995). Chronic splenomegaly is associated with thrombocytopenia in 35% of children and 50% of adults with HbSC and may cause recurrent abdominal pain. Hypersplenism may impede optimal treatment with transfusion or hydroxycarbamide (Zimmerman & Ware, 2000; Aquino et al, 1997).
Acute splenic sequestration crisis (ASSC), precipitated by obstruction of splenic outflow with sickled erythrocytes, causes massive pooling of blood within the splenic sinusoids. ASSC occurs in 6-12% of children with HbSC. Affected patients present with rapid, progressive and, especially in older patients, painful splenomegaly (Aquino et al, 1997). Platelet counts fall with worsening anaemia, typically 20 g/l or more below baseline. Splenic infarction, characterized by moderate splenomegaly, left upper quadrant pain and wedge-shaped defects on liver-spleen scan, occurs spontaneously or is triggered by high-altitude exposure. Splenic infarcts may mimic pain crises and are likely under diagnosed (Aquino et al, 1997; Githens et al, 1977). Primary treatment for ASSC is RBC transfusion, with cautious hydration due to the risk of high output congestive heart failure. Anticipating that Hb will increase as RBCs are released from the enlarged spleen (“auto-transfusion”), conservative simple RBC transfusions of 5 ml/kg are advised. Splenomegaly improves within hours to days after transfusion (Koduri & Nathan, 2006). Recurrent sequestration is common and may require complete or partial splenectomy, although strong evidence in HbSC is lacking (Englum et al, 2016).
The incidence and optimal management of HbSC splenic sequestration is unknown (Koduri & Nathan, 2006). Splenectomy improves anaemia and thrombocytopenia. Whether this causes an injurious increase in viscosity for HbSC patients is unclear (Subbannan et al, 2009). Splenectomy is ideally performed at least two weeks after vaccination against encapsulated organisms, and post-splenectomy antibiotic prophylaxis is recommended. Caregivers and patients should learn splenic palpation and recognition of the signs of sequestration (Aquino et al, 1997; Zimmerman & Ware, 2000).
Osteonecrosis
Osteonecrosis, or avascular necrosis (AVN), is common, occurring in 12 - 24% of HbSC patients (Lionnet et al, 2012; Mukisi-Mukaza et al, 2009). AVN typically affects large joints, such as hips and shoulders, but can occur in other joints, including the spine. Bilateral hip disease is more common in HbSS than in HbSC. Hyperviscosity may contribute to the development of AVN, though high-grade evidence is lacking (Milner et al, 1991; Lemonne et al, 2013). Alpha thalassaemia trait, while a risk factor for AVN in HbSS, is less strongly associated in HbSC, and may even be protective (Powars et al, 2002; Milner et al, 1991; Ballas et al, 1989). Evidence does not support surveillance x-rays in asymptomatic patients.
AVN usually presents as focal pain, but it can precipitate diffuse pain crisis and should be considered when diffuse pain resolves, but focal pain persists (Almeida & Roberts, 2005). Initial evaluation in symptomatic patients is with plain radiographs; however, magnetic resonance imaging (MRI) is the most sensitive diagnostic modality and delineates the extent of joint damage. Original AVN scoring systems, developed prior to the availability of MRI, have been adapted (Steinberg & Steinberg, 2004).
Reducing pain and disability are the primary management goals of AVN. Unfortunately, high-level evidence about timing or type of joint intervention is lacking (Almeida & Roberts, 2005; Stoica et al, 2009). Depending upon the extent and progression of joint injury, management may include analgesia, physical therapy and surgery. Localized lidocaine- or opiate-eluting patches may decrease systemic opiate intake. Patients with progressive AVN should be referred to orthopaedics for evaluation. Core decompression may alleviate pain and delay arthroplasty (Mukisi-Mukaza et al, 2009). When femoral head damage progresses to collapse, total hip arthroplasty may be indicated. Several recent studies investigated the optimal type and timing of joint repair or replacement without definitive conclusions (Almeida & Roberts, 2005; Stoica et al, 2009).
Central Nervous System Complications
Neurological complications, including headache, overt ischaemic stroke, haemorrhagic stroke, and silent cerebral infarcts (SCI), are common in SCD (DeBaun & Kirkham, 2016). Risk factors for ischaemic stroke include hypertension (adults and children), hyperlipidaemia, renal disease and atrial fibrillation. Risk factors for haemorrhagic stroke include hypertension (all ages), kidney disease, and coagulopathy (Strouse et al, 2009). Stroke rates for HbSC, while much lower than in SCA, peak in older adults, in whom haemorrhagic stroke is more common (Strouse et al, 2009; DeBaun & Kirkham, 2016).
Few data distinguish the neurological sequelae of HbSC from all SCD. However patients with HbSC are known to suffer overt stroke (2%) and SCI (8 – 13.5%) (Wang et al, 2001; Guilliams et al, 2015) at higher rates than in the general population. Baseline transcranial Doppler (TCD) values are reportedly lower in children with HbSC (Deane et al, 2008). Children with HbSS and HbSC without evidence of stroke on brain MRI scored slightly lower, but within the population means, on neurocognitive testing (Gold et al, 2008), whereas HbSS and HbSC patients with SCI scored 9-10 points lower on neuropsychological testing. Impairment was associated mostly with frontal lobe tasks requiring attention and concentration. Children also had problems with hyperactivity, cognitive and academic performance, and executive functioning (Gold et al, 2008). Although annual TCD screening and intervention with chronic RBC transfusions have reduced ischaemic stroke rates in children with SCA, neither is validated in HbSC disease.
Little is known about headaches in HbSC disease, as most studies do not report genotype-specific outcomes. Up to 36% of children and adolescents with SCD experience chronic headaches; in adults with SCD, chronic headache is associated with opioid use and vaso-occlusive events (DeBaun & Kirkham, 2016). Neurology consultation may help guide chronic headache treatment with therapies, such as pizotifen, propranolol, topiramate or valproic acid. Tryptans are avoided because of concerns about exacerbating cerebral ischaemia (DeBaun & Kirkham, 2016).
Increased incidence of sensorineural hearing loss, sometimes associated with retinopathy, is reported in HbSC (Leveziel et al, 2012; Onakoya et al, 2010; Lionnet et al, 2012). We maintain a high index of suspicion for hearing loss or vestibular problems, especially in patients with retinopathy, and in the absence of high-level evidence, support a low threshold for audiological testing in these patients.
Sexual development and Pregnancy
Young women with HbSC may have marginally delayed menarche compared to unaffected controls (median age 13.7 vs. 13 years) (Serjeant et al, 2005). The CSSCD found comparable onset of menarche across sickle cell genotypes consistent with constitutional growth delay (Platt et al, 1984). In women with HbSS, menses may precipitate painful crises and infertility rates are increased (Stimpson et al, 2016; Yoong & Tuck, 2002; Smith-Whitley, 2014). These complications are unexplored in HbSC. Koduri (2003) hypothesizes that menstrual blood losses lower iron stores and reduce blood viscosity thereby improving HbSC disease severity.
