Abstract
Numerous studies have shown that certain types of striatal interneurons play a crucial role in selection and regulation of striatal output. Striatal Fast-Spiking Interneurons (FSIs) are parvalbumin positive, GABAergic interneurons that constitute less than 1% of the total striatal population. It is becoming increasingly evident that these sparsely distributed neurons exert a strong inhibitory effect on Medium Spiny projection Neurons (MSNs). MSNs in lateral striatum receive direct synaptic input from regions of cortex representing discrete body parts, and show phasic increases in activity during touch or movement of specific body parts. In the present study, we sought to determine whether lateral striatal FSIs identified by their electrophysiological properties, i.e., short-duration spike and fast firing rate (FR), display body part sensitivity similar to that exhibited by MSNs. During video recorded somatosensorimotor exams, each individual body part was stimulated and responses of single neurons were observed and quantified. Individual FSIs displayed patterns of activity related selectively to stimulation of a discrete body part. Most patterns of activity were similar to those exhibited by typical MSNs, but some phasic decreases were observed. These results serve as evidence that some striatal FSIs process information related to discrete body parts and participate in sensorimotor processing by striatal networks that contribute to motor output.
Keywords: Dorsolateral Striatum, Fast Spiking Interneurons, Parvalbumin, Electrophysiology
1. Introduction
Medium Spiny projection Neurons (MSNs) are the principal neurons of the striatum and constitute over 90% of its neural population (Groves, 1983; Rymar et al., 2004). MSNs receive synaptic input from virtually all areas of the cortex, thalamus and brain stem (Tepper & Plenz, 2006) and synapse in the globus pallidus and substantia nigra pars reticulata. MSNs also possess rich local axon collaterals (Kreitzer, 2009) and it has therefore been hypothesized that striatal output is controlled via lateral inhibition between MSNs (Groves , 1983; Wickens, Kötter & Alexander, 1995; Plenz, Wickens, & Kitai, 1996). Nevertheless, inhibition between MSNs has been shown to be weak and therefore is unlikely to account for the magnitude of GABAergic inhibition that has been recorded from MSNs (Jaeger et al., 1994; Tunstall et al., 2002; Koos et al., 2002; Koos et al., 2004; Plenz 2003).
Recent striatal research has targeted a class of GABAergic, parvalbumin positive neurons, called Fast Spiking Interneurons (FSIs) (Kawaguchi et al., 1995). FSIs are medium sized, aspiny cells that form symmetrical gap junctions enabling them to fire in synchrony. Studied in vitro, they emit fast, short duration spikes. Even a single FSI spike can delay MSN firing. Despite their presumed strong effect, FSIs constitute less than 1% of the striatal population. In addition, they are distributed more densely in the dorsolateral striatum (DLS) as compared to the medial striatum (Gerfen et al., 1985, Luk and Sadikot, 2001; Rymar et al., 2004). Extracellular recordings in striatum of awake, behaving rats (Berke, 2004) demonstrated a distinct group of neurons that closely resembled parvalbumin cells recorded in vitro (Kawaguchi, 1993). In contrast to typically silent MSNs, this group of neurons was characterized by high firing rates, a tonic firing pattern and characteristic narrow waveforms. It was later confirmed by juxtcellular labeling that those cells indeed represent parvalbumin neurons (Mallet et al, 2005).
Interactions between FSIs and MSNs have been demonstrated in vitro, but more evidence is needed regarding direct interactions or similarity of firing patterns under naturalistic conditions. MSNs in the DLS receive direct synaptic input from regions of cortex representing individual body parts (Kincaid et al., 1998). As a consequence, clusters of neighboring Type IIb MSNs (Kimura, 1990) respond to stimulation or movement of the same body part (Liles & Updyke, 1985; Alexander & Delong, 1985; Carelli & West, 1991; Mittler et al., 1994; Cho & West, 1997). Inhibition of type IIB MSNs by FSIs is hypothesized to be particularly important for controlling motor behavior. Post-mortem studies have shown decreased numbers of parvalbumin interneurons in striatum of Tourette syndrome patients (Kalanithi et al., 2005; Kataoka et al., 2010). Ablation of striatal parvalbumin interneurons in the mouse has led to anxiety triggered increases in stereotyped movements (Xu et al. 2016). In addition, pharmacological inactivation of parvalbumin interneurons was associated with dystonia-like symptoms (Gittis et al., 2011). Numerous questions arise regarding how the firing of FSIs might influence the relations of MSNs to movement, their clustering, and their organization into a patchy somatotopy (Flaherty & Graybiel, 1993, 1994). However, no studies to date have examined FSI activity using the type of approach often used for studying MSNs, i.e., by testing responsiveness to stimulation of body parts in awake animals. The purpose of the present study was to test whether FSIs exhibit such responsiveness. Single neurons were recorded in freely moving rats and the firing of FSIs identified using standard online electrophysiological measures was tested during a somatosensorimotor exam. As expected, the majority of neurons recorded in the DLS exhibited electrophysiological characteristics typical of MSNs: a slow baseline firing rate (often slower than 1 spike per second) and broad waveform shape (valley-to-peak width often greater than 150 μs). A subset exhibited characteristics of FSIs that have been published by several laboratories (Berke et al. 2008, Gage et al., 2010, Wiltschko et al., 2010, Gittis et al., 2011), i.e., narrow waveforms, measured by the waveform’s peak width (often less than 110 μs) and fast firing rates (faster than 2 spikes per second). This subset was preferentially recorded and tested. Given the reported sensitivity of FSIs to cortical input (Bennet & Bolam, 1994, Mallet et al., 2005, Parthasarathy & Graybiel,1997) and putative role in controlling surrounding MSNs, we anticipated that a proportion of FSIs in DLS would show changes in firing during stimulation or movement of individual body parts. Given the uniformly excitatory responses of MSNs to body part stimulation, we expected that some proportion of FSIs, if responsive, might show decreased firing centered on the onset of stimulation (i.e., that concomitant decreases in firing by FSIs might disinhibit MSNs).
