Abstract
We explored transient elastography (TE) and enhanced liver fibrosis (ELF™) score with standard markers of liver function to assess liver damage in 193 well patients with sickle cell disease (SCD). Patients with HbSS or HbSβ0 thalassaemia (sickle cell anaemia, SCA; N=134), had significantly higher TE results and ELF scores than those with HbSC (N=49) disease (TE, 6.8 vs 5.3, p<0.0001 and ELF, 9.2 vs 8.6 p <0.0001). In SCA patients, TE and ELF correlated significantly with age and all serum liver function tests (LFTs). Additionally, (weak) positive correlation was found with lactate dehydrogenase (TE: r = 0.24, p=0.004; ELF: r = 0.26 p=0.002), and (weak) negative correlation with haemoglobin (TE: r= −0.25, p=0.002; ELF: r = −0.25 p=0.004). In HbSC patients, correlations were weaker or not significant between TE or ELF, and serum LFTs. All markers of iron loading correlated with TE values when corrected for sickle genotype (serum ferritin, β = 0.25, p <0.0001, total blood transfusion units, β = 0.25, p <0.0001 and LIC β = 0.32, p=0.046).
The exploratory study suggests that, while TE could have a role, the utility of ELF score in monitoring liver damage in SCD, needs further longitudinal studies.
Keywords: Sickle cell anaemia, liver disease, haemoglobinopathy, cirrhosis, fibrosis
Introduction
Survival in patients with sickle cell disease (SCD) has improved in recent years but it still falls behind that of the general population (Hassell 2010). Sickle-related complications affecting multiple organs, including the liver, contribute to the early mortality (Gardner et al, 2016; Powars et al, 2005). Liver fibrosis stage, particularly the development of cirrhosis, has been shown to be a significant predictor of morbidity and mortality in the majority of chronic liver disease and we postulate this can be extended to sickle-related liver disease.
Apart from the disorder itself, therapeutic interventions, such as blood transfusion, have the potential to impact upon liver function in SCD. Blood transfusion is increasingly used to prevent and treat the complications of SCD in both the acute and chronic settings, an important complication of which is iron overload (Adamkiewicz et al, 2009; Ballas 2001; Drasar et al, 2011), which predominantly affects the liver leading to fibrosis and cirrhosis. The iron overload-related fibrosis partly results from increased levels of non-transferrin bound iron which has a high propensity to induce reactive oxygen species, causing cellular damage and depleting nitric oxide (NO) levels, leading to vasoconstriction and endothelial dysfunction (Reiter and Gladwin 2003).
Earlier diagnosis of significant liver fibrosis would enable earlier intervention and, potentially, a better outcome for patients. While liver biopsy remains the gold standard for diagnosis and monitoring of fibrosis, it is an invasive technique, accompanied by an increased risk of bleeding in SCD compared to other groups, particularly in the acute setting (Zakaria et al, 2003). Standard liver function tests (either serially or as a single observation) are not of proven clinical utility in predicting which patients will go on to develop chronic liver disease (Fracanzani et al, 2008). A number of alternative, non-invasive assessment techniques have been validated in viral liver disease where they now have a role in routine care (Castera 2015; European Association for the Study of the Liver 2015; Friedrich-Rust et al, 2008; Friedrich-Rust et al, 2010; Lichtinghagen et al, 2013; Trepo et al, 2011).
Transient elastography (TE; Echosens, Paris, France) using ultrasound and low frequency (50Hz) elastic waves with a propagation velocity directly related to the stiffness of the liver, is a useful method of detecting fibrosis. It is routinely used as an alternative to liver biopsy in hepatitis C, correlates well with the existing gold standard of liver biopsy and is extremely reproducible (Boursier et al, 2008; Castera et al, 2005). Outside of SCD, two systematic reviews have highlighted its diagnostic capability in advanced fibrosis in a variety of liver disorders (European Association for the Study of the Liver 2015; Friedrich-Rust et al, 2008). In the SCD setting, Voskaridou et al (2010) studied 110 patients and showed that TE correlated with liver iron loading as assessed by magnetic resonance imaging (MRI) T2* values, serum ferritin and number of transfusions, as well as with markers of haemolysis and liver function. Another study showed that severity of liver biopsy fibrosis (Ishak score) correlated with an increase in TE values during acute painful episodes in patients with SCD (Koh et al, 2013). This study also reported that TE values increased twofold in patients in vaso-occlusive crises as compared with those who were studied during a clinically stable phase, suggesting that liver stiffness can be affected by factors not related to fibrosis, such as rheological influences, sickled erythrocytes and inflammation of the local vascular endothelium.
While TE examines the amount of existing fibrosis (or more accurately deposited extracellular matrix and other factors not related to fibrosis), the enhanced liver fibrosis (ELF™) score (iQUR, London, UK), examines the balance between matrix deposition and degradation, i.e. the current activity of the fibrotic process. ELF score combines serum levels of hyaluronic acid, amino-terminal propeptide-of-type-III-collagen (PIIINP) and tissue-inhibitor of matrix-metaloproteinase-1 (TIMP1), and has an area under receiver operated characteristic (ROC) curve of 0.8 (Friedrich-Rust et al, 2010; Parkes et al, 2010; Rosenberg et al, 2004; Trepo et al, 2011). These three proteins, markers of hepatic matrix metabolism, are then analysed using a patented formula to calculate a score for hepatic fibrosis, based on the Ishak modified scoring system (0 = normal, 1 = moderate and 2 = severe). Other scoring ranges have been proposed, depending on whether established fibrosis/cirrhosis (Lichtinghagen et al, 2013) or likelihood of developing cirrhosis (Parkes et al, 2010) are considered as end-points. The utility of the ELF score as a non-invasive marker of liver flbrosis has been derived mainly from studies of patients with chronic hepatitis C virus infection and there have been no reports of the use of this technique in SCD. As with TE, it is likely that factors unrelated to fibrosis can influence ELF raising the possibility that scores or cutoff values used to indicate fibrosis/cirrhosis in chronic hepatitis C virus infection cannot be reliably applied in other disease processes.