Women with HbSC, like all women with SCD, are at increased risk of maternal and fetal morbidity and mortality. They may experience sickle and non-sickle related complications during pregnancy including acute pain, pre-eclampsia, acute chest syndrome (ACS) and urinary tract infections (Oteng-Ntim et al, 2015a, 2015b). Babies born to mothers with HbSC are more often born at term, with fewer fetal complications than those born to mothers with HbSS (Oteng-Ntim et al, 2015b; Wilson et al, 2012; Serjeant et al, 2005). In a cohort of Brazilian women with HbSC, erythrocytapheresis or manual exchange in the third trimester reduced maternal and fetal complications (Benites et al, 2016), but high-level evidence for transfusion during pregnancy is lacking (Okusanya & Oladapo, 2013).
Education about the heritability of HbSC, contraception options and the need for high-risk obstetrics care should begin in early adolescence (Stimpson et al, 2016). A 2010 survey of women with HbSS in the UK found that 53% experienced an unintended pregnancy (Eissa et al, 2015). High quality evidence to identify the optimal forms of contraception in women with SCD is needed, particularly given the risk of venous thromboembolism (VTE) with oestrogen-containing contraception. Depot medroxyprogesterone-acetate use is associated with decreased rates of pain crises in SCD (Manchikanti et al, 2007), but whether reversible changes in bone mineral density exacerbate disease related complications is unknown (Kaunitz et al, 2008).
Priapism
Priapism, an unwanted erection lasting four hours or more, occurs in 20% of men with HbSC and is a clinical emergency (Lionnet et al, 2012). Priapism often occurs as young men reach sexual maturity (Adeyoju et al, 2002), and may be the presenting sign of HbSC disease in previously undiagnosed men (Anele et al, 2015). Typically caused by acute ischaemic injury, priapism may be continuous, intermittent or recurrent (stuttering priapism). Treatment goals are detumescence and pain reduction to prevent future erectile dysfunction and impotence. If conservative management with aggressive hydration and analgesia is unsuccessful, treatments include oral alpha-adrenergic agonists, phosphodiesterase-5 inhibitors and urological consultation for penile aspiration or corporeal irrigation with alpha-adrenergic agonists. Transfusion has no demonstrated efficacy for management of priapism and in HbSS, there may be an association between priapism, exchange transfusion and neurological complications (Merritt et al, 2006).
Cardiovascular and Pulmonary Complications
Cardiovascular complications of SCD arise from complex interactions between blood and vascular endothelium and from the systemic response to anaemia (Pecker & Ackerman, 2016). Blood pressure (BP) in patients with HbSC is similar to healthy controls whereas in HbSS it is lower (Pegelow et al, 1997; Lionnet et al, 2012). Near normal BP might be expected in HbSC, where milder anaemia probably improves vascular tone. Patients with all sickle cell genotypes require monitoring and treatment of hypertension due to stroke risk (Pegelow et al, 1997; Yawn et al, 2014; James et al, 2014). SCD-related changes in cardiac function may reflect intrinsic myocardial injury, the sequelae of chronic high-output demand or elevated tricuspid valve regurgitant jet velocity (TRJV) (Voskaridou et al, 2012; Niss et al, 2016). Echocardiogram, electrocardiogram (EKG) and serum markers, such as brain natriuretic peptide (BNP) and troponins are difficult to interpret in the setting of SCD, and clinical condition dictates management (Voskaridou et al, 2012). Routine EKGs, echocardiograms and TRJV are not recommended for asymptomatic patients (Yawn et al, 2014).
Acute and chronic pulmonary complications contribute to morbidity and mortality in SCD (Saraf et al, 2014; Mehari & Klings, 2016). Hyper-reactive airways, asthma, ACS, pulmonary embolism (PE), pulmonary hypertension, sleep disordered breathing, obstructive sleep apnoea, reduced Hboxygen saturation and compromised pulmonary function are described more commonly in SCA cohorts (Saraf et al, 2014; Mehari & Klings, 2016). In a prospective evaluation of pulmonary hypertension in SCA, Gladwin et al. (2004), reported statistically significantly lower TRJV in subjects with HbSC, presumably due to relatively reduced haemolysis. ACS occurs in HbSC at similar or lower rates than in HbSS (Waltz et al, 2013; Lionnet et al, 2012; Vichinsky et al, 1997). In a study examining resting and exercise-induced oxygen desaturation, 50% of HbSS subjects, but none with HbSC, had baseline oxygen saturation <98%. After a six-minute walk test, baseline oxygen saturation fell 3% in children with HbSC and HbSS (18% vs. 34%) (Waltz et al, 2013). Lower oxygen saturation in children with HbSC correlated with reduced RBC deformability, but not blood viscosity, Hb, ACS rates or vaso-occlusive events. Severe ACS is an indication for blood transfusion, and hydroxycarbamide reduces the recurrence of ACS in patients with SCA, but these disease-modifying treatments are unstudied for HbSC disease (Yawn et al, 2014). Research is needed to establish optimal diagnostic, prognostic, and therapeutic approaches for lung disorders in SCD, particularly in HbSC.
Thrombosis
HbSC is a hypercoagulable state associated with arterial thrombosis and VTE complications and elevations in fibrinogen (Ajuwon et al, 2014), tissue factor expression, D-dimer and thrombin-antithrombin complexes (Colella et al, 2015). Alterations in protein C, protein S, nitric oxide, microparticles and markers of endothelial activation at steady state and in crisis may contribute to a prothrombotic state in SCD and in HbSC (Piccin et al, 2015). Deep vein thrombosis (DVT) and PE, which constitute VTE, are increased in HbSC, as are AVN, stroke and pregnancy-related complications (Colella et al, 2015). Retrospective cohort studies identify higher rates of non-catheter-related VTE in HbSC than HbSS (Lionnet et al, 2012; Naik et al, 2014a). In an autopsy study of 44 HbSC patients, PE was the second leading cause of death (Manci et al, 2003). Risk factors for VTE in HbSC include higher Hb and splenectomy (Yu et al, 2016). Therapeutic phlebotomy is used at some centres to reduce disease complications associated with increased blood viscosity and higher Hb in HbSC (Lionnet et al, 2012; Yu et al, 2016), but whether this reduces thrombotic complications is unknown. Oestrogen-based hormone therapy generally increases baseline thrombosis risk, but there is no SCD-specific data (Manchikanti et al, 2007).
Clinicians should have a high index of suspicion for thrombosis in HbSC and follow general expert guidelines for prevention and detection of thromboembolic disease, recognizing that D-dimer cannot be reliably interpreted due to chronic and fluctuating activation of coagulation (Naik et al, 2014a; Kearon et al, 2016). Patient education includes VTE prevention strategies and recognizing signs and symptoms of venous and arterial thrombosis. SCD-specific VTE prophylaxis guidelines do not exist and no known disease-modifying interventions alter the risk of thrombosis in patients with HbSC disease.