2. Results
Six hundred forty-eight striatal neurons were recorded and of these, 90 neurons’ waveforms were isolated during offline spike sorting to undergo further analysis (the remainder of recorded neurons exhibited characteristics of MSNs, studied extensively via body exam in previous studies). Fifty-eight of the isolated striatal neurons were sampled with fixed microwire arrays and 32 neurons were sampled with drivable microelectrode arrays. All 90 isolated neurons’ waveforms were entered into a cluster analysis to obtain an objective breakdown and subgrouping of the neural population. Cluster analysis revealed that the average neuronal waveforms could be grouped into eight discrete clusters based on pseudo F statistics. Post-hoc graphical analyses revealed two main clusters, encompassing 63.3% and 28.9%, respectively, of the entire set of neurons (total 93.2%). The 26 neurons grouped together into the smaller cluster exhibited a distinctive, sharp waveform shape, with a short valley width [M=58.41 μs (±3)], as well as short valley-to-peak latency [M=110 (±4) μs] and fast spontaneous FR [M=4.3 (±0.9) spikes/second], and thus were classified as FSIs. The 57 neurons in the larger cluster exhibited a waveform shape typical of MSNs with a broader valley width [M=81.05 (±2.09) μs] and broader valley-to-peak latency [M=168.42 (±2.69) μs], as well as a slower spontaneous FR [M=1.11 (±0.15) spikes per second]. Figure 1 displays average overlaid waveforms separately for the two clusters of interest. Figure 2 presents a scatterplot of data along the three dimensions, valley width, valley-to-peak latency and FR. Seventeen of the 26 neurons initially classified as FSIs showed FRs higher than 2 spikes per second. Further analysis focused on those 17 neurons.
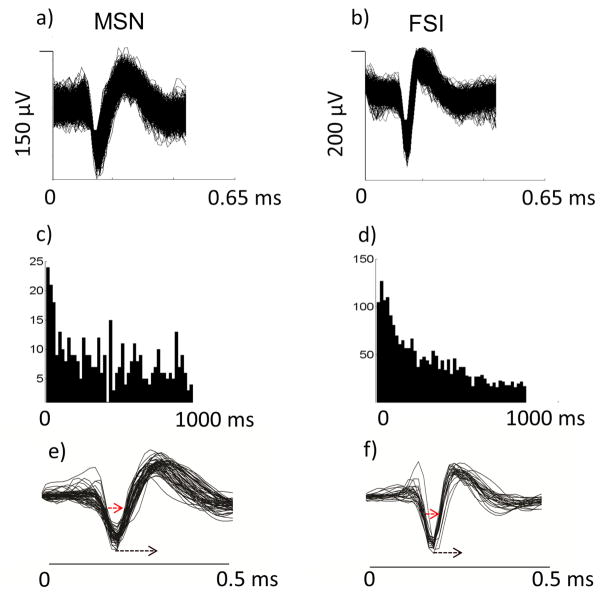
Figure 1.
a) & (b) overlaid single spike waveforms of an example MSN and example FSI. (c) & (d) corresponding inter-spike interval histograms for the example MSN and FSI. (e) & (f) overlaid averaged waveforms of 26 presumed FSIs and 57 presumed MSNs. Red dashed lines: measurements used for cluster analysis.
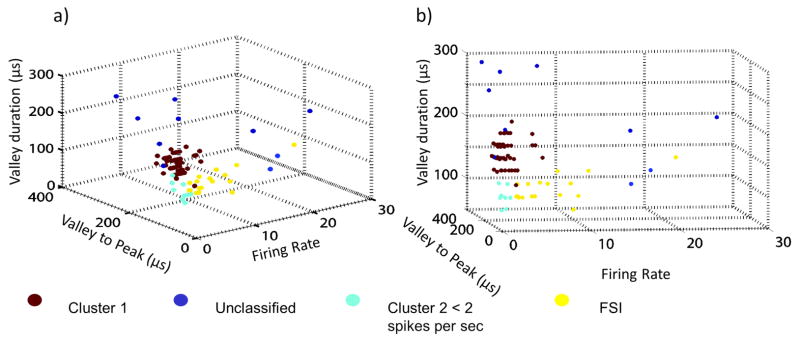
Figure 2.
a) & (b) three-dimensional scatter plots of entire distribution of recorded waveforms along the three dimensions used in cluster analysis: FR, valley duration, valley to peak duration from two perspectives. Yellow dots: 17 neurons classified as FSIs (with FR> 2 spikes/sec), dark red dots: neurons classified as MSNs. Remaining neurons belong to minor clusters.
