Abstract
Abdominal actinomycosis is an uncommon pediatric infection that often manifests with a tumor-like lesion. We describe a previously healthy 11-year-old girl who presented with right lower quadrant abdominal pain and drainage. Computed tomography scan showed an abdominal wall mass. Surgical debridement cultures grew Actinomyces meyeri. Literature review identified 18 additional pediatric cases since 1964 that we have summarized.
Keywords: actinomycosis, abdomen, pediatric, sinus drainage, abdominal wall mass
Introduction
Actinomycosis is a rare, indolent and progressive infection caused by a Gram positive, anaerobic or microaerophillic bacteria. Actinomyces species may be found as normal colonizers of the oropharynx, gastrointestinal tract or urogenital tract. The first documented human actinomycosis case occurred in Israel in 1878 (1). Actinomycosis mostly affects middle-aged adults (2). Commonly affected parts are the cervicofacial, thoracic, abdominopelvic areas and the central nervous system (3). Pediatric actinomycotic is rare, accounting for less than 3% of all actinomycosis cases; 20% of pediatric cases occur in the abdomen (4). We present a case of abdominal actinomycosis in a previously healthy 11-year-old girl.
Case report
An 11-year-old, previously healthy girl presented to the emergency department of the Children’s Hospital Los Angeles with 6 weeks of intermittent right hip and leg pain, 3 days of abdominal pain, and a swollen, indurated, tender, darkened area on her right lower abdomen that began draining bloody, yellowish, foul smelling liquid. The patient lost 4 pounds over the previous month. Parents denied prior illness, fever or any other symptoms.
The family denied pets or travel outside of southern California. She had no known ill contacts. Immunizations were up-to-date. The patient had poor dentition and had 6 cavities filled 2 days before admission. The patient denied ingestion of any foreign objects. She reported falling onto a purse with a metal clasp that poked into her right hip near the affected site approximately 3 months prior. The wound had completely healed before the start of her symptoms.