As yet, there have been no reported studies of the utility of the ELF score in SCD and limited experience of the use of TE in clinically stable patients. We undertook the use of these two non-invasive methods to explore how they correlate with standard liver function tests and estimates of iron overload in a cohort of patients with SCD presenting consecutively in an outpatient setting.
Patients and Methods
The study was approved by the National Research Ethics Committee London-East (11/LO/0065). All participants gave written informed consent and the research was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008.
Patients were recruited sequentially from adults attending the specialist clinic at King’s College Hospital, south London, UK. All patients were clinically stable with no acute symptoms requiring treatment or hospitalisation in the preceding 6 weeks, i.e. were in steady-state to avoid the known increase in TE results associated with acute sickle crisis as described by Koh et al (2013). All diagnoses of SCD were confirmed by haematological and DNA analyses, as appropriate. Patients were excluded from the study if they were pregnant, had an active implantable medical device, and acute or chronic viral hepatitis. Laboratory data, including haematological profile, lactate dehydrogenase (LDH), fetal haemoglobin (HbF), reticulocyte count, ferritin, liver function tests [aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), alanine transaminase (ALT), conjugated bilirubin and albumin] markers of liver function and markers of renal function were collected retrospectively from routine blood results from 1 January 2010 to 31 December 2012 with the mean of three contemporaneous values being used in the analyses. Clinical data regarding hydroxycarbamide (also termed hydroxyurea) and transfusion therapy was also recorded for each patient. The electronic patient record was examined for liver imaging (ultrasound and computed tomography (CT) scans) performed during the study period. Results were classified as abnormal if there was evidence of hepatomegaly, cirrhosisor fibrosis or, on ultrasound, heterogeneous appearances of the liver parenchyma.
Transient elastography (TE) and enhanced liver fibrosis (ELF) score
TE was performed using a FibroScan® 502 by two independent, appropriately trained individuals in accordance with technical recommendations. Ten separate measurements were taken from each patient as per Echosens™ guidance. Results were recorded as a continuous variable in kilopascals (kPa) and correspond to the mean of 10 valid measurements completed within a 5-min period. The success rate of each sampling event was calculated as the number of successful measurements over the number of acquisitions. Only sampling events with a success rate of 75% and an interquartile range of less than 0.3 were considered valid (Piscaglia et al, 2014). Ten patients initially recruited had their initial results rejected due to sampling issues, i.e. results outside the above parameters. Four patients had their FibroScan® repeated and were included in the analysis. Five patients with satisfactory results had their FibroScan® repeated after several weeks (to enable assessment of intra-subject variability). The results were within 2 kPa for all subjects.
All serum samples for ELF score testing were collected within three months of the TE being performed; the majority were sampled on the same day. Whole blood samples were allowed to clot for a minimum of 30 min and then centrifuged at 1300g for 10 min at room temperature. Serum was frozen in 2 ml aliquots at −80°C. ELF testing was performed using a patented enzyme-linked immunosorbent assay technique (ADVIA Centaur Systems, Siemens Healthcare Diagnostics, Surrey, UK) and ELF score calculated.
Statistical analyses
Data were manipulated in Excel (Microsoft, Seattle, WA) and SPSS version 21 (IBM Corp., Armonk, NY). Variables were log transformed where appropriate to obtain a normal distribution. TE and ELF measurements were analysed as continuous variables and categorical variables (grouped according to the classifications outlined in Table I). The student’s t test and the one-way ANOVA were used to compare differences in mean values between sub-groups. Association between two continuous variables was assessed using Pearson’s correlation. Associations between two continuous variables corrected for a categorical variable (e.g. sickle cell genotype) were performed using linear regression. For the development of the screening tool, abnormal TE and abnormal ELF score were used as the dependant (outcome) variables. For all analyses a p value of <0.05 was considered significant.
Table I.