Renal
Renal complications in HbSC occur later, more gradually, and less commonly than in HbSS and include hyposthenuria, acute papillary necrosis and microalbuminuria. The sickle nephropathy of HbSC is distinct from HbSS in that it occurs later in life and rarely leads to end-stage-renal-disease (ESRD) (Aygun et al, 2011; Lionnet et al, 2012). Microalbuminuria occurs in 30% of patients with HbSC, and ESRD develops in 2-3% by their 5th decade (Powars et al, 1991, 2002; Lionnet et al, 2012; Drawz et al, 2016). Children with HbSC typically develop irreversible hyposthenuria at 10 - 15 years of age and have evidence of glomerular damage and medullary injury (Iyer et al, 2000; Drawz et al, 2016). Acute HbSC renal complications include renal papillary necrosis and renal infarction. Renal medullary carcinoma, associated with HbAS, but not HbSS, is also reported in HbSC (Davis et al, 1995; Dimashkieh et al, 2003), raising intriguing questions that are beginning to be explored about mechanisms of renal injury in people with one HbS gene (Naik et al, 2014b).
The management of renal complications is not HbSC-specific (Sasongko et al, 2015). Annual screening for proteinuria starts at age 10 years for all children with SCD. Rapid deterioration in renal function is inconsistent with the natural history of HbSC disease and should prompt investigation of another aetiology (Sharpe & Thein, 2014). Hydroxycarbamide may improve the concentrating defect in children with SCA (Aygun et al, 2011), but its effects in HbSC are unclear (Iyer et al, 2000).
Hepatobiliary disease
Gallbladder-related complications are less common in HbSC (15-20%) than in HbSS (50%), probably because of lower haemolytic rates (Walker et al, 2000; Powars et al, 2002). The incidence of cholelithiasis increases with age, occurring in 3% of HbSC children under age 14 years, and in nearly 20% by age 20 years (Walker et al, 2000). Inheritance of alpha thalassaemia trait is associated with lower rates and later onset of symptomatic gallbladder disease (Powars et al, 2002).
DISEASE MODIFYING THERAPIES
Hydroxycarbamide and RBC transfusion are currently the most available and under-utilized disease modifying therapies in SCD (Yawn et al, 2014). For people with HbSC, the use of disease modifying RBC transfusions (Estcourt et al, 2016a), phlebotomy (Lionnet et al, 2012) and hydroxycarbamide are supported by low-level evidence of safety and efficacy (Luchtman-Jones et al, 2016). Haematopoietic stem cell transplant (HSCT) for HbSC is described for patients with an additional diagnosis like cancer, but has not been reported in patients with isolated HbSC disease, probably because the risks of HSCT are thought to outweigh potential benefits (Kamble et al, 2006).
Red Cell Transfusion
Most adults with HbSC receive a RBC transfusion in their lifetime, but the efficacy of transfusions is not well demonstrated for prevention or treatment of disease complications (Estcourt et al, 2016a, 2016b). A retrospective analysis of HbSC patients in the CSSCD showed benefit for preoperative RBC transfusions (Koshy et al, 1995); however a prospective nonrandomized clinical trial of 75 HbSC patients transfused at the discretion of their physician did not show benefit, except in moderate risk procedures (such as abdominal surgery) (Neumayr et al, 1998). Low quality evidence reviewed in a recent meta-analysis prevented conclusions about perioperative transfusions in HbSC disease (Estcourt et al, 2016b). The use of empiric transfusion in the UK is inconsistent (Buck et al, 2005). Higher potential risk has been attributed to certain surgeries where acidosis, dehydration and tissue hypoxia seem likely, such as cardiac bypass surgery. Case reports detail preoperative or intraoperative exchange transfusion procedures, usually to reduce HbS + C to less than 30% (Marchant et al, 2001; Law et al, 2007)
Acute and chronic RBC transfusion modalities are important in HbSC, where higher Hb and hyperviscosity are concerns. Simple transfusion raises Hb and blood viscosity. Exchange transfusions remove sickled RBCs and replace them with allogeneic RBCs, lowering or minimally increasing blood viscosity. Partial volume exchange, performed manually or by apheresis, facilitates rapid reduction in the %HbS while maintaining a stable Hb concentration. Therapeutic targets for RBC transfusion in HbSC are derived from HbSS, where the goals are Hb of 100 g/l and HbS < 30%. Higher baseline Hb in HbSC may unacceptably increase blood viscosity and Hb after simple transfusion, while marginally reducing the HbS%. Exchange transfusion is favoured, often with a goal of reaching a HbS% of 15 - 25, so that HbS + C% is 30 or 50, respectively.
Risks of RBC transfusion include infection, transfusion reactions, alloimmunization, iron overload and vascular access problems. Exchange transfusion and apheresis procedures are associated with vascular access issues and higher donor exposure rates because more RBC units are required. A knowledge gap exists about alloimmunization rates in transfused HbSC patients (Chou & Fasano, 2016). RBC transfusion in HbSC for management of refractory pain, ACS or other vaso-occlusive complications remains empiric.
Decisions about preoperative transfusion should include consideration of the type of procedure and published complications rates, the severity of the patient’s HbSC disease in general and a frank discussion with the patient and family about the risks and benefits of preoperative transfusion. Given that hypoxia and acidosis are predicted to precipitate sickling, reduction of HbS + C to < 30% will probably remain usual practice for high risk surgical procedures until better evidence is available.
Phlebotomy
Because of a possible association between higher Hb concentration and sickle complications, phlebotomy targeting a reduction in Hb to 95 - 100 g/l has been empirically implemented for some HbSC patients. Phlebotomy reduces blood viscosity and iron stores. In a retrospective, nonrandomized study of 64 HbSC patients with clinical complications including pain, ACS, priapism and thrombosis, Lionnet et al (2012) reported clinical improvement in 71% after serial phlebotomy procedures. Whether this benefit is attributable to hyperviscosity is unclear because actual measured blood viscosity may not correlate with hyperviscosity predicted by using factors like haemoglobin, RBC deformability and aggregation. Lemonne et al (2015) measured blood viscosity directly in a cohort of 90 HbSC patients and found no significant association between the direct measurement of hyperviscosity and hyperviscosity as predicted by high Hb values). Venesection-induced iron restriction could decrease blood viscosity by decreasing Hb, MCV and MCHC, but compensatory thrombocytosis might at least partially counteract the positive benefits. Iron restriction in paediatric SCD patients, who have an increased risk of neurocognitive impairment at baseline, limits the study of this intervention in children (Lukowski et al, 2010). Phlebotomy and vascular access are often technically challenging. Randomized, controlled clinical trials are needed to determine the safety, tolerability and efficacy of phlebotomy in the management of acute and chronic complications in HbSC disease.
Hydroxycarbamide
NHLBI guidelines strongly recommend hydroxycarbamide use for adults with SCA and severe or recurrent ACS, frequent sickle cell pain or chronic anaemia and make a moderate-strength recommendation in asymptomatic infants, children and adolescents with SCA. A paucity of safety and efficacy data in HbSC prevented recommendations for its use (Yawn et al, 2014). Before 2014, five retrospective and prospective publications described the effects of hydroxycarbamide in 71 adult and paediatric patients with HbSC. Hydroxycarbamide was well tolerated, but the dose, treatment duration and laboratory and clinical efficacy varied. Concerns remained about whether treatment could dangerously increase blood viscosity, Hb and/or MCV, and whether increased HbF and clinical efficacy were possible in HbSC. Hydroxycarbamide may improve RBC rheology despite higher MCV, reduce circulating microparticles and inflammation, lower absolute neutrophil count (ANC) and ARC and enhance nitric oxide release in the vasculature (Luchtman-Jones et al, 2016).
