Abstract
Vasopressin exerts important cardio–renal effects, but remains problematic to measure. Copeptin is a more stable peptide derived from the same precursor molecule. In this case-control study from the Type 1 Diabetes Exchange (T1DX) Biobank registry, men with T1D and albuminuria had greater copeptin concentrations than men with normoalbuminuria.
Introduction
Diabetic kidney disease (DKD) is a major cause of mortality in type 1 diabetes (T1D) (1), and emerging data suggest the arginine vasopressin (AVP) system plays an important role in the development of cardio-renal disease (2). Measuring AVP is cumbersome due to its relatively small size and short half-life. However, copeptin, a more stable peptide derived from the same precursor molecule as AVP, is recognized as a surrogate marker for AVP (2). We previously reported that among adults with T1D in The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study, greater copeptin concentrations were associated with greater odds of atherosclerosis, albuminuria and impaired GFR (eGFR ≤ 60ml/min/1.73m2) (3).
Herein, we present data from a matched case-control study of 38 males with type 1 diabetes and albuminuria (urinary albumin-to-creatinine ratio [UACR] ≥ 30mg/g) and 38 males with type 1 diabetes with normoalbuminuria (UACR <10mg/g) recruited from the T1D Exchange Biobank.
Methods
Samples were collected from participants in the T1D Exchange Biobank. Informed consent was obtained from all subjects according to IRB-approved protocols. Data were collected from the T1D Exchange Registry and the T1D Exchange Biobank (4). Age at blood draw, diabetes duration, LDL-cholesterol (LDL-C), systolic blood pressure (BP) [SBP], and diastolic BP (DBP), UACR, serum creatinine, ACEi/ARB usage was obtained from the T1D Exchange Clinic Registry database. Race/ethnicity, BMI (height/weight), HbA1c, insulin modality (multiple daily injections vs. insulin pump) and history of cardiovascular disease (CVD) [defined as ever been treated for high blood pressure, high cholesterol, heart attack or stroke] was obtained from the Living Biobank standard question set/demographic form. This investigation was a case-control study (cases: microalbuminuria [≥30mg/g], controls: normoalbuminuria [<10mg/g]) (5, 6) matched by age, and diabetes duration. Due to the known interaction between copeptin and sex, only males were selected for the study. Cases were defined as at least one occurrence of clinic-reported micro/macroalbuminuria or 2 consecutive elevated ACR values from most recent visit. Controls were defined as clinic-reported normoalbuminuria or all available ACR < 10 mg/g. The selection of participants was not random. We limited the pool of Living Biobank participants to those potentially eligible and recruited these participants until the sample size was met.
Serum copeptin was measured on all participants. Blood was collected, centrifuged, separated, and serum aliquots were stored at −80°C. Copeptin was measured by an ultrasensitive assay on KRYPTOR Compact Plus analyzers using the commercial sandwich immunoluminometric assays (Thermo Fisher Scientific, Waltham, MA). Estimated GFR (eGFR) was calculated by CKD-EPI creatinine. Impaired GFR was defined as eGFR <60mL/min/1.73m2.
Analyses were performed in SAS (version 9.4 for Windows; SAS Institute, Cary, NC). Variables were checked for the distributional assumption of normality using normal plots. Variables that were positively skewed (e.g. ACR and copeptin) were natural log-transformed for the analyses. Differences in continuous parametric and log-transformed variables between matched cases and controls were examined using linear mixed models, unadjusted and adjusted for HbA1c and eGFR, in order to account for the matched design. Because cases and controls were matched based on age and duration, these variables were not included as covariates in the multivariable models with albuminuria as outcome. To examine the relationships between copeptin and cases/controls we used univariable and multivariable (HbA1c and eGFR) conditional logistic regression models. The relationship between copeptin and impaired GFR were examined with univariable and multivariable (adjusted for T1D duration, HbA1c, ACEi/ARB usage and case/control status) generalized linear mixed models with random effect for pairing to account for the correlation between members of a case-control pair. P-value < 0.05 was considered statistically significant.