Serum liver function tests in study cohort and SCA and HbSC sub-groups
| Serum liver function test | Whole cohort | SCA | HbSC | p value |
|---|---|---|---|---|
| Albumin (n) NR (35–50 g/l) | 193 | 134 | 49 | |
| Range (mean) | 33 – 48 (43.4) | 33 – 48.1 (43.2) | 35 – 47 (43.7) | N/S |
| Abnormal, n (%) | 2 (1) | 2 (2) | 0 (0) | |
| ALT (n) NR (5 – 55 iu/l) | 164 | 111 | 42 | |
| Range (mean) | 2 – 111 (24.0) | 2 – 65 (22.7) | 9 – 111 (27.1) | N/S |
| Abnormal, n (%) | 3 (1.5) | 1 (1) | 2 (4) | |
| AST (n) NR (10–50 iu/l) | 193 | 134 | 49 | |
| Range (mean) | 17 – 85.5 (37.0) | 21 – 85.5 (40.7) | 17 – 70 (28.6) | <0.0001 |
| Abnormal, n (%) | 30 (16) | 28 (21) | 2 (4) | |
| ALP (n) (NR 20–130 iu/l) | 193 | 134 | 49 | |
| Range (mean) | 26 – 268 (87.8) | 26 – 268 (92.6) | 42 – 139 (71.4) | <0.0001 |
| Abnormal (> 130), n (%) | 18 (9) | 15 (11) | 1 (2) | |
| GGT (n) (NR 1–55 iu/l) | 193 | 134 | 49 | |
| Range (mean) | 3 – 294 (49.6) | 4 – 297 (55.3) | 9 – 145 (35) | <0.002 |
| Abnormal (> 55), n (%) | 54 (28) | 44 (33) | 8 (16) | |
| Conjugated bilirubin (NR <4 μmol/l) | 164 | 114 | 39 | |
| Range (mean) | 2 – 31 (11.0) | 3–31 (12.4) | 2 – 16 (8.2) | <0.0001 |
| Abnormal (> 4), n (%) | 159 (82) | 113 (84) | 36 (73) |
The p value refers to the difference in mean value between the sickle cell anaemia (SCA) and HbSC groups, The (SCA) sub-group includes 133 HbSS and one HbSβ0 thalassaemia patients.
ALP = alkaline phosphatase; ALT = alanine transaminase; AST = aspartate transaminase; GGT = gamma-glutamyl transferase; N/S = not significantNR = normal range; ULN = upper limit of normal.
Results
One hundred and ninety-three patients who met the inclusion criteria were studied, of which 78 (40%) were male. Patients’ age ranged from 17 to 72 years at the time of the last blood sample (mean 35 years), and included 133 (69%) HbSS, 49 (25%) HbSC, 10 (5%) HbSβ+ thalassaemia, and 1 (1%) HbSβ0thalassaemia. For the purposes of this study, patients with HbSS and Hb Sβ0 thalassaemia were analysed as a group (sickle cell anaemia, SCA) due to same clinical phenotype. The HbSβ+ thalassaemia patients were not included in the separate sub-group analysis due to low numbers. As alcohol consumption was generally low (mean intake 1 unit per week, range 0–20) in our population, it was not included as a variable in further analyses.
Of the 193 patients, 58% had received at least 1 unit of blood since our records began (1 January 1991). Eight of the patients were on transfusion programmes, 3 on simple transfusion and the remainder were on exchange programmes. The 3 patients on top-up programmes were treated with chelation therapy but adherence was poor. Serum ferritin of the 3 patients on blood transfusion programmes ranged from 6.9 to 9873.1 μg/l (mean 415.6 μg/l SD± 903) with 13 (7%) patients having serum ferritin values of greater than 1000 μg/l. Liver iron concentration (LIC, as measured by R2 MRI, FerriScan™, Resonance Health Limited and Resonance Health Analysis Services Pty Limited, Claremont, Australia) was available in 35/193 (18%) patients and ranged from 0.6 to 43 mg/g dry weight (mean 9.1 mg/g dry weight SD±13.5).
Conventional liver imaging, a combination of CT scans and ultrasound, was available in 75 patients (62 SCA and 13 HbSC). Thirty of the 75 patients (24 SCA and 6 HbSC) were found to have abnormal liver parenchyma and the remainder (38 SCA and 7 HbSC patients) were reported as normal.
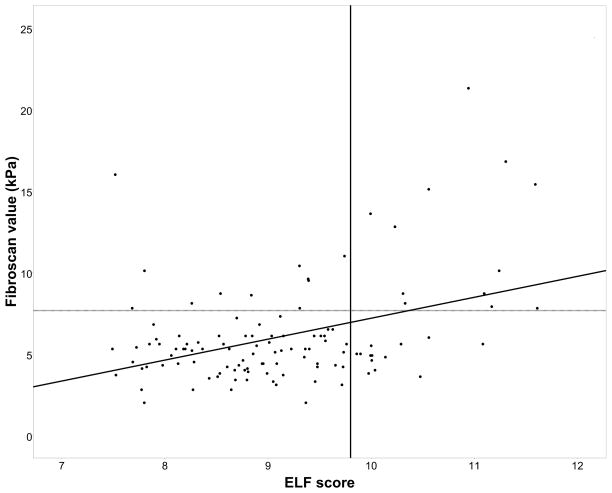
TE and ELF score were performed in all 193 patients. TE moderately correlated with ELF score, r = 0.37 (p<0.0001) (Figure 1).
Figure 1. Association between Transient elastography and ELF score in the SCA and HbSC patients (n=183).
The dashed horizontal reference line indicates an abnormal Transient Elastography result [(≥7.66kPa; Friedrich–Rust et al (2008)]. The solid vertical line indicates an abnormal enhanced liver fibrosis (ELF) score [≥ 9.8; Lichtinhagen et al (2013)]
Mean serum LFTs were available for all patients, apart from ALT and conjugated bilirubin, which were introduced as tests half way through the study period. Patients with SCA had significantly higher AST (40.7 vs 28.6 iu/l p<0.0001), ALP (92.6 vs 71.4 iu/l p<0.0001), GGT (55.3 vs 35.0 iu/l p=0.002) and conjugated bilirubin (12.4 vs 8.2 μmol/l p<0.0001) compared to HbSC patients (Table I), although all mean values were within the normal range of our laboratory. Abnormalities in GGT were most commonly observed, being present in a third (n=44) of SCA patients. ALT values were available in 85% of our cohort and was rarely abnormal (3 patients). Altogether, 124 (93%) SCA patients had at least 1 abnormal serum LFT result (52% - one, 26% - two, 13% - three, and 2% - 4 abnormal LFTs).