In 2016, investigators from 18 US sickle cell centres reported retrospective data on 133 paediatric and young adult patients with HbSC taking a median hydroxycarbamide dose of 20mg/kg/day. Luchtman-Jones et al (2016) found laboratory changes and potential efficacy in reducing painful events in treated patients. Laboratory results included stable Hb concentration, increased HbF and MCV and reductions in white blood cell counts, ANC and ARC (Table I). Subtle changes in RBC morphology can be seen on peripheral blood smear (Figure 1B). Overall, hydroxycarbamide was well tolerated, with reversible cytopenias (neutropenia and thrombocytopenia) occurring in 22% of patients, similar to published rates in HbSS (Luchtman-Jones et al, 2016). Hydroxycarbamide treatment for the subset of HbSC patients with severe or refractory painful events or, less commonly, ACS remains common but empiric (Luchtman-Jones et al, 2016; Summarell & Sheehan, 2016). Summarell & Sheehan (2016) reported a series of 14 paediatric HbSC patients with clinically severe disease treated with hydroxycarbamide. Four patients who did not respond were treated with a combination of daily hydroxycarbamide, monthly phlebotomy and iron supplementation with positive clinical effect (Summarell & Sheehan, 2016). A prospective single-centre study of hydroxycarbamide in children with HbSC is ongoing (NCT02336373).
There is a knowledge gap about the safety of hydroxycarbamide in pregnant or lactating women. Hydroxycarbamide use is not recommended for these women. Counselling regarding contraception use is necessary for men and women while they are taking hydroxycarbamide (Yawn et al, 2014).
Conclusions
Considered a milder SCD variant, HbSC is associated with significant comorbidities, including high rates of retinopathy, AVN, pain and priapism. Although 30% of patients with SCD in the US and UK have HbSC, disease-specific data are lacking. In the era of precision medicine, studies that combine HbSC patients with other types of SCD obfuscate genotypic differences among the sickle haemoglobinopathies. There is an urgent need for HbSC-specific baseline data, followed by high quality clinical trials that include efficacy, safety and HRQL outcomes to define best practices and treatment options. Research funding should prompt validation of currently used disease modifying therapies, but also serve as a catalyst for discovery of novel therapeutics targeting disease-specific mechanisms.
Acknowledgements
Each author (LHP, BS, LLJ) contributed to the literature review, writing and revisions of this manuscript. The authors would like to thank Dr. Karen Kalinyak and Dr. Russell Ware for review of this manuscript and to Dr. D. Ashley Hill for pictures of HbSC blood smears.
Dr. Pecker was supported by Award Number T32HL110841-04. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose.
References
- Adeyoju AB, Olujohungbe ABK, Morris J, Yardumian A, Bareford D, Akenova A, Akinyanju O, Cinkotai K, O’Reilly PH. Priapism in sickle-cell disease; incidence, risk factors and complications – an international multicentre study. BJU International. 2002;90:898–902. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- Ajuwon MD, Olayemi E, Benneh AA. Plasma levels of some coagulation parameters in steady state HBSC disease patients. The Pan African Medical Journal. 2014;19:289. doi: 10.11604/pamj.2014.19.289.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Roberts I. Bone involvement in sickle cell disease. British Journal of Haematology. 2005;129:482–490. doi: 10.1111/j.1365-2141.2005.05476.x. [DOI] [PubMed] [Google Scholar]
- Anele UA, Le BV, Resar LM, Burnett AL. How I treat priapism. Blood. 2015;125:3551–3558. doi: 10.1182/blood-2014-09-551887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino VM, Norvell JM, Buchanan GR. Acute splenic complications in children with sickle cell-hemoglobin C disease. The Journal of Pediatrics. 1997;130:961–965. doi: 10.1016/s0022-3476(97)70284-1. [DOI] [PubMed] [Google Scholar]
- Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE. Glomerular Hyperfiltration and Albuminuria in Children with Sickle Cell Anemia. Pediatric nephrology (Berlin, Germany) 2011;26:1285–1290. doi: 10.1007/s00467-011-1857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin. 1989;13:649–655. doi: 10.3109/03630268908998842. [DOI] [PubMed] [Google Scholar]
- Barrett AN, McDonnell TCR, Chan KCA, Chitty LS. Digital PCR Analysis of Maternal Plasma for Noninvasive Detection of Sickle Cell Anemia. Clinical Chemistry. 2012;58:1026–1032. doi: 10.1373/clinchem.2011.178939. [DOI] [PubMed] [Google Scholar]
- Benites BD, Benevides TCL, Valente IS, Marques JF, Gilli SCO, Saad STO. The effects of exchange transfusion for prevention of complications during pregnancy of sickle hemoglobin C disease patients. Transfusion. 2016;56:119–124. doi: 10.1111/trf.13280. [DOI] [PubMed] [Google Scholar]
- Buchanan GR, Smith SJ, Holtkamp CA, Fuseler JP. Bacterial infection and splenic reticuloendothelial function in children with hemoglobin SC disease. Pediatrics. 1983;72:93–98. [PubMed] [Google Scholar]
- Buck J, Casbard A, Llewelyn C, Johnson T, Davies S, Williamson L. Preoperative transfusion in sickle cell disease: a survey of practice in England. European Journal of Haematology. 2005;75:14–21. doi: 10.1111/j.1600-0609.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- Bunn HF. Sickle Cell Disease: Basic Principles and Clinical Practice. Raven Press; New York: 1994. Hemoglobin Structure, Function, and Assembly; pp. 19–32. [Google Scholar]
- Chatuverdi S, DeBaun MR. Evolution of sickle cell disease from a life-threatening disease of children to chronic disease of adults: The last 40 years. Am J Hematol. 2016;91:5–14. doi: 10.1002/ajh.24235. [DOI] [PubMed] [Google Scholar]
- Chou ST, Fasano RM. Management of Patients with Sickle Cell Disease Using Transfusion Therapy. Hematology/Oncology Clinics of North America. 2016;30:591–608. doi: 10.1016/j.hoc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Colella MP, de Paula EV, Machado-Neto JA, Conran N, Annichino-Bizzacchi JM, Costa FF, Olalla Saad ST, Traina F. Elevated hypercoagulability markers in hemoglobin SC disease. Haematologica. 2015;100:466–71. doi: 10.3324/haematol.2014.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114:2861–2868. doi: 10.1182/blood-2009-04-210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. The American Journal of Surgical Pathology. 1995;19:1–11. doi: 10.1097/00000478-199501000-00001. [DOI] [PubMed] [Google Scholar]