Results
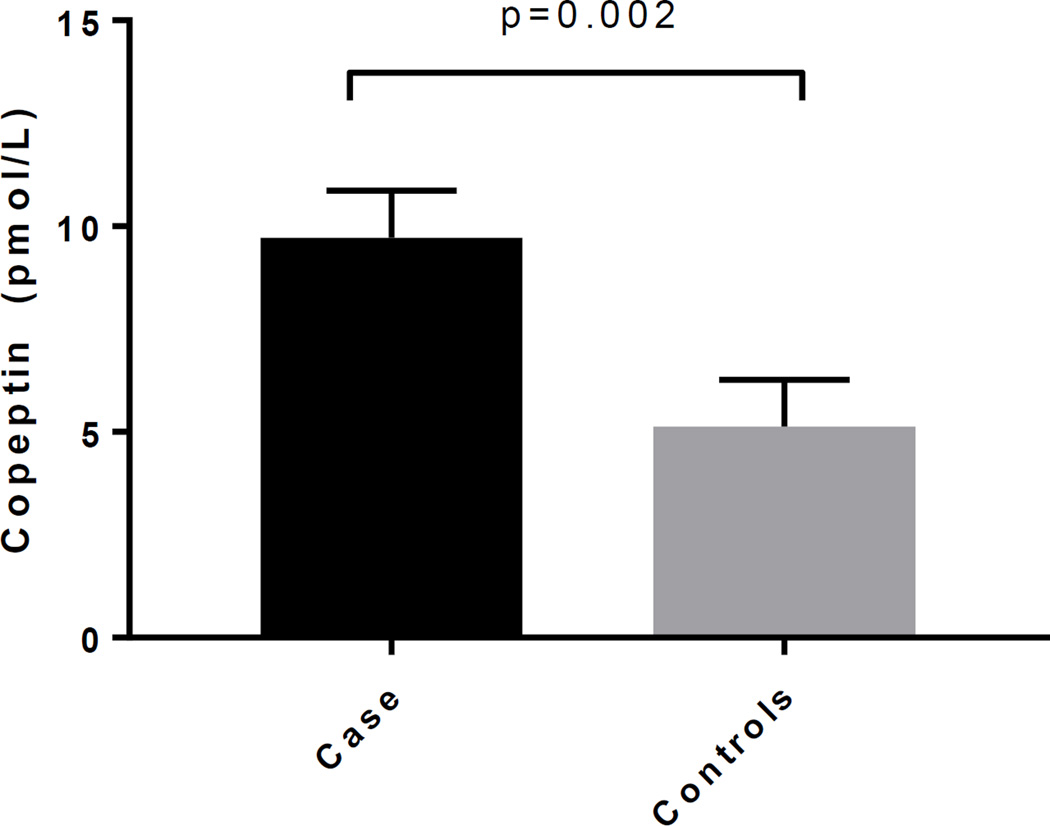
Table 1 shows the participant characteristics of cases and controls. The mean copeptin concentration was over 2-fold greater in cases compared to controls (10.54, 95% CI 8.08–13.75 vs. 4.60, 95% CI 3.52–6.00, p<0.0001). The difference in natural log of copeptin between the matched cases and controls was significant after adjusting for HbA1c, eGFR and ACEi/ARB use (Figure 1).
Table 1.