Of 17 neurons classified as FSIs with FR > 2 spikes/sec, 9 received a thorough, video-recorded body exam. For 6 out of 9 neurons the body exam revealed a correlated body part to which the neuron selectively responded. The correlated body parts were the following: upward head movement (2 neurons), downward head movement, left vibrissae, snout, and left front paw (Fig. 3). For 3 of the 9 neurons the body exam did not reveal any body part with which FR was correlated.
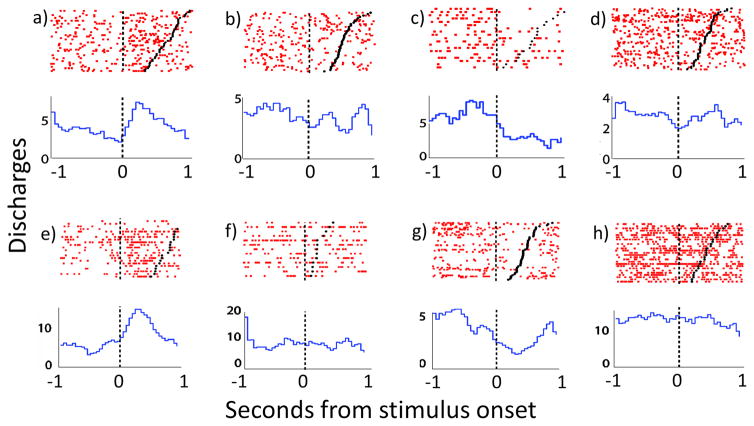
Figure 3.
Rasters and peri-event time histograms (PETHs) centered on the onset of body part stimulation (represented by black vertical dashed line at time 0) displaying firing of body part responsive FSIs. Black dots represent the offset of body part stimulation. (a) raster-PETH displaying firing of a responsive FSI that exhibits clear increase in firing during downward passive head movement, (b) lack of modulation for same neuron during upward head movement. (c) Another FSI exhibits clear decrease related to upward paw movement, but (d) a lack of modulation during upward head movement. e) FSI showing increased FR during upward head movement, (f) and no modulation during whisker stimulation. (g) Another FSI showing large decrease in FR during upward head movement, (h) but no change during left paw lifts.
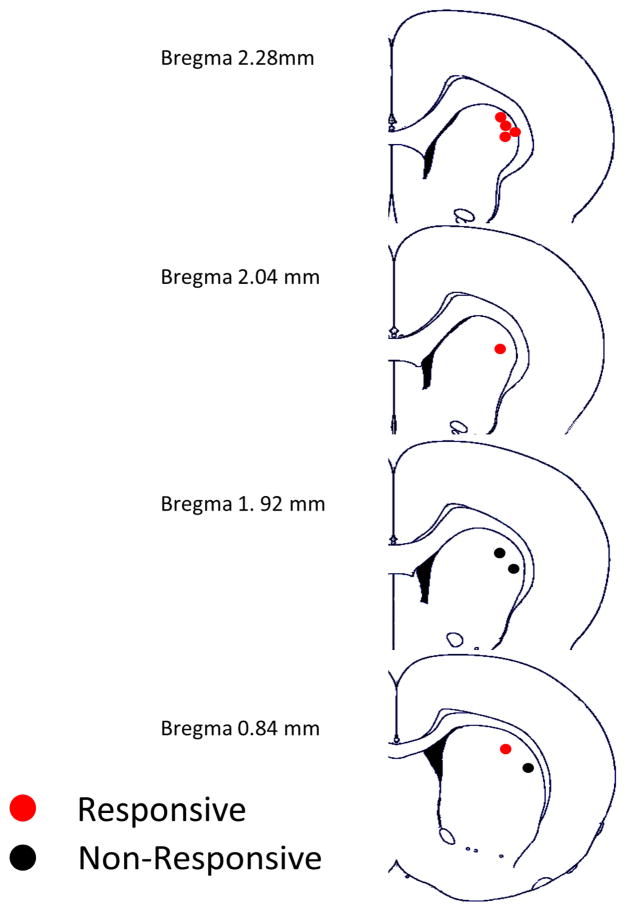
The following analysis focused on the 6 neurons for which the body exam resulted in the experimenter’s subjective determination that FR was selectively sensitive to stimulation of one body part (‘Related’) but insensitive to other body parts (‘Unrelated’). The |fold Δ FR| values for the ‘Related’ and ’Unrelated’ body parts were entered into a mixed model ANOVA, which revealed a significant difference in the magnitude of FR change from Baseline to Test periods between ‘Unrelated’ and 'Related' body parts, F(1,11)=15.91, p=.0021. Post-hoc Sidak corrected confidence interval (CI) tests indicated that Unrelated body part stimulation did not cause a significant change in magnitude of FR from Baseline to Test periods, t(11)=0.78, 95% CI [−3.05, 4.82], fold Δ of 1 = no change. In contrast, responses to stimulation of 'Related' body parts showed a significant change in magnitude of FR from Baseline to Test periods, t(11)=4.96, 99% CI [2.97, 17.10]. The magnitude of this effect for ‘Related’ and ‘Unrelated’ body parts is shown in Figure 4. These results provide statistical validation of the experimenter’s qualitative assessments of FR change during the body exam, which have been consistently applied to studies of striatal MSNs, extended here to FSIs for the first time. Locations of the nine FSIs tested are shown in Figure 5. Results of analyses comparing FRs of FSIs depending on whether simultaneously recorded MSN FR was zero vs non-zero showed a trend toward a negative (reciprocal) relationship, but were not significant.