- Deane CR, Goss D, O'Driscoll S, Mellor S, Pohl KRE, Dick MC, Height SE, Rees DC. Transcranial Doppler scanning and the assessment of stroke risk in children with HbSC [corrected] disease. Archives of disease in childhood. 2008;93:138–141. doi: 10.1136/adc.2007.125799. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood. 2016;127:829–838. doi: 10.1182/blood-2015-09-618579. [DOI] [PubMed] [Google Scholar]
- Dimashkieh H, Choe J, Mutema G. Renal Medullary Carcinoma: A Report of 2 Cases and Review of the Literature. Archives of Pathology & Laboratory Medicine. 2003;127:e135–e138. doi: 10.5858/2003-127-e135-RMCARO. [DOI] [PubMed] [Google Scholar]
- Drawz P, Ayyappan S, Nouraie M, Saraf S, Gordeuk V, Hostetter T, T Gladwin M, Little J. Kidney Disease among Patients with Sickle Cell Disease, Hemoglobin SS and SC. Clinical journal of the American Society of Nephrology: CJASN. 2016;11:207–215. doi: 10.2215/CJN.03940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa AA, Tuck SM, Rantell K, Stott D. Trends in family planning and counselling for women with sickle cell disease in the UK over two decades. The Journal of Family Planning and Reproductive Health Care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists. 2015;41:96–101. doi: 10.1136/jfprhc-2013-100763. [DOI] [PubMed] [Google Scholar]
- Elmariah H, Garrett ME, De Castro LM, Jonassaint J, Ataga K, Eckman J, Ashley-Koch AE, Telen MJ. Factors associated with survival in a contemporary adult sickle cell disease cohort. American Journal of Hematology. 2014;89:530–535. doi: 10.1002/ajh.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury S, Dozy A, Miller J, Davis J, Kleman K, Preisler H, Vichinsky E, Lande W, Lubin B, Kan Y, Mentzer W. Concurrent sickle-cell anemia and alpha-thalassemia: effect on severity of anemia. The New England journal of medicine. 1982;306:270–4. doi: 10.1056/NEJM198202043060504. [DOI] [PubMed] [Google Scholar]
- Embury SH, Clark MR, Monroy G, Mohandas N. Concurrent sickle cell anemia and alpha-thalassemia. Effect on pathological properties of sickle erythrocytes. Journal of Clinical Investigation. 1984;73:116–123. doi: 10.1172/JCI111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englum BR, Rothman J, Leonard S, Reiter A, Thornburg C, Brindle M, Wright N, Heeney MM, Smithers CJ, Brown RL, Kalfa T, Langer JC, Cada M, Oldham KT, Scott JP, St. Peter SD, Sharma M, Davidoff AM, Nottage K, Bernabe K, Wilson DB, Dutta S, Glader B, Crary SE, Dassinger MS, Dunbar L, Islam S, Kumar M, Rescorla F, Bruch S, Campbell A, Austin M, Sidonio R, Blakely ML, Rice HE. Hematologic outcomes after total splenectomy and partial splenectomy for congenital hemolytic anemia. Journal of Pediatric Surgery. 2016;51:122–127. doi: 10.1016/j.jpedsurg.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estcourt LJ, Fortin PM, Hopewell S, Trivella M. Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews. Cochrane Database Systematic Review. 2016a;2:CD012082. doi: 10.1002/14651858.CD012082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estcourt LJ, Fortin PM, Trivella M, Hopewell S. Preoperative blood transfusions for sickle cell disease. Cochrane Database Systematic Review. 2016b;4:CD003149. doi: 10.1002/14651858.CD003149.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine LC, Petrovic VV, Irvine AR, Bhisitkul RB. Correction-spontaneous central retinal artery occlusion in hemoglobin SC disease. American Journal of Ophthalmology. 2000;130:906–907. doi: 10.1016/s0002-9394(00)00798-4. [DOI] [PubMed] [Google Scholar]
- Gallagher PG. Transporting down the road to dehydration. Blood. 2015;126:2775–2776. doi: 10.1182/blood-2015-10-675488. [DOI] [PubMed] [Google Scholar]
- Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, Zarkowsky H, Vichinsky E, Iyer R, Lobel JS. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. New England Journal of Medicine. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- Githens JH, Gross GP, Eife RF, Wallner SF. Splenic sequestration syndrome at mountain altitudes in sickle/hemoglobin C disease. The Journal of Pediatrics. 1977;90:203–206. doi: 10.1016/s0022-3476(77)80630-6. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- Gold JI, Johnson CB, Treadwell MJ, Hans N, Vichinsky E. Detection and assessment of stroke in patients with sickle cell disease: neuropsychological functioning and magnetic resonance imaging. Pediatric Hematology and Oncology. 2008;25:409–421. doi: 10.1080/08880010802107497. [DOI] [PubMed] [Google Scholar]
- Gualandro SFM, Fonseca GHH, Yokomizo IK, Gualandro DM, Suganuma LM. Cohort study of adult patients with haemoglobin SC disease: clinical characteristics and predictors of mortality. British Journal of Haematology. 2015;171:631–637. doi: 10.1111/bjh.13625. [DOI] [PubMed] [Google Scholar]
- Guilliams KP, Fields ME, Hulbert ML. Higher-than-expected prevalence of silent cerebral infarcts in children with hemoglobin SC disease. Blood. 2015;125:416–417. doi: 10.1182/blood-2014-10-605964. [DOI] [PubMed] [Google Scholar]
- Hassell KL. Population estimates of sickle cell disease in the U.S. American Journal of Preventive Medicine. 2010;38:S512–521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Iyer R, Baliga R, Nagel RL, Brugnara C, Kirchner K, Hogan S, Steinberg MH. Maximum urine concentrating ability in children with Hb SC disease: Effects of hydroxyurea. American Journal of Hematology. 2000;64:47–52. doi: 10.1002/(sici)1096-8652(200005)64:1<47::aid-ajh8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Kamble RT, Tin-U CK, Carrum G. Successful mobilization and transplantation of filgrastim mobilized hematopoietic stem cells in sickle cell-hemoglobin C disease. Bone Marrow Transplantation. 2006;37:1065–1066. doi: 10.1038/sj.bmt.1705376. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM, Arias R, McClung M. Bone density recovery after depot medroxyprogesterone acetate injectable contraception use. Contraception. 2008;77:67–76. doi: 10.1016/j.contraception.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- Koduri PR. Iron in sickle cell disease: A review why less is better. American Journal of Hematology. 2003;73:59–63. doi: 10.1002/ajh.10313. [DOI] [PubMed] [Google Scholar]
- Koduri PR, Nathan S. Acute splenic sequestration crisis in adults with hemoglobin S-C disease: a report of nine cases. Annals of Hematology. 2006;85:239–243. doi: 10.1007/s00277-005-0061-5. [DOI] [PubMed] [Google Scholar]
- Koduri PR, Agbemadzo B, Nathan S. Hemoglobin S-C disease revisited: clinical study of 106 adults. American journal of hematology. 2001;68:298–300. doi: 10.1002/ajh.10001. [DOI] [PubMed] [Google Scholar]
- Koshy M, Weiner SJ, Miller ST, Sleeper LA, Vichinsky E, Brown AK, Khakoo Y, Kinney TR. Surgery and anesthesia in sickle cell disease. Cooperative Study of Sickle Cell Diseases. Blood. 1995;86:3676–3684. [PubMed] [Google Scholar]