On examination at hospital admission, her oral temperature was 37.6°C, pulse 80 beats/minute, respiratory rate 17 breaths/minute, blood pressure 128/67 mm Hg, weight 24.6 kg (<3rd percentile) and height 136 cm (5th percentile). She was alert, thin-appearing and in no distress. Her dentition was poor with multiple fillings. She had no cervical lymphadenopathy and a normal heart and lung examination. She had a tender, erythematous firm area on her right lower abdominal wall (Figure 1) with some discharge of turbid bloody fluid. Remainder of abdomen was nontender without organomegaly. Examinations of extremities, back and neurologic functions were normal.
Figure 1.
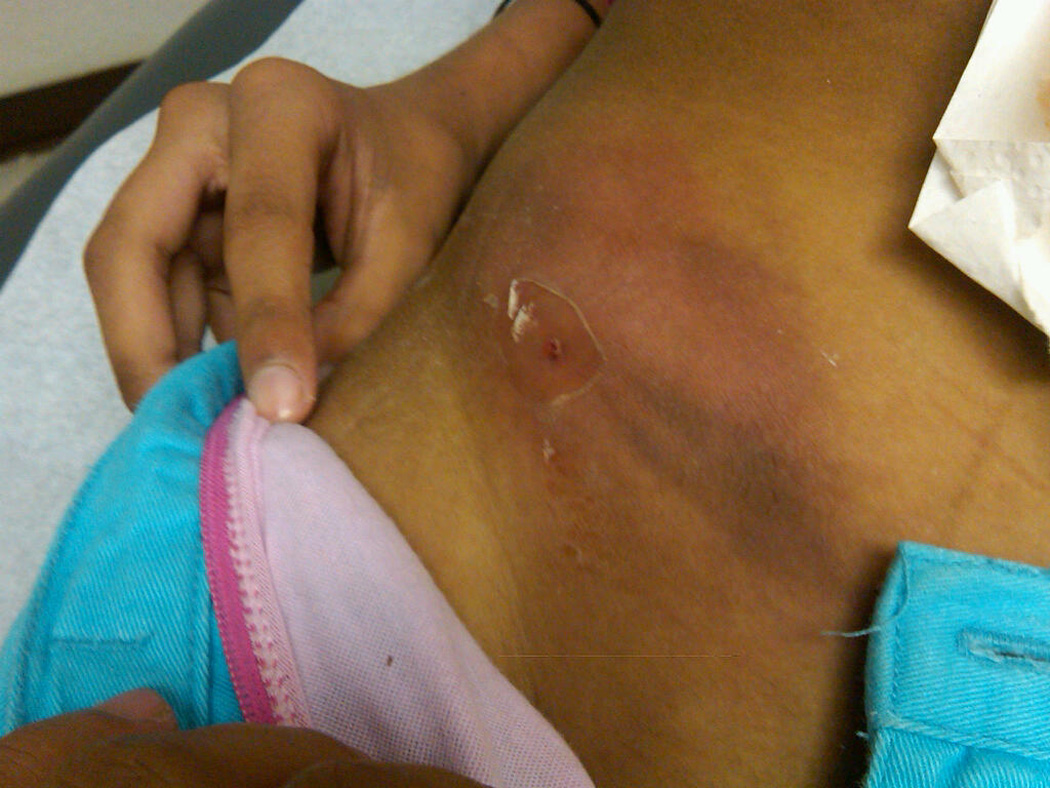
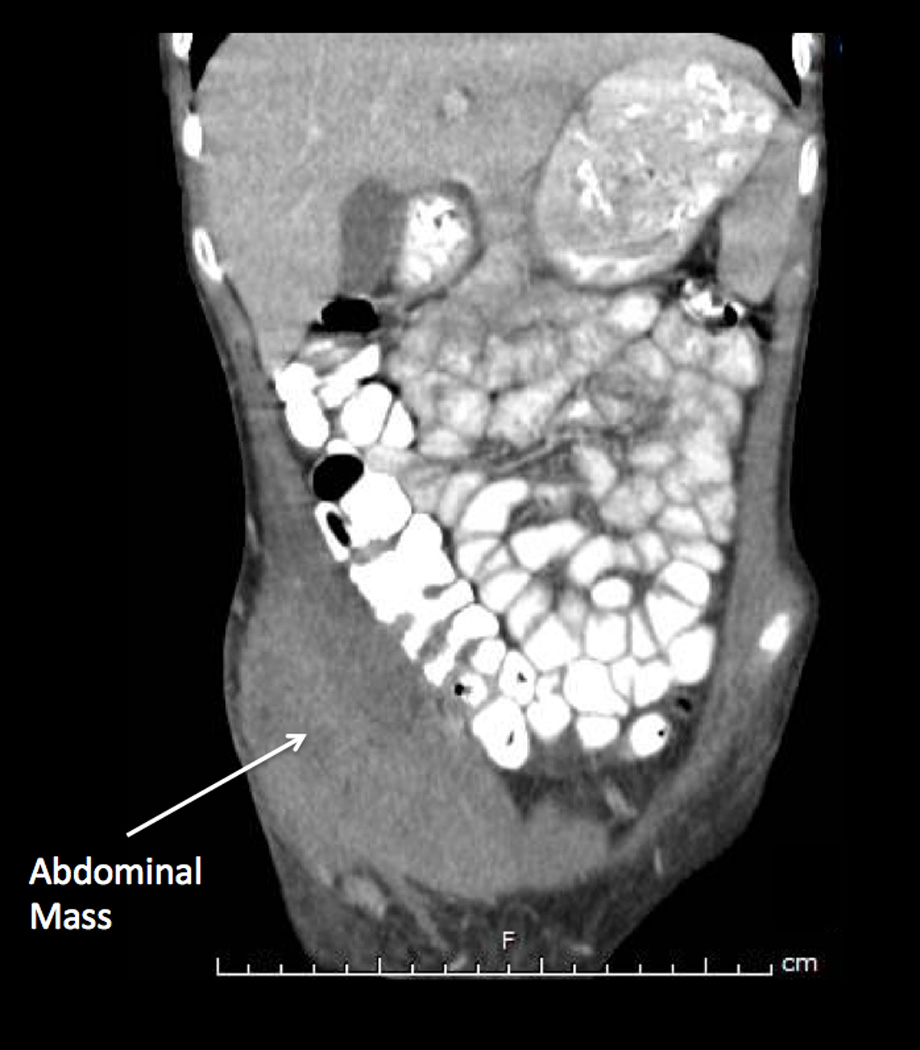
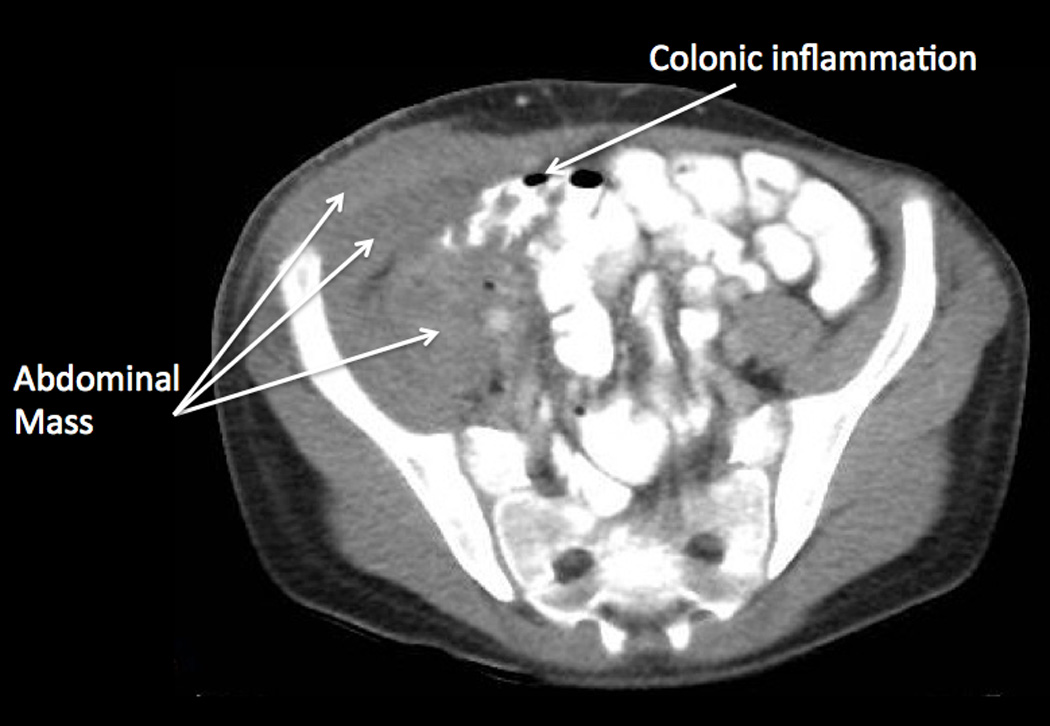
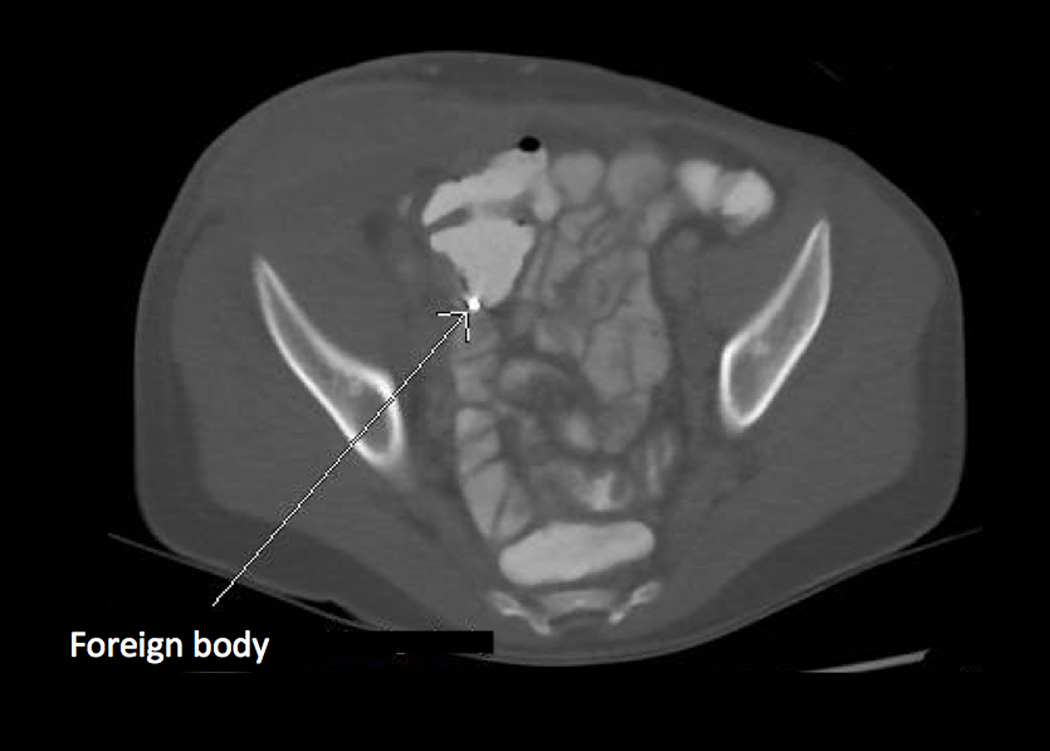
(a) Initial examination of right lower abdomen. (b and c) Initial CT scan showed a complex appearing heterogeneous mass involving the right abdominal wall with extension into the abdomen involving the right-sided iliacus and psoas muscles, and mild thickening of the cecum. (d) A small metallic foreign body was seen located within the lumen or wall of the cecum.