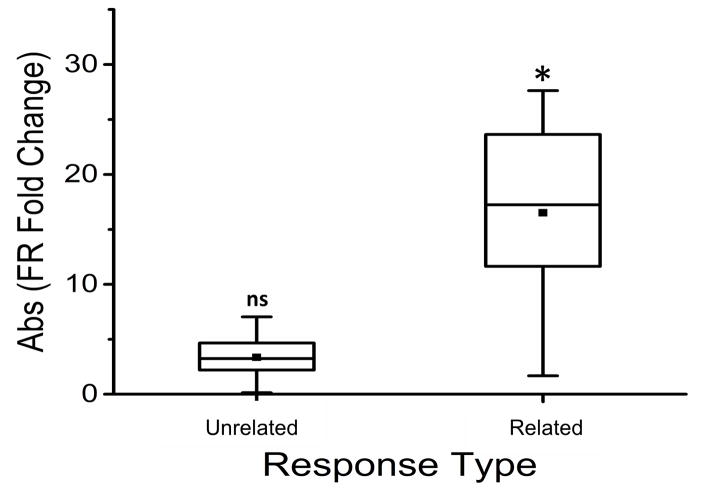
Figure 4.
Box plot displaying mean absolute fold change in FR of FSI with respect to baseline, plotted separately for unrelated body parts versus related body parts. * indicates significant difference (p = 0.01) in magnitude of FR from Baseline to Test for Related but not Unrelated (ns = not significant) categories (absolute FR fold change value of 1 = no change).
Figure 5.
Diagram of locations where FSIs responsive and non-responsive to body part stimulation were recorded. Red dots mark locations of responsive FSIs, whereas black dots mark locations of non-responsive FSIs.
3. Discussion
As in previous studies (Wilson et al, 1990; Inokawa et al, 2010), a large sample of recorded neurons yielded a small sample of interneurons targeted for study. In the present case, a subset of neurons met criteria widely recognized as characteristic of striatal FSIs (short valley durations, short valley to peak durations, high FRs). The clear, selective responsiveness to body part stimulation by a proportion of those neurons provides the first evidence during behavior that discrete body parts are represented by the firing of striatal FSIs.
Nine out of the 17 neurons classified as FSIs were carefully tested with a thorough somatic sensorimotor body exam. Six of the 9 recorded neurons responded selectively to stimulation of one individual body part. Three neurons exhibited clear and selective increases in FR during stimulation: upward head movement, downward head movement and whiskers, respectively (neurons exhibiting decreases are discussed below). These increases in activity were not dissimilar to responses of typical MSNs to movement or stimulation of the related body part (Figure 3a): 1) the magnitude of change was an increase on the order of several spikes per second from baseline. This reaffirms FSI sensitivity to cortical input, but we note that we did not assess whether sensitivity was greater than that of MSNs (Parthasarathy & Graybiel, 1997; Mallet et al, 2005); 2) increases were selective in nature with respect to the stimulated body part. For example the vibrissae sensitive FSI did not show altered firing during snout or peri-oral stimulation; 3) selectivity was also found for the direction of movement. Neurons showing an increase during downward head movement did not display any changes in firing during upward or horizontal head movement (Figure 3a–b).