- Kuliev A, Pakhalchuk T, Verlinsky O, Rechitsky S. Preimplantation genetic diagnosis for hemoglobinopathies. Hemoglobin. 2011;35:547–555. doi: 10.3109/03630269.2011.608457. [DOI] [PubMed] [Google Scholar]
- Lane PA, O’Connell JL, Lear JL, Rogers ZR, Woods GM, Hassell KL, Wethers DL, Luckey DW, Buchanan GR. Functional asplenia in hemoglobin SC disease. Blood. 1995;85:2238–2244. [PubMed] [Google Scholar]
- Law MA, Dreyer Z, Heinle JS, Dickerson HA. Staged single-ventricle palliation in an infant with hemoglobin SC disease. Texas Heart Institute Journal. 2007;34:439–441. [PMC free article] [PubMed] [Google Scholar]
- Lemonne N, Lamarre Y, Romana M, Mukisi-Mukaza M, Hardy-Dessources M-D, Tarer V, Mougenel D, Waltz X, Tressières B, Lalanne-Mistrih M-L, Etienne-Julan M, Connes P. Does increased red blood cell deformability raise the risk for osteonecrosis in sickle cell anemia? Blood. 2013;121:3054–3056. doi: 10.1182/blood-2013-01-480277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonne N, Lamarre Y, Romana M, Hardy-Dessources M-D, Lionnet F, Waltz X, Tarer V, Mougenel D, Tressieres B, Lalanne-Mistrih M-L, Etienne-Julan M, Connes P. Impaired blood rheology plays a role in the chronic disorders associated with sickle cell-hemoglobin C disease. Haematologica. 2014;99:74–5. doi: 10.3324/haematol.2014.104745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonne N, Billaud M, Waltz X, Romana M, Hierso R, Etienne-Julan M, Connes P. Rheology of red blood cells in patients with HbC disease. Clinical Hemorheology and Microcirculation. 2015;61:571–577. doi: 10.3233/CH-141906. [DOI] [PubMed] [Google Scholar]
- Leveziel N, Lalloum F, Bastuji-Garin S, Binaghi M, Bachir D, Galacteros F, Souied E. [Sickle-cell retinopathy: Retrospective study of 730 patients followed in a referral center] Journal francais d'ophtalmologie. 2012;35:343–347. doi: 10.1016/j.jfo.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Lionnet F, Hammoudi N, Stojanovic KS, Avellino V, Grateau G, Girot R, Haymann J-P. Hemoglobin SC disease complications: a clinical study of 179 cases. Haematologica. 2012;97:1136–1141. doi: 10.3324/haematol.2011.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionnet F, Hammoudi N, Stojanovic KS, Avellino V, Grateau G, Girot R, Haymann J-P. Iron restriction is an important treatment of hemoglobin SC disease. American Journal of Hematology. 2016;91:E320. doi: 10.1002/ajh.24380. [DOI] [PubMed] [Google Scholar]
- Luchtman-Jones L, Pressel S, Hilliard L, Brown RC, Smith MG, Thompson AA, Lee MT, Rothman J, Rogers ZR, Owen W, Imran H, Thornburg C, Kwiatkowski JL, Aygun B, Nelson S, Roberts C, Gauger C, Piccone C, Kalfa T, Alvarez O, Hassell K, Davis BR, Ware RE. Effects of hydroxyurea treatment for patients with hemoglobin SC disease: Hydroxyurea Treatment for HbSC. American Journal of Hematology. 2016;91:238–242. doi: 10.1002/ajh.24255. [DOI] [PubMed] [Google Scholar]
- Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutritional Neuroscience. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, Taomoto M, Cao J, McLeod DS, Vanderslice P, McIntyre BW, Fabry ME, Nagel RL. Inhibition of TNF-α–induced Sickle RBC Retention in Retina by a VLA-4 Antagonist. Investigative ophthalmology & visual science. 2001;42:1349–1355. [PubMed] [Google Scholar]
- Manchikanti A, Grimes DA, Lopez LM, Schulz KF. Steroid hormones for contraception in women with sickle cell disease. The Cochrane Database of Systematic Reviews. 2007:CD006261. doi: 10.1002/14651858.CD006261.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manci EA, Culberson DE, Yang Y-M, Gardner TM, Powell R, Haynes J, Shah AK, Mankad VN, Investigators of the Cooperative Study of Sickle Cell Disease Causes of death in sickle cell disease: an autopsy study. British Journal of Haematology. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- Marchant WA, Wright S, Porter JB. Coronary artery bypass graft surgery in a patient with haemoglobin SC disease. Anaesthesia. 2001;56:667–669. doi: 10.1046/j.1365-2044.2001.01912-2.x. [DOI] [PubMed] [Google Scholar]
- Mason K, Gibson F, Gardner R-A, Serjeant B, Serjeant GR. Prevention of sickle cell disease: observations on females with the sickle cell trait from the Manchester project, Jamaica. Journal of Community Genetics. 2016;7:127–132. doi: 10.1007/s12687-015-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy PR, Sherman AS. Irreversibly sickled cells and red cell survival in sickle cell anemia: a study with both DF32P and 51CR. The American Journal of Medicine. 1978;64:253–258. doi: 10.1016/0002-9343(78)90053-0. [DOI] [PubMed] [Google Scholar]
- McCurdy PR, Mahmood L, Sherman AS. Red cell life span in sickle cell-hemoglobin C disease with a note about sickle cell-hemoglobin O ARAB. Blood. 1975;45:273–279. [PubMed] [Google Scholar]
- Mehari A, Klings ES. Chronic Pulmonary Complications of Sickle Cell Disease. Chest. 2016;149:1313–1324. doi: 10.1016/j.chest.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt AL, Haiman C, Henderson SO. Myth: Blood transfusion is effective for sickle cell anemia—associated priapism. CJEM. 2006;8:119–122. doi: 10.1017/s1481803500013609. [DOI] [PubMed] [Google Scholar]
- Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, Bellevue R, Koshy M, Moohr JW, Smith J. Sickle Cell Disease as a Cause of Osteonecrosis of the Femoral Head. New England Journal of Medicine. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- Modiano D, Luoni G, Sirima BS, Simporé J, Verra F, Konaté A, Rastrelli E, Olivieri A, Calissano C, Paganotti GM, D’Urbano L, Sanou I, Sawadogo A, Modiano G, Coluzzi M. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A, Hofrichter J, Eaton WA. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science (New York, N.Y.) 1987;237:500–506. doi: 10.1126/science.3603036. [DOI] [PubMed] [Google Scholar]