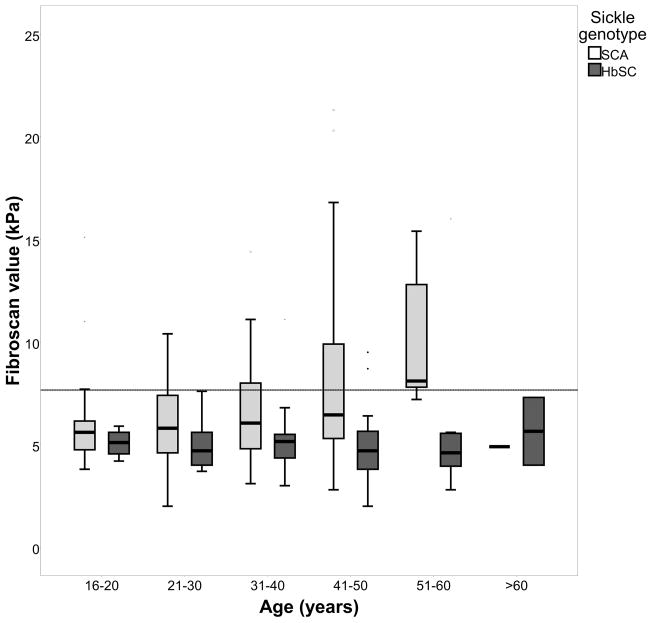
There was a significant positive association between TE and age (when corrected for sickle genotype) β = 0.19, p = 0.006 (Figure 2a). SCA patients had significantly higher TE values compared to HbSC patients (6.8 vs 5.3, p<0.0001), moderate or severe stiffness was present in 27% SCA patients (Table II). TE value increases with age in SCA patients while age has no effect on TE values in HbSC patients (Figure 2a). In SCA patients, TE values correlated moderately with serum LFTs (Albumin r = −0.35, p<0.0001, AST r = 0.44, p<0.0001, ALP r = 0.29, p<0.0001, GGT r = 0.40, p<0.0001, conjugated bilirubin r = 0.26, p = 0.004). Weak positive correlation was also found with LDH (r = 0.24 p = 0.004) and weak negative correlation with Hb (r= −0.25, p = 0.002). No significant correlations were found with other biological variables including HbF levels. Using a cut-off of 550 iu/l LDH (a value of 1 SD above population mean), SCA patients in the ≥ 550 iu/l group had significantly higher TE values compared to those with LDH in the <550 iu/l group, 8.77 kPa SD±3.99 vs 5.98 kPa SD±2.65 (p <0.0001). In HbSC patients, significant associations of TE values were seen between white blood cell (WBC) count (R = 0.39, p = 0.02), and reticulocyte count (R = 0.35, p = 0.01), although for LFTs there were only correlations with AST (r = 0.39, p = 0.004) and ALP (r = 0.30, p = 0.03).
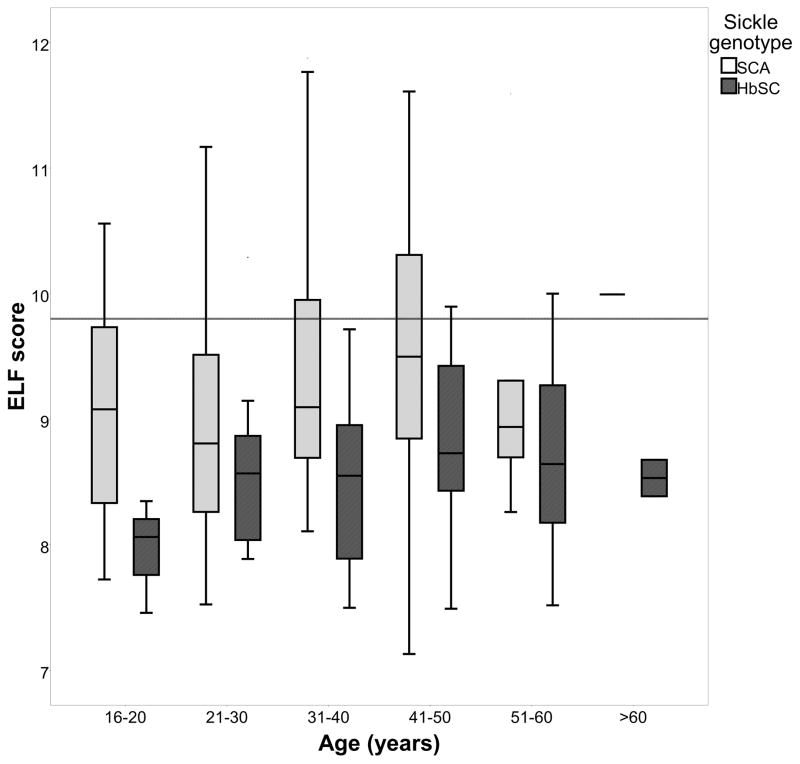
Figure 2. Effect of age and sickle genotype on a) Transient Elastography value and b) ELF score.