According to feed-forward inhibition theory as applied to the striatum, FSIs receive or respond to cortical input before MSNs do and delay or entirely abolish firing of MSNs (Plenz & Kitai, 1998; Koos and Tepper, 1999; Mallet et al, 2005). Despite receiving the same cortical input, the theory predicts that firing of pairs of FSIs and MSNs should be negatively correlated due to inhibitory effects of FSIs on MSNs. This has been observed in a number of studies where periods of high FR of an FSI were associated with decreased probability of an action potential in a neighboring MSN (e.g., Gage et al., 2010). The present assessment, capable only of revealing a general relationship between the two types of neurons in likelihood to fire, separated FSI FRs during baseline according to MSN FR recorded simultaneously (MSN FR=0 vs MSN FR>0), and revealed no difference in average FSI FR. Testing more specific linkages and timing were outside the present scope, given distances exceeding several hundred microns across the array of recording electrodes, and low temporal resolution (30 frames/sec) of video recordings. Certain other results of the present study are in fact consistent with feed-forward inhibition theory. Notably, some proportion of FSIs, as indicated by the small sample observed in the present study, selectively and substantially decreased in activity during movement of an individual body part. This is in contrast with MSNs, which uniformly increase in activity during their related movement. FSIs showing decreases in FR could disinhibit MSNs, whereas FSIs that show increases in firing related to a movement could inhibit groups of MSNs representing body parts unrelated to that particular movement. Also consistent with the theory is the large differential in tonic FR, whereby high spontaneous FRs of FSI may be responsible for the very low FRs of MSNs during non-movement.
In conclusion, we demonstrated that electrophysiologically defined FSIs exhibit robust changes in FR related selectively to stimulation of an individual body part. Determining the actual prevalence of somatomotor responsive FSIs was outside the present scope and will await further study. The majority of fully tested FSIs showed increases in firing related to body part stimulation similar to those typically observed in MSNs. However, a subset of tested FSIs showed changes in FR that differed from those typically observed in MSNs: decreases in activity related to movement of the related body part. The current study does not address the question of how sensorimotor responses of FSIs are synaptically linked to those of MSNs. Thus it is not yet possible to interpret the meaning of FR changes exhibited by FSIs during body part stimulation. Nonetheless, we have provided novel information that now makes this question one that needs answering, and informs future studies of the fact that a portion of FSIs recorded in awake animals are sensitive to activity of body parts.
4. Methods and Materials
Subjects
Male Long Evans rats (N=17; Charles Rivers Laboratories, Wilmington, MA) were studied. Prior to surgery, animals were individually housed on a 12 hour light/dark cycle with lights on from 10:00 a.m. to 10:00 p.m. Animals were given ad libitum water and restricted food access to maintain body weight of 350g throughout the experiment. All protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publications 865–23) and were approved by the Institutional Animal Care and Use Committee, Rutgers University.
Procedures
Surgery
Thirteen animals were implanted with microwire arrays (Microprobes Inc., Gaithersburg, MD) in the right dorsolateral striatum (DLS) through a rectangular craniotomy, with the following corners relative to Bregma [(mediolateral (ML)mm, anteroposterior (APmm), (2.8, 2.5) (3.4, 2.6) (3.4, −0.5) (4.0, −0.4)] (details of surgery in Barker et al., 2014). The array was implanted using a computerized stereotaxic device (Coffey et al. 2013) and lowered to the depth of 3.9 mm. Four other animals were implanted with tungsten microelectrode arrays at the same coordinates. Each microelectrode array consisted of a set of fifteen, 125 μm diameter microelectrodes and a ground wire. The microelectrode array was connected to an EDDS Microdrive System (Microprobes Inc., Gaithersburg, MD), enabling post-surgical repositioning of the array along the dorsoventral axis. The maximum depth of lowering was 7 mm from skull level. Each array was initially lowered 1.5 mm below skull level. The array casing was sealed to the surface of the skull with cyanoacrylate and attached to the front and back skull screws using dental cement. Recovery took place in individual Plexiglas chambers, which served as the animals’ home cages and recording cages for the duration of the experiment.
Somatosensorimotor exam
After eight days of post-surgical recovery, all animals underwent a somatosensorimotor exam during which individual body parts (head, chin, neck, trunk, forelimb, forepaws, whiskers) both contralaterally and ipsilaterally were stimulated by the experimenter. Body parts underwent stimulation in a variety of types, e.g., cutaneous touch (gently poked or touched, using a cotton swab), passive manipulation, or active movement. A stimulus was defined as any active or passive movement of, or experimenter contact with, a particular body part. Stimulation of each body part was performed repeatedly over the period of one hour. Each body part was stimulated at least ten times in a single series (further details are described in Carelli & West, 1991; Coffey et al., 2016). To confirm spiking during each individual body part stimulation, neuronal signals were recorded, amplified, and played through a pair of headphones. Body part sensitivity was judged online during the exam through auditory inspection of the neuronal activity heard through the head phones (Carelli & West, 1991; Prokopenko et al, 2004). Animals were required to remain still in order to enable detection of phasic changes in FR. For a neuron to be considered body part sensitive, a noticeable change in firing was required during the active or passive manipulation of that body part selectively. Video recordings of body exams were time-stamped (30 frames/sec) by the same computer that time-stamped neuronal discharges. Using video recordings of the exam, recorded neural data were quantified post hoc with raster plots centered on the onset of each discrete body part stimulation.
Microwire Array Recordings
On the day of or the day after the body exam, spontaneous FR and waveform parameters were obtained by connecting animals to a recording harness at the onset of the light phase of the light cycle, and recording for an hour while the animal was uninterrupted by body exam procedures.