Laboratory evaluation upon hospital admission showed 8.87 × 103 white blood cells per µL (differential was not done), hemoglobin 11.1 g/dL, hematocrit 33.8%, and platelet count 624 × 103 per µL. Blood chemistry and liver panel were normal. C-reactive protein was 8.5 grams/dL. An abdominal ultrasound showed a 6.6 cm × 1.6 cm complex appearing collection mainly involving the right abdominal wall with sinus tract extending to the abdomen. A computed tomography (CT) (Figure 1) showed the heterogeneous right abdominal wall mass with intraabdominal extension into the right-sided iliacus and psoas muscles. There was also mild wall thickening of the ascending colon, cecum and appendix. A round small metallic foreign body was seen located within the lumen or wall of the cecum.
The patient was empirically treated with pipercillin-tazobactam and taken to the operating room for incision and drainage on hospital day (HD) 1. A moderate amount of purulent fluid was drained. The underlying muscle was indurated. Exploratory laparoscopy did not find any intestinal inflammation, perforation or trauma on visualization. A small hard object was palpated within the intestinal lumen at the location seen on the CT scan and left alone. The patient was taken back to the OR for wound packing change on HD2 and HD3 where the muscle was noted to remain remarkably rigid. A wound vacuum assisted closure (VAC) was placed.
Wound cultures from the initial incision and drainage grew few Actinomyces meyeri, moderate Fusobacterium nucleatum, and rare Clostridium perfringens after 2 days. Histological evaluation demonstrated classic sulfur granules consistent with actinomycosis (Figure 2). Antimicrobial therapy was narrowed to intravenous penicillin G. The patient was taken back to the operating room on HD 10. The wound was copiously irrigated. Non-viable tissue was removed. However, a portion of the infected but viable tissue was not debrided because full debridement of all involved tissues would entail resection of the patient’s psoas muscle as well as a large part of the patient’s abdominal wall and pelvis that would result in significant loss of function.