In Figure 2a, the area above horizontal limit line indicates abnormal Transient Elastography value [(≥7.66kPa; Friedrich–Rust et al (2008)]. In Figure 2b, the area above the horizontal limit line indicates abnormal enhanced liver fibrosis (ELF)score - current presence of fibrosis [≥9.8; Lichtinhagen et al (2013)].
SCA, sickle cell anaemia
Table II.
Range and mean transient elastography results and ELF scores for study cohort and SCA and HbSC sub-groups.
| Whole cohort n = 193 (%) | SCA n = 134 (%) | HbSC n = 48 (%) | p value | |
|---|---|---|---|---|
| FibroScan results (kPa) | ||||
| Mean (range) | 6.2 (2.0 – 21.3) | 6.8 (2.0 – 21.3) | 5.3 (2.0 – 16.0) | < 0.0001 |
| None/mild (0–7.65) | 153 (79) | 98 (73) | 43 (90) | |
| Moderate (7.66–13.00) | 33 (17) | 29 (22) | 5 (8) | |
| Severe (≥13.01) | 8 (4) | 7 (5) | 1 (2) | |
| ELF score | ||||
| Mean (range) | 9.1 (7.1 – 11.6) | 9.2 (7.1 – 11.6) | 8.6 (7.5 – 10.3) | <0.0001 |
| iQur™ | ||||
| None/mild (≤7.7) | 8 (4) | 3 (2) | 5 (10) | |
| Moderate (7.8 – 9.7) | 149 (77) | 98 (73) | 41 (84) | |
| Severe (≥9.8) | 37 (19) | 33 (25) | 3 (6) | |
| Lichtinhagen et al (2013) | ||||
| None/mild (≤9.7) | 164 (84) | 107 (79) | 47 (96) | |
| Moderate (9.8 – 11.2) | 27 (14) | 24 (19) | 2 (4) | |
| Severe (≥11.3) | 3 (2) | 3 (2) | 0 (0) | |
| Parkes et al (2010) (risk of developing fibrosis) | ||||
| None/low (≤8.34 HR = 2.1) | 44 (22) | 27 (20) | 16 (33) | |
| Moderate (8.35 – 10.42 HR = 5.1) | 137 (71) | 94 (70) | 33 (67) | |
| High (≥10.43 HR = 75) | 13 (7) | 13 (10) | 0 (0) |
Number and percentage of patients in each group with each separate stage of fibrosis according to the meta-analysis by Friedrich-Rust et al (2008) (Fibroscan data) and iQur™, Lichtinhagen et al (2013) and Parkes et al (2010) (ELF score data). p value indicates significant difference between the mean results for SCA and HbSC sub-groups. The sickle cell anaemia (SCA) sub-group includes 133 HbSS and one HbSβ0 thalassaemia patients.
ELF = enhanced liver fibrosis: HR = hazard ratio
In the SCA sub-group, there were weak but significant correlations between TE and all markers of iron loading (ferritin r=0.24, p = 0.006, total top-up units r=0.18 p = 0.02, total units transfused r=0.2 p = 0.02, and LIC r=0.18 and p=0.04). Repeat analysis of TE values using a one-way ANOVA examined differences between ferritin sub-groups of <500 μg/l (mean TE 6.28 SD±2.72), 500–1000 μg/l (mean TE 6.79 SD±3.09) and >1000 μg/l (mean TE 8.79 SD±4.08). A significant association was confirmed with increasing ferritin (p = 0.02). In the Hb SC sub-group there were no significant correlations using Pearson’s analysis with markers of iron loading and TE. For the whole cohort, using linear regression correcting for age and sickle genotype, all markers of iron loading were associated with TE values (serum ferritin β = 0.25, p <0.0001, total top-up units β = 0.22, p = 0.001, total units transfused β = 0.25, p <0.0001 and LIC β = 0.32, p = 0.046).
In the SCA subgroup TE was significantly higher in patients with abnormal scans from conventional imaging (mean 8.82 SD±4.5) than those with normal scans (5.7 SD±2.3) p = 0.007. There was no significant difference between the two groups TE values (11.93 SD± 6.6 in the abnormal group vs 6.10 SD± 1.9 in the normal imaging group). The lack of statistical significance is probably due to small patient numbers (only 6 in each group).
There was significant association between ELF score and increasing age when corrected for sickle genotype (β = 0.2, p = 0.005) (Figure 2b). There was no significant effect of gender on ELF score, corrected for sickle genotype. Patients with SCA had significantly higher mean ELF scores than the HbSC patients (9.2 vs 8.6 p <0.0001) (Table II and Figure 2b).
In SCA patients, ELF score correlated strongly with serum LFTs, although less strongly than TE values (Albumin r = −0.30, p<0.0001, AST r = 0.39, p<0.0001, ALP r = 0.25, p = 0.003, GGT r = 0.28, p = 0.001, conjugated bilirubin r = 0.36, p<0.0001). Positive correlation was seen with ELF score and LDH (r = 0.26, p = 0.002) and negative correlation with Hb (r = −0.25, p = 0.004). In contrast to TE, negative correlation was also seen with HbF levels (r = −0.24, p = 0.01). In the HbSC group, there were no significant correlations with either ELF score or serum LFTs. However associations were seen between ELF score and LDH (r = 0.40, p = 0.004) and Hb level (r = −0.31, p = 0.01).