Microdrive Recordings
Four animals implanted with movable microelectrode arrays underwent a slightly modified procedure. Eight days following surgery, each animal was connected to a recording harness and the microelectrode array was slowly lowered (~70 μm per minute) until neurons were first detected. The number and FR of cortical neurons were characterized at each point during lowering of the array. The number of turns until the 'quiet zone' (Carelli and West, 1991), corresponding to subcortical white matter, was estimated and the array was lowered further until spiking striatal neurons were detected. Each time the array was lowered by a quarter of a turn (70μm), the number of neurons observed was recorded and their profile was characterized. The array was lowered until it detected neurons matching the criteria of FSIs. i.e., narrow waveform and fast firing. If a neuron matching the criteria of an FSI was observed, a body exam, identical to one for animals with microwire implants, was conducted. Neurons matching the criteria of FSIs were specifically targeted for the body exam. After the body exam was complete, recording continued for a period of an hour to gather data consisting of spontaneous FRs and waveform parameters uninterrupted by body exam. At the end of each day the array was lifted until cortical neurons were detected.
Fluorescent Immunohistochemical labelling
Following all recordings (~30 days after surgery) animals were anesthetized and perfused transcardially with 0.9% phosphate buffered saline followed by 4% paraformaldehyde. Brains were post-fixed for 48 hours in 4% paraformaldehyde and transferred to a 30% sucrose solution. Brains of animals implanted with microwires were sliced into 30 μm coronal sections, whereas brains implanted with tungsten microelectrode arrays into 50 μm coronal sections. Fluorescent immunohistochemistry was performed on free floating brain tissue. Slices were incubated for an hour in a 4% Bovine Serum Albumin (BSA) and 0.3% Triton X-100 in phosphate buffer. Thirty μm thick sections were rinsed and incubated overnight in a 4% BSA with mouse anti-parvalbumin antibody and rabbit anti-GFAP antibody. Next, tissue was rinsed and incubated in anti-mouse secondary antibody conjugated to a green fluorophore (Alexa Fluor ® 488) and an anti-rabbit antibody conjugated to a red fluorophore (Alexa Fluor ®555). Subsequently, tissue was washed with phosphate buffer (PB) and mounted on a slide using mounting medium containing Dapi (nucleic acid stain), which served as a counter stain. Fifty μm thick slices were mounted using Dapi stain. All pictures were recorded with a Zeiss Axiovert 200M, Fluorescence microscope.
Analyses
Neural data
Continuous electrophysiological data were amplified (700x) and band pass filtered between 450 Hz and 10 kHz with a roll off of 1.5 dB per octave below 1 kHz and 6 dB per octave above 11 kHz. Signals were digitized at a 50-kHz sampling frequency and were recorded using DataWave Technologies hardware and software (Longmont, CO, USA). Following recording sessions, data were displayed for assessment of waveform stability on a computer simulated oscilloscope. Parameters such as peak time, peak amplitude, spike time, spike height, and principle component analysis were used to detect and isolate neural spikes. Neuronal signals were considered recorded from one single neuron only if the following criteria were met: (1) Signals recorded from the same wire were of similar amplitude and shape. (2) The putative neuron exhibited a signal-to-noise ratio greater than 2:1. (3) The auto-correlation revealed a minimum interspike interval (ISI) ≥ 1.6 ms (natural refractory period). A cross-correlation was performed if several waveform profiles were detected in the recordings from a single microwire. Waveforms were designated as belonging to two separate neurons if each individual waveform showed a refractory period containing zero spikes within 1.6 ms and discharges occurred within the first 1.6 ms in the cross-correlation. Otherwise, the signals were combined and considered originating from one single neuron.
Cluster Analysis
For each individual neuron, all action potential waveforms were averaged together and three parameters were computed: the neuron’s average FR, the waveform’s valley width (μsec), and valley to peak length (μsec). A cluster analysis, using SAS PROC CLUSTER, was conducted on these three standardized parameters using average linkage based on Euclidian distances. A plot of pseudo F statistics was used to determine the cutoff for the number of clusters to retain. In order to be conservative in our analyses, neurons with FRs slower than 2 spikes per second were not considered FSI-candidates (Wiltschko et al., 2010).
Analyses of Firing during Body Exam
During body exams that were video recorded and audio-monitored through headphones, each body part was stimulated multiple times and the neuron’s online responsiveness resulted in designation by the experimenter as either ‘Related’ or ‘Unrelated’ to that body part. 'Related' body parts were designated as those that produced an audible change in a neuron’s FR when stimulated, while ‘Unrelated’ body parts were designated as those that did not produce any detectable change in firing. A trial was defined as a single stimulation of an individual body part. The inter-trial interval was >1 sec. The onset and offset of each individual stimulus was subsequently established using post-hoc frame-by-frame video analysis (30 frames/sec). FR from onset to offset of the stimulus was defined as Test firing. Baseline firing was defined as FR during the 500 ms prior to the onset of each stimulus, in order to be comparable to the average duration of individual stimuli. For each stimulus, change in FR from Baseline to Test was assessed by computing a fold-change statistic, in which the higher FR of the two, Baseline or Test, was divided by the other. A sign of change was applied to accommodate either increasing or decreasing Test FRs with respect to Baseline FR, such that if Baseline FR was greater than Test FR, then Baseline FR to Test FR ratio was given a negative sign of change. On the other hand, if Test FR was greater than Baseline FR, then the Test FR to Baseline FR ratio was given a positive sign of change. The magnitude of change in FR was analyzed by taking the absolute value of the fold-change statistic (|fold Δ FR|). The absolute values of the fold-changes in FR were collapsed across all trials within a test session by using the trimmed mean (10%).