Figure 2.
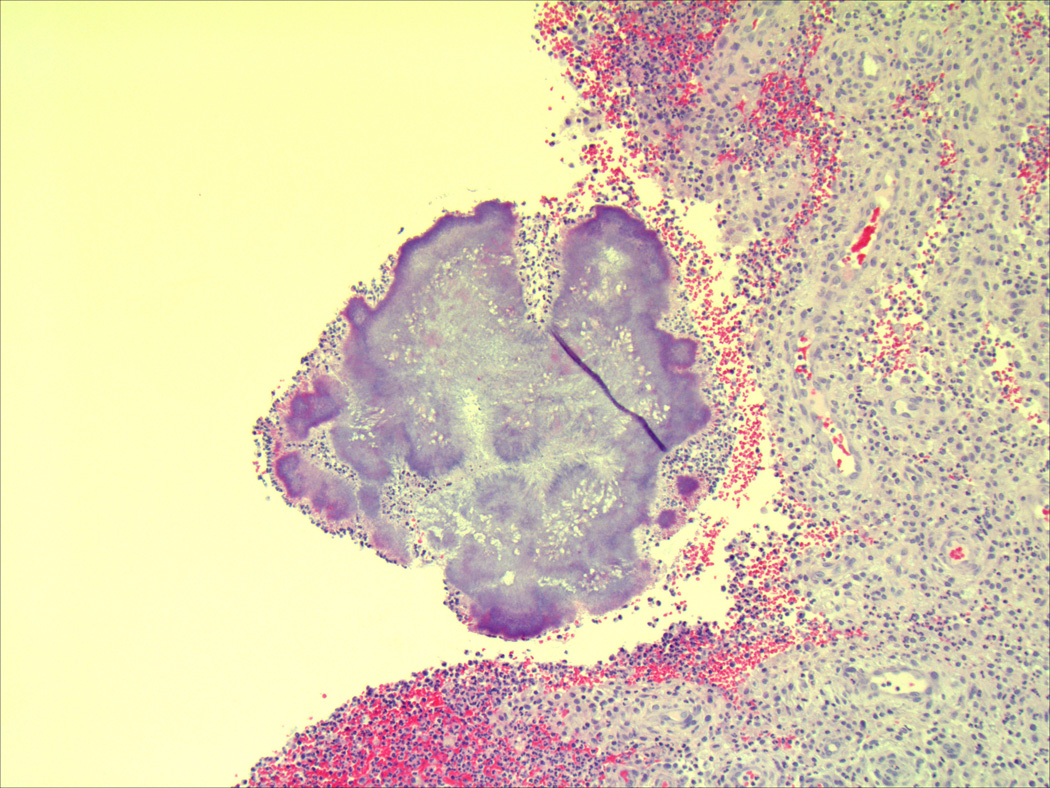
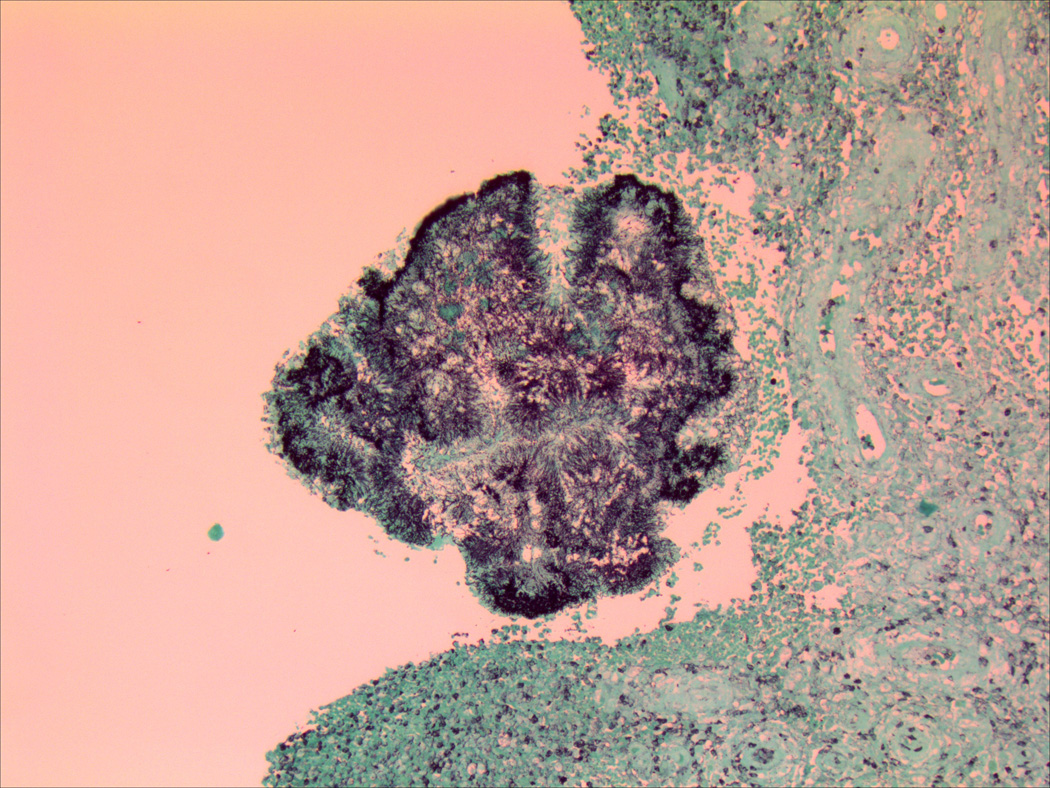
Histological evaluation of surgical specimens shows classic sulfur granules consistent with actinomycosis using (a) hematoxylin and eosin staining and (b) Gomori methenamine silver staining.
Patient was discharged with a PICC line on HD 27 on intravenous penicillin G at home. After 3 months of intravenous penicillin G, she completed an additional 11.5 months of oral penicillin. The child did not develop bone marrow suppression or other adverse effects associated with long-term penicillin therapy. MRI at the end of therapy showed complete resolution of previous infectious/inflammatory changes. Mild soft tissue prominence and enhancement remained which were thought to be secondary to chronic changes or granulation tissue. The patient has had no evidence of recurrence after 4 years of follow-up to date.
Materials and Methods
A literature search was conducted in Pubmed with search terms actinomycosis, Actinomyces, abdomen, abdominal, pediatrics, child and children in various combinations. The age of patients was limited to 0–18 years. Additional search was done using the references of identified literature. We limited the review to literature published in the English language. Only cases with positive culture for Actinomyces species or confirmed histology from a biopsy specimen or drainage were included.
Demographic and clinical data were summarized including patient age, sex, clinical presentation, microbiologic and/or histologic reports, surgical interventions and antimicrobial chemotherapy.
Results
Our literature search found 15 articles that reported 18 additional pediatric abdominal actinomycosis cases from 1964 to 2015. Nineteen cases including our patient have been described (Table 1) (1–15). Half (53%) occurred in females. The mean age of patients (years ± SD) was 11.9 ± 3.5 (11.3 ± 3.0 for boys and 12.5 ± 3.9 for girls).