- Mukisi-Mukaza M, Manicom O, Alexis C, Bashoun K, Donkerwolcke M, Burny F. Treatment of Sickle cell disease’s hip necrosis by core decompression: A prospective case-control study. Orthopaedics & Traumatology: Surgery & Research. 2009;95:498–504. doi: 10.1016/j.otsr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Myint KT, Sahoo S, Thein AW, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. The Cochrane Database of Systematic Reviews. 2015:CD010790. doi: 10.1002/14651858.CD010790.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Reviews. 2003;17:167–178. doi: 10.1016/s0268-960x(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Naik RP, Streiff MB, Haywood C, Jr, Nelson JA, Lanzkron S. Venous Thromboembolism in Adults with Sickle Cell Disease: A Serious and Under-recognized Complication. The American Journal of Medicine. 2014a;126:443–449. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RP, Derebail VK, Grams ME, Franceschini N, Auer PL, Peloso GM, Young BA, Lettre G, Peralta CA, Katz R, Hyacinth HI, Quarells RC, Grove ML, Bick AG, Fontanillas P, Rich SS, Smith JD, Boerwinkle E, Rosamond WD, Ito K, Lanzkron S, Coresh J, Correa A, Sarto GE, Key NS, Jacobs DR, Kathiresan S, Bibbins-Domingo K, Kshirsagar AV, Wilson JG, Reiner AP. ASsociation of sickle cell trait with chronic kidney disease and albuminuria in african americans. JAMA. 2014b;312:2115–2125. doi: 10.1001/jama.2014.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumayr L, Koshy M, Haberkern C, Earles AN, Bellevue R, Hassell K, Miller S, Black D, Vichinsky E. Surgery in patients with hemoglobin SC disease. Preoperative Transfusion in Sickle Cell Disease Study Group. American Journal of Hematology. 1998;57:101–108. doi: 10.1002/(sici)1096-8652(199802)57:2<101::aid-ajh2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Niss O, Quinn CT, Lane A, Daily J, Khoury PR, Bakeer N, Kimball TR, Towbin JA, Malik P, Taylor MD. Cardiomyopathy With Restrictive Physiology in Sickle Cell Disease. JACC: Cardiovascular Imaging. 2016;9:243–252. doi: 10.1016/j.jcmg.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottage KA, Hankins JS, Faughnan LG, James DM, Richardson J, Christensen R, Kang G, Smeltzer M, Cancio MI, Wang WC, Anghelescu DL. Addressing challenges of clinical trials in acute pain: The Pain Management of Vaso-occlusive Crisis in Children and Young Adults with Sickle Cell Disease Study. Clinical Trials (London, England) 2016;13:409–16. doi: 10.1177/1740774516636573. [DOI] [PubMed] [Google Scholar]
- Okusanya BO, Oladapo OT. Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy. The Cochrane Database of Systematic Reviews. 2013;12:CD010378. doi: 10.1002/14651858.CD010378.pub2. [DOI] [PubMed] [Google Scholar]
- Onakoya PA, Nwaorgu OGB, Shokunbi WA. Hearing impairment in persons with the hemoglobin SC genotype. Ear, Nose, & Throat Journal. 2010;89:306–310. [PubMed] [Google Scholar]
- Oteng-Ntim E, Ayensah B, Knight M, Howard J. Pregnancy outcome in patients with sickle cell disease in the UK--a national cohort study comparing sickle cell anaemia (HbSS) with HbSC disease. British Journal of Haematology. 2015a;169:129–137. doi: 10.1111/bjh.13270. [DOI] [PubMed] [Google Scholar]
- Oteng-Ntim E, Meeks D, Seed PT, Webster L, Howard J, Doyle P, Chappell LC. Adverse maternal and perinatal outcomes in pregnant women with sickle cell disease: systematic review and meta-analysis. Blood. 2015b;125:3316–3325. doi: 10.1182/blood-2014-11-607317. [DOI] [PubMed] [Google Scholar]
- Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatric Blood & Cancer. 2012;59:377–385. doi: 10.1002/pbc.24176. [DOI] [PubMed] [Google Scholar]
- Pecker LH, Ackerman HC. Cardiovascular Adaptations to Anemia and the Vascular Endothelium in Sickle Cell Disease Pathophysiology. In: Costa FF, Conran N, editors. Sickle Cell Anemia: From Basic Science to Clinical Practice. Springer International Publishing Switzerland; 2016. pp. 129–175. [Google Scholar]
- Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, Vichinsky E. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. The American Journal of Medicine. 1997;102:171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- Perlado S, Bustamante-Aragonés A, Donas M, Lorda-Sánchez I, Plaza J, Rodríguez de Alba M. Fetal Genotyping in Maternal Blood by Digital PCR: Towards NIPD of Monogenic Disorders Independently of Parental Origin. PloS One. 2016;11:e0153258. doi: 10.1371/journal.pone.0153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin A, Murphy C, Eakins E, Kunde J, Corvetta D, Di Pierro A, Negri G, Guido M, Sainati L, Mc Mahon C, Smith OP, Murphy W. [Accessed July 26, 2016];Circulating microparticles, protein C, free protein S and endothelial vascular markers in children with sickle cell anaemia. Journal of Extracellular Vesicles. 2015 Nov 23;4 doi: 10.3402/jev.v4.28414. [Online] 2015. Available at: http://www.journalofextracellularvesicles.net/index.php/jev/article/view/28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB, Howes RE, Patil AP, Nyangiri OA, Gething PW, Bhatt S, Williams TN, Weatherall DJ, Hay SI. [Accessed February 11, 2016];The distribution of haemoglobin C and its prevalence in newborns in Africa. Scientific Reports. 2013 3 doi: 10.1038/srep01671. Article number: 1671 Available at: http://www.nature.com/articles/srep01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. The New England Journal of Medicine. 1984;311:7–12. doi: 10.1056/NEJM198407053110102. [DOI] [PubMed] [Google Scholar]
- Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Annals of Internal Medicine. 1991;115:614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- Powars DR, Hiti A, Ramicone E, Johnson C, Chan L. Outcome in hemoglobin SC disease: a four-decade observational study of clinical, hematologic, and genetic factors. American Journal of Hematology. 2002;70:206–215. doi: 10.1002/ajh.10140. [DOI] [PubMed] [Google Scholar]
- Rees DC, Thein SL, Osei A, Drasar E, Tewari S, Hannemann A, Gibson JS. The clinical significance of K-Cl cotransport activity in red cells of patients with HbSC disease. Haematologica. 2015;100:595–600. doi: 10.3324/haematol.2014.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ZR, Buchanan GR. Bacteremia in children with sickle hemoglobin C disease and sickle beta(+)-thalassemia: is prophylactic penicillin necessary? The Journal of Pediatrics. 1995;127:348–354. doi: 10.1016/s0022-3476(95)70062-5. [DOI] [PubMed] [Google Scholar]
- Saraf SL, Molokie RE, Nouraie M, Sable CA, Luchtman-Jones L, Ensing GJ, Campbell AD, Rana SR, Niu XM, Machado RF, Gladwin MT, Gordeuk VR. Differences in the clinical and genotypic presentation of sickle cell disease around the world. Paediatric Respiratory Reviews. 2014;15:4–12. doi: 10.1016/j.prrv.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasongko TH, Nagalla S, Ballas SK. Angiotensin-converting enzyme (ACE) inhibitors for proteinuria and microalbuminuria in people with sickle cell disease. The Cochrane database of systematic reviews. 2015:CD009191. doi: 10.1002/14651858.CD009191.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant GR, Hambleton I, Thame M. Fecundity and pregnancy outcome in a cohort with sickle cell-haemoglobin C disease followed from birth. BJOG: an international journal of obstetrics and gynaecology. 2005;112:1308–1314. doi: 10.1111/j.1471-0528.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Sharpe CC, Thein SL. How I treat renal complications in sickle cell disease. Blood. 2014;123:3720–3726. doi: 10.1182/blood-2014-02-557439. [DOI] [PubMed] [Google Scholar]