To test whether there was a statistically significant difference between ‘Related’ vs ‘Unrelated’ categories as designated by the experimenter’s assessment via headphones, a one-way mixed model ANOVA compared the trimmed means of the fold-changes in FR between the ‘Unrelated’ and ‘Related’ responses. Neuron was specified as a random effect, and ‘Unrelated’ and ‘Related’ stimulations associated with an individual neuron were nested together. Post-hoc confidence interval (CI) tests based on the results of one way t-tests using Sidak’s Type I error correction were conducted, in which the means for ‘Unrelated’ and ‘Related’ were tested against a value of 1 (fold Δ = 1 indicates no change from Baseline to Test). CI tests were required because the means of the distributions had to be tested against a value of 1, whereas standard post hoc, one-sample t-tests test only against a value of 0. All analyses were conducted using SAS PROC GLIMMIX.
Analyses of the Baseline Period
Inter-stimulus-intervals (onset-to-onset) were longer than 1 second. However, stimulus durations could last several hundred milliseconds. The latency from offset of stimulation until the onset of stimulation on the next trial was termed offset-to-onset latency. Fewer than 4% of trials had latencies <500ms and fewer than 14% of trials had latencies <800ms; 20% of trials had latencies <1 sec. To investigate the possibility that a “rebound” or residual following stimulus offset could have influenced our measure of baseline firing rate (FR), the large number of trials preceded by greater than 1 second free of any stimulation were selected. The assumption was that, after a full second, any putative rebound effects have ended. For each of those trials, baseline FR was computed and compared to FR during the subsequent five 100 ms bins after the offset of stimulation on that trial. In order to compare Post-Offset to Baseline FR, the standardized change in FR (FRSc) between Post-Offset and Baseline FR was computed for each Post-Offset interval, . This yields a scale from -1 to 1, in which 0 indicates no difference in FR. FRSc values were analyzed using a generalized linear mixed model (GLMM) using SAS PROC GLIMMIX (SAS 9.4, SAS Institute), in which Body Part Response (Control vs. Target) and Time Period were the independent variables. Because Target body part responses were specifically of interest, planned post-hoc simple effects were carried out that analyzed the overall difference of FRSc across all Time Periods for the Target condition. Further, planned post-hoc t-tests comparing average FRSc values for each individual Time Period in the Target condition against a value of 0 were conducted, where 0 indicates no difference from baseline FR. Type I error was controlled using the Sidak correction for multiple comparisons. The GLMM showed that there was no statistically significant interaction for Body Party Response by Time Period, F(4, 4280) = 0.21, p = .933, and no significant main effects for Body Part Response, F(1, 4280) = 0.03, p = .860, and Time Period, F(4, 4280) = 0.46, p = 0.766. The post-hoc simple effect test for Target across all Time Periods was not significant, F(4, 4280) = 1.02, p = 0.396. Post-hoc t-tests for the average FRSc values for each Time Period showed no statistically significant difference from 0, i.e., no change in FRSc between Baseline and the Time Period: 0 – 100 msec, t(4280) = −1.72, p = .357; 100 – 200 msec, t(4280) = −2.26, p = .114; 200 – 300 msec, t(4280) = −2.12, p = .158; 300 – 400 msec, t(4280) = −2.14, p = .153; 400 – 500 msec, t(4280) = −1.92, p = .244. These data confirmed that there was no residual "rebound" in FR after stimulus offset that could have influenced our measure of baseline FR on the subsequent trial.
Analyses of MSN and FSI interaction
To investigate a possible dependency between MSN and FSI FRs, the FR recorded during the 500 ms pre-stimulus period (baseline period) of all FSIs exhibiting a ‘Related’ body part were sorted into two categories depending on whether the MSN(s) recorded simultaneously on the same array was firing or not during that trial (MSN FR >0 vs MSN FR =0). Subsequently average FR for each FSI was computed for each category.
Statement of Significance.
Parvalbumin positive, striatal FSIs are hypothesized to play an important role in behavior by inhibiting MSNs. We asked a fundamental question regarding information processed during behavior by FSIs: whether FSIs, which preferentially occupy the sensorimotor portion of the striatum, process activity of discrete body parts. Our finding that they do, in a selective manner similar to MSNs, begins to reveal the types of phasic signals that FSI feed forward to projection neurons during striatal processing of cortical input regarding a specific sensorimotor event. These findings suggest new avenues for testing feed-forward inhibition theory as applied to striatum in naturalistic conditions, such as whether FSI decreases facilitate excitation of MSNs related to the current movement while FSI increases silence MSNs unrelated to the current movement.