Table 1.
Demographic and clinical characteristics from pediatric abdominal actinomycosis
| Reference | Year | Case* | Age | Sex | Actinomyces species | Co-pathogens | Histopathology |
|---|---|---|---|---|---|---|---|
| Pheils (1) | 1964 | 2/6 | 16 | Female | No growth | Gram positive bacteria with mycelia |
|
| 16 | Female | No growth | Sulfur granule | ||||
| Davies (7) | 1973 | 2/7 | 17 | Male | Actinomyces spp. | §Consistent | |
| 13 | Male | Actinomyces spp. | Consistent | ||||
| Hadary (2) | 1986 | 1/1 | 11 | Male | A. israelii, | Bacteroides spp. | Consistent |
| Shah (14) | 1987 | 1/4 | 8 | Male | Actinomyces spp. | Sulfur granule | |
| Benammar (4) | 1995 | 1/2 | 9 | Female | A. israelii |
Actinobacillus actinomycetemcomitans |
Consistent |
| Radhi (12) | 1997 | 1/1 | 7 | Male | No growth |
Actinobacillus actinomycetemcomitans |
Gram positive Filamentous organism |
| Schmidt (13) | 1999 | 1/1 | 15 | Female | No growth | Bacteroides fragilis | Sulfur granule |
| Campo (6) | 2001 | 2/2 | 11 | Male | No growth | Consistent | |
| 11 | Male | No growth |
Enterococcus faecalis, Klebsiella pneumoniae |
Beaded filamentous gram positive rods |
|||
| Wali (15) | 2002 | 1/1 | 10 | Male | A. israelii | Sulfur granule | |
| Buyukavci (5) | 2004 | 1/1 | 11 | Female | Actinomyces spp. | Sulfur granule | |
| Latawiec-Mazurkiewicz (3) | 2005 | 1/4 | 12 | Female | No growth | Consistent | |
| Guven (8) | 2007 | 1/1 | 5 | Female | No growth | Sulfur granule | |
| Karateke (11) | 2013 | 1/3 | 18 | Female | No growth | Sulfur granule | |
| Hirayama (9) | 2013 | 1/1 | 12 | Female | No growth | Consistent | |
| Karakus (10) | 2014 | 1/1 | 14 | Male | Culture not reported | Sulfur granule | |
| Our study | 2015 | 1/1 | 11 | Female | A. meyeri |
Fusobacterium nucleatum, Clostridium perfringens |
Sulfur granule |
Number of pediatric case aged 0–18yr/total reported case,
Histopathology “consistent with actinomycosis” is reported by the study’s authors without further description.
A.-Actinomyces, spp.-species
The most commonly described presentations among the 19 patients were abdominal pain in 15 (79%) and a lump/mass in 13 (68%) children. Additional presenting features included fever in 10 (53%), draining sinus in 7 (37%) and weight loss in 6 (32%) children. Past medical history prior to diagnosing actinomycosis was significant for appendectomy in 4 (21%) children. Dental caries were also noted in 2 (11%) patients including our case.
Time to diagnosis ranged from one day to 6 years and 11 months in the 17 cases with reported duration of symptoms. Twelve cases (70%) were diagnosed within 3 months, 3 cases (18%) were diagnosed within 4– 12 months and 2 cases (12%) took more than 1 year.
Surgical intervention was performed in 16 cases (84 %) including exploratory laparotomy (8 [50%]), sole appendectomy (3 [19%]), needle aspiration (2 [12%]), incision and drainage (1 [6%]), open biopsy (1 [6%]) and total excision (1 [6%]). Specimens for histopathology or culture were obtained by these interventions or spontaneous wound drainage. Actinomyces was identified on bacterial cultures from 8 (42%) patients, with consistent histopathology in 6 patients. Eleven (58%) were diagnosed by histopathology alone. Sulfur granule was the most commonly reported finding on histopathology (47%). Histopathological specimens from three patients (16%) had Gram positive bacteria consistent with Actinomyces. Seven (37%) reported “histopathology consistent with Actinomycosis” without a description.