ELF scores showed weak but significant correlations with ferritin (r=0.16, p = 0.04, total top-up units r=0.2 p = 0.02, total units transfused r=0.18 p = 0.02 but not LIC, in the SCA patients. A one-way ANOVA analysis confirmed significant association (p = 0.03) with increasing ferritin when the results were divided into <500 μg/l (mean ELF 9.08 SD±0.91), 500–1000 μg/l (mean ELF 9.37 SD±0.87) and >1000 μg/l (mean ELF 9.74 SD±0.98) sub groups. There were no significant correlations with markers of iron loading and ELF score in the HbSC group. Analysis using linear regression correcting for sickle genotype and age, revealed significant correlations with ELF score and serum ferritin (β = 0.25, p <0.0001), total top-up units (β = 0.24 p = 0.001) and total units transfused (β = 0.24 p = 0.001). LIC was not significantly correlated with ELF score.
In contrast to TE there were no significant differences in mean values of ELF between patients with abnormal conventional liver imaging and those with normal imaging. In the SCA cohort the mean ELF score was 9.14 (SD±1.3) in the group with abnormal imaging and 9.28 (SD±0.84) in the group with normal imaging. This was also found in the HbSC sub-group with mean ELF being 9.77 (SD±1.6) in the abnormal group and 9.49 (SD±1.64) in those with normal liver parenchyma on standard imaging.
45/134 SCA patients were treated with hydroxycarbamide. No HbSC patients were treated with hydroxycarbamide. There was no significant difference between the mean TE results for patients on hydroxycarbamide (6.60 SD±0.42) compared to those untreated at the time of the study (6.58±0.33). There was also no significant difference between the mean ELF results in the hydroxycarbamide group (9.19 SD±0.87) and untreated group (9.17±0.96). This lack of significance remained when regression analysis included age as a covariate.
We explored the utility of serum liver function tests as indicators for further investigations using TE and ELF as non-invasive methods of assessing fibrosis. The numbers of true positives (a), false positives (b), false negatives (c) and true negatives (d) were calculated. Sensitivity (a/[a+c]), specificity (d/[b+d]), positive (a/[a+b]) and negative (d/[c+d]) predictive values were assessed using the binary classification test with the outcome measure of presence or absence of fibrosis. The number of abnormal LFTs, number of LFTs >1.5x the upper limit of normal, and the number of LFTs twice the upper limit of normal were examined for association with TE result and abnormal ELF score (using levels indicative of or predictive of moderate or severe fibrosis). Liver function tests appear to have a moderate negative predictive value but a weak positive predictive value for moderate or severe liver stiffness using either non-invasive method (Table III), but the values are too broad for routine application in clinical practice.
Table III.
Value of abnormal liver function tests in predicting abnormal transient elastography or ELF Score results
| Transient Elastography | ELF Score (Lichtinhagen et al 2013) | ELF Score (Parkes et al 2010) | ||||
|---|---|---|---|---|---|---|
| Abnormal liver function tests | Positive predictive value (%) | Negative predictive value (%) | Positive predictive value (%) | Negative predictive value (%) | Positive predictive value (%) | Negative predictive value (%) |
| 1 LFT 1.5x ULN | 23 | 95 | 34 | 87 | 11 | 96 |
| 1 LFT 2x ULN | 25 | 84 | 28 | 89 | 14 | 98 |
| 2 or more abnormal LFTs | 32 | 85 | 24 | 90 | 18 | 95 |
ELF = enhanced liver fibrosis; LFT = liver function test; ULN = upper limit of normal
Six patients in the cohort were known to have chronic sickle hepatopathy as defined by Gardner et al (2014) (severe right upper quadrant pain, acute hepatomegaly, coagulopathy, extreme conjugated hyperbilirubinemia and moderately elevated liver enzymes) but were not acutely unwell at the time of the study. Three patients in the cohort were on regular transfusion programmes to treat other sickle-related complications (stroke, pulmonary hypertension and severe anaemia) and had a prolonged history of significant iron overload (LICs ranging from 31 to over 43 mg/g dry weight) with a history of poor compliance with chelation. All had abnormal findings on liver imaging, including hepatomegaly, heterogeneous liver pattern and, in one case, cirrhosis. These patients with known sickle hepatopathy represented 6/7 SCA patients with severe fibrosis as defined by FibroScan® with results ranging from 13.6 to 21.3 (mean 16.2) kPa. The other patient in this severe group had not had contemporaneous liver imaging performed and it was not possible to make a confident diagnosis. In contrast, the ELF scores for all 6 patients were in the moderate risk category using the Parkes criteria, while 3 had moderate fibrosis and 3 had severe fibrosis, using the Lichtinhagen criteria.
Discussion
Chronic liver disease in SCD is highly variable in phenotype, and often presents at an advanced stage when the potential for intervention is limited. It is thus worthwhile to explore and develop a screening tool that could be routinely applied in clinic. This would have three benefits. Firstly, the identification of patients with established fibrosis would prompt immediate treatment, avoiding unnecessary liver biopsy. Secondly, a screening tool would facilitate longitudinal assessment in the treated group providing insights on response to therapy and guiding the timing of more invasive tests (including liver biopsy) and risky treatments (such as liver transplant). Thirdly, longitudinal assessment would provide insights on the natural history of liver disease in patients with SCD, and could be potential biomarkers in prediction and prognosis.