Characteristics of Cases and Controls
| Variables | Case (n=38) Albuminuria |
Controls (n=38) Normoalbuminuria |
|---|---|---|
| Age (years) | 54±18 | 52±17 |
| T1D duration (years) | 37±15 | 30±13 |
| Ethnicity (NHW) | 92% | 100% |
| Insulin pump (yes, %) | 61% | 74% |
| Smoking | ||
| - Never | 55% | 74% |
| - In the past | 37% | 26% |
| - Currently | 8% | 0% |
| Ever been treated for CVD (yes, %)† | 82% | 50% |
| HbA1c (%) | 8.0±1.7 | 7.2±0.8 |
| BMI (kg/m2) | 27±5 | 26±4 |
| LDL-C (mg/dL) | 87±28 | 91±25 |
| SBP (mm Hg) | 128±20 | 122±11 |
| DBP (mm Hg) | 70±8 | 73±8 |
| ACE/ARB usage (yes, %) | 86% | 70% |
| Anti-hypertensive usage (yes, %) | 63% | 57% |
| Anti-dyslipidemia usage (yes, %) | 58% | 50% |
| ACR* (mg/g) | 145 (84–249) | 4 (3–5) |
| CKD-EPI eGFR (ml/min/1.73m2) | 76±42 | 90±24 |
| Time of blood collection for copeptin analysis‡ |
10:00 am (9:00–11:00 am) | 9:15 am (8:00–11:00 am) |
Geometric means and 95% CI
Defined as ever been treated for a CVD (e.g., high blood pressure, high cholesterol, heart attack or stroke)
Median and p25–75
Figure 1.
HbA1c, eGFR and ACE/ARB use adjusted means of copeptin in cases and controls
Adjusted p-value p=0.002
In conditional logistic regression models, one standard deviation (SD) increase in copeptin was associated with greater odds of albuminuria (OR: 3.57, 95% CI 1.56–8.17, p=0.003), and this relationship remained significant after adjusting for GFR and HbA1c (OR: 3.52, 95% CI 1.34–9.23, p=0.01). In a generalized linear mixed model, one SD increase in copeptin was associated with greater odds of impaired GFR (OR: 2.94, 95% CI 1.39–6.21, p=0.006), and this relationship remained significant after multivariable adjustments (T1D duration, ACEi/ARB use, HbA1c and case/control status) [OR: 5.79, 95% 1.64–20.42, p=0.008].
Discussion
In this matched cross-sectional case-control study, copeptin was significantly higher in men with T1D and albuminuria compared to those with normoalbuminuria. The odds of albuminuria in participants with a 1 SD increase in natural log of copeptin was greater than 3 times as high as the odds of albuminuria in those with mean values of natural log of copeptin. Furthermore, higher copeptin concentrations conferred greater odds of impaired GFR, independent of other important risk factors. These findings from a cohort of people with T1D across the US are consistent with our observations in CACTI (3), and from GENEDIAB and GENESIS (7).
AVP concentrations are higher in people with T1D and type 2 diabetes (T2D) compared to healthy non-diabetic counterparts (8, 9). Copeptin is co-secreted with AVP from the neurohypophysis, reflects changes in AVP, and appears to increase the risk of cardiovascular mortality (10, 11) and DKD in T1D and T2D. While AVP has classically been thought of as a hormone primarily involved in volume and osmolality regulation, emerging data suggest that chronically elevated vasopressin levels cause renal and vascular injuries (11, 12). In fact, the administration of vasopressin induces glomerular hyperfiltration and albuminuria in laboratory animals and in humans (2, 13, 14).
Our study does have important limitations including the small sample size and cross-sectional design which prevents determination of causality; whether the association holds true longitudinally requires evaluation. Due to the pilot nature of the study, the study was limited to men with T1D. For these reasons the data should be viewed as hypothesis generating. Results from this study may also not be generalizable to youth or women with T1D. However, the results are consistent and supportive of our findings in CACTI (3).
In conclusion, men with T1D and albuminuria had greater concentrations of copeptin compared to men with T1D and normoalbuminuria. Greater copeptin concentrations were associated with albuminuria and impaired GFR independent of important risk factors. Further studies are needed to determine whether copeptin plays a causal role in the development of DKD.
Acknowledgments
We wish to acknowledge the T1D Exchange Biobank, investigators and staff in the T1D Exchange Clinic Network for subject recruitment.