Parenteral penicillin (intravenous or intramuscular) was the most common antibiotic used to treat actinomycosis. Fifteen (13 parenteral and 2 without a described route) received penicillin monotherapy. One received penicillin in combination with cefuroxime, 1 received ampicillin in combination with gentamicin, 1 received triple antibiotics (no detail given), and our patient was initially treated with intravenous piperacillin/tazobactam before being switched to intravenous penicillin. Two children who were allergic to penicillin were treated with either erythromycin or tetracycline. Duration of parenteral antibiotics varied from 3 weeks to 3 months in the 58% of cases who reported duration. Eight cases reported use of oral antibiotics ranging from 3 to 12 months after intravenous antibiotics. Oral penicillin was the most commonly used (62 %). Four cases were successfully treated without additional oral antibiotics. Seven cases did not report whether or not oral antibiotics followed intravenous therapy.
Discussion
Pediatric actinomycosis is a rare disease accounting for less than 3% of all reports of Actinomyces infections (3). The most affected areas in pediatrics are head and neck (50–60%), chest/lung (15–20%) and abdomen (20–22%) (3). Our review of the literature found only 19 reported pediatric abdominal actinomycosis cases including our patient described above. Abdominal actinomycosis usually occurs by invasion of perforated, breached or necrotic tissue. Risk factors include penetrating trauma, gut perforation, or recent surgery (16). Abdominopelvic actinomycosis also has been associated with presence of an intrauterine contraceptive device in women of childbearing age (8, 17, 18). Our patient did not have any definitive risk factor. We questioned the fall on a metal clasp shortly before this infection as a possible penetrating trauma source or the metallic object seen on CT scan as a risk factor from within the intestines but there was no evidence of intestinal wall perforation on surgical inspection.
Sung et al. (19) reported that the most common clinical presentations of abdominal actinomycosis in patients 6 to 75 years of age are abdominal pain (74%) and palpable mass (17.4%). Our review in children shows similar frequency of abdominal pain (79%) but a much higher number with palpable mass (68%) in children at presentation. Sinus drainage especially after surgical intervention or biopsy is a classic sign for diagnosis (7). Over one third (37%) of children had sinus drainage. Initial misdiagnoses of malignancy, appendicitis, tuberculosis, or ameboma are common (8, 16). Actinomycosis should be considered in the differential diagnosis of children presenting with abdominal pain, and palpable mass with sinus tract in addition to malignancy.
Although a positive culture for Actinomyces is the gold standard, growing Actinomyces may be difficult in the clinical setting. Culture for Actinomyces needs freshly obtained specimens, specific anaerobic transport medium, and longer duration for growth. Other studies have reported positive cultures ranging from 23% to 60% (19, 20). Our review in children demonstrated a positive culture in 42%.
Among the four Actinomyces that were speciated in our literature review, 3 were A. israelii. A. israelii is the most commonly isolated species for all forms of human actinomycosis. Interestingly, our patient had A. meyeri. A. meyeri, first identified in 1984 (20), is not uncommon (21). A. meyeri is also found as a colonizing organism of the mouth, esophagus, respiratory tract and genitourinary tract. It tends to cause more disseminated disease (22).
Actinomyces species are frequently found as co-pathogens with other anaerobic bacteria. Bartlett (23) reviewed a case series of thoracic actinomycosis and reported that the most common co-pathogen was Actinobacillus actinomycetemcomitans. Other anaerobic bacteria including Fusobacterium, Bacteroides, Eikenella and Peptostreptococcus were additional co-pathogens in thoracic actinomycosis (24). In our review of pediatric abdominal actinomycosis, we found that A. actinomycetemcomitans was a co-pathogen in only 10%. Bacteroides, Fusobacterium, Clostridia, Klebsiella, and Enterococcus species were additional co-pathogens.
Surgical intervention is important in the management of actinomycosis for diagnosis and treatment (19). Less than 10% of cases are diagnosed before surgery (14, 19). Furthermore, abdominal actinomycosis usually mimics malignant tumors that need to be differentiated (3, 9, 14, 15). Surgical treatment may be limited to incision and drainage for abscesses, but frequently more aggressive surgery to debride all infected tissue is required, especially for severely necrotic lesions or those that fail to resolve with antibiotics alone (7, 11).
Before the era of antibiotics, the outcome for abdominal actinomycosis was unfavorable (1). In the present day, several antibiotics can be used for the treatment of actinomycosis including penicillin, tetracyclines, erythromycin, clindamycin, imipenem, streptomycin, and cephalosporins. Actinomyces species are less susceptible to fluoroquinolones, fosfomycin, and other aminoglycosides (16). Penicillin is the drug of choice and most extensively studied. Dosing and duration of treatment depends on the severity of disease. Most experts recommend 4 of 6 weeks of intravenous penicillin followed by 6 to 12 months of oral therapy (24).