Due to the risks involved in liver biopsies in SCD, we explored the utility of TE and ELF scores as screening tests for fibrosis in sickle cell disease. Although the number of patients with histological data was small, published data does support TE as a surrogate of histologically diagnosed fibrosis in SCD (Koh et al, 2013; Voskaridou et al, 2010). The ranges for abnormal values were taken from the meta-analysis by Friedrick-Rust et al (2008), with the target to select patients with either moderate or severe fibrosis (TE of 7.66 kPa and above). There is no published data on the use of ELF score in SCD. However, the potential for prediction of active or future development of initial fibrosis makes it an attractive outcome measure as it could potentially enable earlier detection of fibrosis and therefore earlier intervention.
We have compared the results of two novel non-invasive techniques in our SCD population with standard liver function tests, estimates of iron loading and standard imaging techniques. Liver dysfunction appears to be a potentially significant problem amongst the SCD population, with 89% having at least 1 abnormal serum LFT, increasing to 93% in the SCA subgroup. We have shown that the majority of measures of liver function, including serum liver tests, TE and ELF score, are significantly higher in SCA compared with HbSC, consistent with the more severe phenotype of this genotypic sub-group. TE results indicate a significant prevalence of underlying liver stiffness in our population, with at least 27% of our patients having moderate or severe liver stiffness using this method of assessment.
The ELF score has less clearly defined normal and abnormal ranges, with different values indicating abnormality depending whether ongoing fibrosis or future liver morbidity/mortality is the defined outcome. Unfortunately there is a lack of consensus in the hepatology community even when used within the hepatitis C positive population (where there is most experience). Based on the manufacturer’s value ranges (no/mild fibrosis ≤ 7.7, moderate fibrosis 7.8 – 9.7 and severe fibrosis ≥ 9.8), 96% of patients in our cohort have moderate or severe fibrosis (98% of SCA patients). However, Lichtinghagen et al (2013) suggested that the manufacturer’s value range overestimates the prevalence of fibrosis, and proposed a different range of : no/mild fibrosis ≤ 9.7, moderate fibrosis 9.8 – 11.2, and severe fibrosis ≥ 11.3, which would make the prevalence of moderate or severe fibrosis as 16% in our cohort of SCD patients (21% SCA). Parkes et al (2010) applied longitudinal results to derive hazard ratios (HR) to differentiate the increased risk of liver-related death in each group, using ELF scores of no/mild fibrosis ≤ 8.34 HR = 2.1, moderate fibrosis 8.35 – 10.42 HR = 5.1 and severe fibrosis ≥ 10.43 HR = 75, as predictive values. Using the Parkes scoring system, 78% of patients in total cohort (or 80% SCA) have a moderate or high risk of developing fibrosis in the future (Table II). In light of the TE results obtained in parallel, the ELF score data seem to represent an overestimate of established fibrosis and this is a significant weakness as a mode of investigation in SCD patients. The role of ELF testing is further thrown into doubt by the lack of significant differences in ELF scores between those with abnormal conventional liver imaging and those without. In our small group of patients with established sickle hepatopathy, ELF score was unable to differentiate this group from the general sickle population, in contrast to TE. However both TE and ELF score correlate significantly with both abnormal liver function tests and haemolytic markers in the SCA population. It is likely that ELF is influenced by factors unrelated to fibrosis (e.g. endothelial inflammation, haemolysis). It has already been shown by Koh et al (2013) that TE increases during periods of increased erythrocyte sickling. ELF scores may well be confounded by the background of ongoing haemolysis and vaso-occlusion present in patients with SCD.
The correlation between TE / ELF and haemolytic markers adds further evidence to the theory that underlying haemolysis contributes to the pathogenesis of sickle liver dysfunction (Belcher et al, 2010). The correlation between age and measures of liver stiffness also point to accrual of liver damage over time, either from sickling, haemolysis or iron accumulation. As patients survive longer, liver damage has the potential to become an increasing cause of mobidity and mortality.
Transfusion and markers of iron overload are weakly but significantly correlated with both TE and ELF score, although the lack of correlation with LIC and ELF needs further investigation and casts further doubt on the utility of ELF score in sickle-related liver damage. This has a potentially significant treatment implication for clinical practice – should we become more aggressive in screening and chelation of patients to attempt to reduce one of the few risk factors for liver disease for which we have a useful intervention? Longitudinal TE and potentially ELF measurements of patients throughout their transfusion programmes and chelation treatment could indicate whether the removal of iron from the liver actually makes a difference to outcome in the form of liver fibrosis and cirrhosis.
We acknowledge that the lack of longitudinal data for TE and ELF score is a significant limitation in this study. We have shown that patients known to have abnormal liver appearances via other imaging modalities have significantly abnormal TE results, however the lack of a clearly defined range for normality/abnormality for ELF score, even in its established setting in hepatitis C, and the significantly abnormal results across the board in sickle cell disease patients may render ELF not useful as a diagnostic measure in this patient group.
In summary, the role of TE in monitoring liver dysfunction in SCD needs to be further evaluated, preferably with long-term longitudinal follow-up and, if possible, histological data. The future of the ELF score as a monitoring method in SCD appears to have less potential following this study, with little clinical correlation and the ranges developed to monitor hepatitis C having no apparent specificity in this situation. However it is likely that the utility of non-invasive investigation will lie in the identification of patients with established significant fibrosis and the longitudinal assessment of all patients with SCD to further target intervention and treatment. The new development of MRI TE may also provide further information about the involvement of the liver in sickle cell disease.