Support: Support for this study was provided by NHLBI grants T32-DK063687 and The T1D Exchange, a program of Unitio, supported by the Leona M. and Harry B. Helmsley Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: PB researched, wrote, contributed to discussion, analyzed data and reviewed/edited the manuscript; RJJ contributed to discussion and reviewed/edited the manuscript; JKSB contributed to the discussion, reviewed/edited the manuscript; AD researched, contributed to discussion and reviewed/edited the manuscript; NF researched, contributed to discussion and reviewed/edited the manuscript; DZC contributed to discussion and reviewed/edited the manuscript; DMM researched, wrote, contributed to discussion, and reviewed/edited the manuscript.
References
- 1.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A Scientific Statement From the American Heart Association and American Diabetes Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 2.Bardoux P, Martin H, Ahloulay M, Schmitt F, Bouby N, Trinh-Trang-Tan MM, et al. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressin-deficient Brattleboro rats. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(18):10397–10402. doi: 10.1073/pnas.96.18.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornstad P, Maahs DM, Jensen T, Lanaspa MA, Johnson RJ, Rewers M, et al. Elevated copeptin is associated with atherosclerosis and diabetic kidney disease in adults with type 1 diabetes. J Diabetes Complications. 2016;30(6):1093–1096. doi: 10.1016/j.jdiacomp.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA, et al. The T1D Exchange clinic registry. The Journal of clinical endocrinology and metabolism. 2012;97(12):4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 5.Maahs DM, Jalal D, Chonchol M, Johnson RJ, Rewers M, Snell-Bergeon JK. Impaired Renal Function Further Increases Odds of 6-Year Coronary Artery Calcification Progression in Adults With Type 1 Diabetes: The CACTI study. Diabetes Care. 2013;36(9):2607–2614. doi: 10.2337/dc12-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velho G, El Boustany R, Lefevre G, Mohammedi K, Fumeron F, Potier L, et al. Plasma Copeptin, Kidney Outcomes, Ischemic Heart Disease, and All-Cause Mortality in People With Long-Standing Type 1 Diabetes. Diabetes Care. 2016 doi: 10.2337/dc16-1003. [DOI] [PubMed] [Google Scholar]
- 8.Zerbe RL, Vinicor F, Robertson GL. Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes. 1979;28(5):503–508. doi: 10.2337/diab.28.5.503. [DOI] [PubMed] [Google Scholar]
- 9.Roussel R, El Boustany R, Bouby N, Potier L, Fumeron F, Mohammedi K, et al. Plasma Copeptin, AVP Gene Variants, and Incidence of Type 2 Diabetes in a Cohort From the Community. The Journal of clinical endocrinology and metabolism. 2016;101(6):2432–2439. doi: 10.1210/jc.2016-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riphagen IJ, Boertien WE, Alkhalaf A, Kleefstra N, Gansevoort RT, Groenier KH, et al. Copeptin, a Surrogate Marker for Arginine Vasopressin, Is Associated With Cardiovascular and All-Cause Mortality in Patients With Type 2 Diabetes (ZODIAC-31) Diabetes Care. 2013 doi: 10.2337/dc12-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark WF, Sontrop JM, Huang SH, Moist L, Bouby N, Bankir L. Hydration and Chronic Kidney Disease Progression: A Critical Review of the Evidence. Am J Nephrol. 2016;43(4):281–292. doi: 10.1159/000445959. [DOI] [PubMed] [Google Scholar]
- 12.Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. 2013;9(4):223–239. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 13.Bouby N, Ahloulay M, Nsegbe E, Dechaux M, Schmitt F, Bankir L. Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. Journal of the American Society of Nephrology : JASN. 1996;7(6):842–851. doi: 10.1681/ASN.V76842. [DOI] [PubMed] [Google Scholar]
- 14.Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, et al. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18(3):497–506. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]