- Smith-Whitley K. Reproductive issues in sickle cell disease. ASH Education Book. 2014;2014:418–424. doi: 10.1182/asheducation-2014.1.418. [DOI] [PubMed] [Google Scholar]
- Steinberg ME, Steinberg DR. Classification systems for osteonecrosis: an overview. Orthopedic Clinics of North America. 2004;35:273–283. doi: 10.1016/j.ocl.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Embury SH. Alpha-thalassemia in blacks: genetic and clinical aspects and interactions with the sickle hemoglobin gene. Blood. 1986;68:985–990. [PubMed] [Google Scholar]
- Steinberg MH, Nagel RL. Disorders of Hemoglobin. Cambridge University Press; 2009. Hemoglobin SC Disease and Hemoglobin C Disorders; pp. 525–548. [Google Scholar]
- Stimpson S-J, Rebele EC, DeBaun MR. Common gynecological challenges in adolescents with sickle cell disease. Expert Review of Hematology. 2016;9:187–196. doi: 10.1586/17474086.2016.1126177. [DOI] [PubMed] [Google Scholar]
- Stoica Z, Dumitrescu D, Popescu M, Gheonea I, Gabor M, Bogdan N. Imaging of avascular necrosis of femoral head: familiar methods and newer trends. Current Health Sciences Journal. 2009;35:23–28. [PMC free article] [PubMed] [Google Scholar]
- Streetly A, Clarke M, Downing M, Farrar L, Foo Y, Hall K, Kemp H, Newbold J, Walsh P, Yates J, Henthorn J. Implementation of the newborn screening programme for sickle cell disease in England: results for 2003-2005. Journal Of Medical Screening. 2008;15:9–13. doi: 10.1258/jms.2008.007063. [DOI] [PubMed] [Google Scholar]
- Strouse JJ, Jordan LC, Lanzkron S, Casella JF. The excess burden of stroke in hospitalized adults with sickle cell disease. American Journal of Hematology. 2009;84:548–552. doi: 10.1002/ajh.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannan K, Ustun C, Natarajan K, Clair B, Daitch L, Fields S, Kutlar F, Kutlar A. Acute splenic complications and implications of splenectomy in hemoglobin SC disease. European Journal of Haematology. 2009;83:258–260. doi: 10.1111/j.1600-0609.2009.01270.x. [DOI] [PubMed] [Google Scholar]
- Summarell CCG, Sheehan VA. Original Research: Use of hydroxyurea and phlebotomy in pediatric patients with hemoglobin SC disease. Experimental Biology and Medicine (Maywood, N.J.) 2016;241:737–744. doi: 10.1177/1535370216639737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrell BL, Lloyd-Puryear MA, Eckman JR, Mann MY. Newborn screening for sickle cell diseases in the United States: A review of data spanning two decades. Seminars in Perinatology. 2015;39:238–251. doi: 10.1053/j.semperi.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- Voskaridou E, Christoulas D, Terpos E. Sickle-cell disease and the heart: review of the current literature. British Journal of Haematology. 2012;157:664–673. doi: 10.1111/j.1365-2141.2012.09143.x. [DOI] [PubMed] [Google Scholar]
- Walker TM, Hambleton IR, Serjeant GR. Gallstones in sickle cell disease: observations from The Jamaican Cohort study. The Journal of Pediatrics. 2000;136:80–85. doi: 10.1016/s0022-3476(00)90054-4. [DOI] [PubMed] [Google Scholar]
- Waltz X, Romana M, Hardy-Dessources MD, Lamarre Y, Divialle-Doumdo L, Petras M, Tarer V, Hierso R, Baltyde KC, Tressieres B, Lalanne-Mistrih M-L, Maillard F, Etienne-Julan M, Connes P. Hematological and hemorheological determinants of the six-minute walk test performance in children with sickle cell anemia. PLoS One. 2013;8:e77830. doi: 10.1371/journal.pone.0077830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Enos L, Gallagher D, Thompson R, Guarini L, Vichinsky E, Wright E, Zimmerman R, Armstrong FD, Cooperative Study of Sickle Cell Disease Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. The Journal of Pediatrics. 2001;139:391–397. doi: 10.1067/mpd.2001.116935. [DOI] [PubMed] [Google Scholar]
- Wang W, Brugnara C, Snyder C, Wynn L, Rogers Z, Kalinyak K, Brown C, Qureshi A, Bigelow C, Neumayr L, Smith-Whitley K, Chui DHK, Delahunty M, Woolson R, Steinberg M, Telen M, Kesler K. The effects of hydroxycarbamide and magnesium on haemoglobin SC disease: results of the multi-centre CHAMPS trial. British Journal of Haematology. 2011;152:771–776. doi: 10.1111/j.1365-2141.2010.08523.x. [DOI] [PubMed] [Google Scholar]
- Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, Odame I, Fuh B, George A, Owen W, Luchtman-Jones L, Rogers ZR, Hilliard L, Gauger C, Piccone C, Lee MT, Kwiatkowski JL, Jackson S, Miller ST, Roberts C, Heeney MM, Kalfa TA, Nelson S, Imran H, Nottage K, Alvarez O, Rhodes M, Thompson AA, Rothman JA, Helton KJ, Roberts D, Coleman J, Bonner MJ, Kutlar A, Patel N, Wood J, Piller L, Wei P, Luden J, Mortier NA, Stuber SE, Luban NLC, Cohen AR, Pressel S, Adams RJ. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet (London, England) 2016;387:661–670. doi: 10.1016/S0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RB, Goldberg MF. Sickle-cell hemoglobin and its relation to fundus abnormality. Archives of ophthalmology (Chicago, Ill: 1960) 1966;75:353–362. doi: 10.1001/archopht.1966.00970050355008. [DOI] [PubMed] [Google Scholar]
- Wilson NO, Ceesay FK, Hibbert JM, Driss A, Obed SA, Adjei AA, Gyasi RK, Anderson WA, Stiles JK. Pregnancy outcomes among patients with sickle cell disease at Korle-Bu Teaching Hospital, Accra, Ghana: retrospective cohort study. The American Journal of Tropical Medicine and Hygiene. 2012;86:936–942. doi: 10.4269/ajtmh.2012.11-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Managment of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. Journal of the American Medical Association. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- Yoong WC, Tuck SM. Menstrual pattern in women with sickle cell anaemia and its association with sickling crises. Journal of Obstetrics and Gynaecology: The Journal of the Institute of Obstetrics and Gynaecology. 2002;22:399–401. doi: 10.1080/01443610220141362. [DOI] [PubMed] [Google Scholar]
- Yu TT, Nelson J, Streiff MB, Lanzkron S, Naik RP. Risk factors for venous thromboembolism in adults with hemoglobin SC or Sβ(+) thalassemia genotypes. Thrombosis Research. 2016;141:35–38. doi: 10.1016/j.thromres.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkowsky HS, Gallagher D, Gill FM, Wang WC, Falletta JM, Lande WM, Levy PS, Verter JI, Wethers D, Cooperative Study of Sickle Cell Disease Bacteremia in sickle hemoglobinopathies. The Journal of Pediatrics. 1986;109:579–585. doi: 10.1016/s0022-3476(86)80216-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman SA, Ware RE. Palpable splenomegaly in children with haemoglobin SC disease: haematological and clinical manifestations. Clinical and Laboratory Haematology. 2000;22:145–150. doi: 10.1046/j.1365-2257.2000.00304.x. [DOI] [PubMed] [Google Scholar]