Acknowledgments
We thank Marlene Allman for help in recruiting subjects for the study. This work was supported by a grant (MRC Grant No. G0001249) from the Medical Research Council, UK to SLT.
Footnotes
Authorship contributions
ED and EF performed experiments; ED and SLT analysed the data; ED, AD, AS, AB, and SLT designed the research study; ED, KG and SLT wrote the paper. All authors contributed to final version of paper.
Conflict of interest
The authors declare that they have no competing interests
References
- Adamkiewicz TV, Abboud MR, Paley C, Olivieri N, Kirby-Allen M, Vichinsky E, Casella JF, Alvarez OA, Barredo JC, Lee MT, Iyer RV, Kutlar A, McKie KM, McKie V, Odo N, Gee B, Kwiatkowski JL, Woods GM, Coates T, Wang W, Adams RJ. Serum ferritin level changes in children with sickle cell disease on chronic blood transfusion are nonlinear and are associated with iron load and liver injury. Blood. 2009;114:4632–4638. doi: 10.1182/blood-2009-02-203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Seminars in Hematology. 2001;38:30–36. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Vineyard JV, Bruzzone CM, Chen C, Beckman JD, Nguyen J, Steer CJ, Vercellotti GM. Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. Journal of Molecular Medicine. 2010;88:665–675. doi: 10.1007/s00109-010-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursier J, Konate A, Gorea G, Reaud S, Quemener E, Oberti F, Hubert-Fouchard I, Dib N, Cales P. Reproducibility of liver stiffness measurement by ultrasonographic elastometry. Clinical Gastroenterology and Hepatology. 2008;6:1263–1269. doi: 10.1016/j.cgh.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Castera L. Noninvasive Assessment of Liver Fibrosis. Digestive Diseases. 2015;33:498–503. doi: 10.1159/000374097. [DOI] [PubMed] [Google Scholar]
- Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Ledinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Drasar E, Igbineweka N, Vasavda N, Free M, Awogbade M, Allman M, Mijovic A, Thein SL. Blood transfusion usage among adults with sickle cell disease - a single institution experience over ten years. British Journal of Haematology. 2011;152:766–770. doi: 10.1111/j.1365-2141.2010.08451.x. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. Journal of Hepatology. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterology. 2010;10:103. doi: 10.1186/1471-230X-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, Suddle A, Kane P, O’Grady J, Heaton N, Bomford A, Thein SL. How we treat sickle hepatopathy and liver transplantation in adults. Blood. 2014;123:2302–2307. doi: 10.1182/blood-2013-12-542076. [DOI] [PubMed] [Google Scholar]
- Gardner K, Douiri A, Drasar E, Allman M, Mwirigi A, Awogbade M, Thein SL. Survival in adults with sickle cell disease in a high-income setting. Blood. 2016;128:1436–1438. doi: 10.1182/blood-2016-05-716910. [DOI] [PubMed] [Google Scholar]
- Hassell KL. Population estimates of sickle cell disease in the U.S. American Journal of Preventive Medicine. 2010;38:S512–521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Koh C, Turner T, Zhao X, Minniti CP, Feld JJ, Simpson J, Demino M, Conrey AK, Jackson MJ, Seamon C, Kleiner DE, Kato GJ, Heller T. Liver stiffness increases acutely during sickle cell vaso-occlusive crisis. American Journal of Hematology. 2013;88:E250–254. doi: 10.1002/ajh.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. Journal of Hepatology. 2013;59:236–242. doi: 10.1016/j.jhep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, Alexander G, Ramage J, Dusheiko G, Wheatley M, Gough C, Burt A, Rosenberg W. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- Piscaglia F, Marinelli S, Bota S, Serra C, Venerandi L, Leoni S, Salvatore V. The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives. Eur J Radiol. 2014;83:450–455. doi: 10.1016/j.ejrad.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Current Opinion in Hematology. 2003;10:99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ European Liver Fibrosis G. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Trepo E, Potthoff A, Pradat P, Bakshi R, Young B, Lagier R, Moreno C, Verset L, Cross R, Degre D, Lemmers A, Gustot T, Berthillon P, Rosenberg W, Trepo C, Sninsky J, Adler M, Wedemeyer H. Role of a cirrhosis risk score for the early prediction of fibrosis progression in hepatitis C patients with minimal liver disease. Journal of Hepatology. 2011;55:38–44. doi: 10.1016/j.jhep.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Voskaridou E, Schina M, Plata E, Christoulas D, Tsalkani M, Dimopoulou M, Dimitrakopoulou H, Mousoulis G, Terpos E. Liver Transient Elastography (FibroScan) Correlates with Liver Iron Concentration and Reflects Liver Fibrosis In Patients with Sickle Cell Disease. Blood. 2010;116:1646–1646. [Google Scholar]
- Zakaria N, Knisely A, Portmann B, Mieli-Vergani G, Wendon J, Arya R, Devlin J. Acute sickle cell hepatopathy represents a potential contraindication for percutaneous liver biopsy. Blood. 2003;101:101–103. doi: 10.1182/blood-2002-06-1823. [DOI] [PubMed] [Google Scholar]