Highlights.
A subset of neurons met physiological criteria characteristic of striatal FSIs.
Putative FSIs showed selective responses to stimulation of discrete body parts.
FSIs exhibited increases or decreases in FR during body part stimulation.
The timing of responses was similar to that exhibited by striatal MSNs.
Acknowledgments
We thank Joshua Stamos, Mary Nguyen, Thomas Grace Sr., Alisa Ray and Jackie Thomas, for excellent assistance. This study was supported by National Institute on Drug Abuse Grant DA006886. The authors declare no competing financial interests. All coauthors have seen and approve of the contents of the manuscript.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Delong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. 1985;53:1417–1430. doi: 10.1152/jn.1985.53.6.1417. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Coffey KR, Ma S, West MO. A Procedure for Implanting Organized Arrays of Microwires for Single-unit Recordings in Awake, Behaving Animals. JoVE. 2014;(84):e51004–e51004. doi: 10.3791/51004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbuminimmunoreactive neurons in the neostriatum of the rat. Neurosci. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43(6):883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Berke JD. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. The Journal of Neuroscience. 2008;28(40):10075–10080. doi: 10.1523/JNEUROSCI.2192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991;309:231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- Cho J, West MO. Distribution of single neurons related to body parts in the lateral striatum of the rat. Brain Res. 1997;756:241–246. doi: 10.1016/s0006-8993(97)00143-1. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Barker DJ, Ma S, West MO. Building an open-source robotic stereotaxic instrument. JoVE. 2013;(80):e51006–e51006. doi: 10.3791/51006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey KR, Nader M, West MO. Single body parts are processed by individual neurons in the mouse dorsolateral striatum. Brain Res. 2016;1636:200–207. doi: 10.1016/j.brainres.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13(3):1120–1137. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Input-output organization of the sensorimotor striatum in the squirrel monkey. The Journal of neuroscience. 1994;14(2):599–610. doi: 10.1523/JNEUROSCI.14-02-00599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activationof striatal fast-spiking interneurons during choice execution. Neuron. 2010;67:466–479. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. PNAS. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD, Kreitzer AC. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. The Journal of neuroscience. 2011;31(44):15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res Reviews. 1983;5:109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Inokawa H, Yamada H, Matsumoto N, Muranishi M, Kimura M. Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience. 2010;168:395–404. doi: 10.1016/j.neuroscience.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. Journal of neurophysiology. 1994;72(5):2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, … Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. Journal of Comparative Neurology. 2010;518(3):277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. TINS. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63:1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual review of neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Liles SL, Updyke BV. Projection of the digit and wrist area of precentralgyrus to the putamen: relation between topography and physiological properties of neurons in the putamen. Brain Res. 1985;339:245–255. doi: 10.1016/0006-8993(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Luk KC, Sadikot AF. GABA promotes survival but not proliferation of parvalbumin-immunoreactive interneurons in rodent neostriatum: an in vivo study with stereology. Neurosci. 2001;104:93–103. doi: 10.1016/s0306-4522(01)00038-0. [DOI] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler T, Cho J, People LL, West MO. Representation of the body in the lateral striatum of the freely moving rat: single neurons related to licking. Experimental brain research. 1994;98(1):163–167. doi: 10.1007/BF00229122. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Aertsen A. Neural dynamics in cortex-striatum co-cultures—I. Anatomy and electrophysiology of neuronal cell types. Neurosci. 1996;70(4):861–891. doi: 10.1016/0306-4522(95)00406-8. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J Neurosci. 1998;18:266–283. doi: 10.1523/JNEUROSCI.18-01-00266.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends in neurosciences. 2003;26(8):436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Prokopenko VF, Pawlak AP, West MO. Fluctuations in somatosensory responsiveness and baseline firing rates of neurons in the lateral striatum of freely moving rats: Effects of intranigral apomorphine. Neurosci. 2004;125:1077–1082. doi: 10.1016/j.neuroscience.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretininimmunoreactiveGABAergic interneurons of the neostriatum. J Comp Neurol. 2004;469:325–339. doi: 10.1002/cne.11008. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Plenz D. Microcircuits: the interface between neurons and global brain function. The MIT Press; Massachusetts, Cambridge: 2006. Microcircuits in the striatum: striatal cell types and their interaction; pp. 127–148. [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. Journal of neurophysiology. 2002;88(3):1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Kotter R, Alexander ME. Effects of local connectivity on striatal function: Simulation and analysis of a model. Synapse. 1995;20(4):281–298. doi: 10.1002/syn.890200402. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Pettibone JR, Berke JD. Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacol. 2010;35:1261–1270. doi: 10.1038/npp.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Li L, Pittenger C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience. 2016;324:321–329. doi: 10.1016/j.neuroscience.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]