Acknowledgments
Funding Sources: P.S.P receives funding from NIH K23 HD072774-02 and AstraZeneca. J.M.B is supported by K12 HD 052954-09. N.W, L.W., D.B. and S.P. have nothing to disclose.
References
- 1.Pheils MT, Reid DJ, Ross CF. Abdominal Actinomycosis. Br J Surg. 1964;51:345–350. doi: 10.1002/bjs.1800510512. [DOI] [PubMed] [Google Scholar]
- 2.Hadary A, Lernau OZ, Nissan S. Abdominal actinomycosis. Z Kinderchir. 1986;41:239–240. doi: 10.1055/s-2008-1043350. [DOI] [PubMed] [Google Scholar]
- 3.Latawiec-Mazurkiewicz I, Juszkiewicz P, Pacanowski J, et al. Tumour-like inflammatory abdominal conditions in children. Eur J Pediatr Surg. 2005;15:38–43. doi: 10.1055/s-2004-830544. [DOI] [PubMed] [Google Scholar]
- 4.Benammar S, Hélardot PG, Sapin E, Adamsbaum C, Raymond J. Childhood actinomycosis: report of two cases. Eur J Pediatr Surg. 1995:180–183. doi: 10.1055/s-2008-1066200. [DOI] [PubMed] [Google Scholar]
- 5.Buyukavci M, Caner I, Eren S, Aktas O, Akdag R. A childhood case of primary hepatic actinomycosis presenting with cutaneous fistula. Scand J Infect Dis. 2004;36:62–63. doi: 10.1080/00365540310017492. [DOI] [PubMed] [Google Scholar]
- 6.Campo JM, Rubio TT, Churchill RB, Fisher RG. Abdominal actinomycosis with hydronephrosis in childhood. Pediatr Infect Dis J. 2001;20:901–903. doi: 10.1097/00006454-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Davies M, Keddie NC. Abdominal actinomycosis. Br J Surg. 1973;60:18–22. doi: 10.1002/bjs.1800600104. [DOI] [PubMed] [Google Scholar]
- 8.Guven A, Kesik V, Deveci MS, Ugurel MS, Ozturk H, Koseoglu V. Post varicella hepatic actinomycosis in a 5-year-old girl mimicking acute abdomen. Eur J Pediatr. 2008;167:1199–1201. doi: 10.1007/s00431-007-0639-0. [DOI] [PubMed] [Google Scholar]
- 9.Hirayama Y, Iinuma Y, Hashizume N, et al. Abdominal actinomycosis masquerading as an omental tumor in a 12-year-old female. J Infect Chemother. 2013;19:158–161. doi: 10.1007/s10156-012-0432-5. [DOI] [PubMed] [Google Scholar]
- 10.Karakus E, Mambet E, Azılı MN, Gülhan B, Tiryaki T, Tezer H. Actinomycosis of the appendix in childhood- an unusual cause of appendicitis. APSP J Case Rep. 2014;5:26. [PMC free article] [PubMed] [Google Scholar]
- 11.Karateke F, Ozyazıcı S, Menekşe E, Daş K, Ozdoğan M. Unusual presentations of actinomycosis anterior abdominal wall and appendix: report of three cases. Balkan Med J. 2013;30:315–317. doi: 10.5152/balkanmedj.2012.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radhi J, Hadjis N, Anderson L, Burbridge B, Ali K. Retroperitoneal actinomycosis masquerading as inflammatory pseudotumor. J Pediatr Surg. 1997;32:618–620. doi: 10.1016/s0022-3468(97)90721-1. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt P, Koltai JL, Weltzien A. Actinomycosis of the appendix in childhood. Pediatr Surg Int. 1999;15:63–65. doi: 10.1007/s003830050515. [DOI] [PubMed] [Google Scholar]
- 14.Shah HR, Williamson MR, Boyd CM, Balachandran S, Angtuaco TL, McConnell JR. CT findings in abdominal actinomycosis. J Comput Assist Tomogr. 1987;11:466–469. doi: 10.1097/00004728-198705000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Wali D, Sanchez J, Gilchrist B, Cash S, Anderson V, Ramenofsky M. Actinomycosis imitating an adrenal tumor. J Pediatr Surg. 2002;37:930–931. doi: 10.1053/jpsu.2002.32917. [DOI] [PubMed] [Google Scholar]
- 16.Smego RA, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255–1261. doi: 10.1086/516337. quiz 1262-1253. [DOI] [PubMed] [Google Scholar]
- 17.Lunca S, Bouras G, Romedea NS, Pertea M. Abdominal wall actinomycosis associated with prolonged use of an intrauterine device: a case report and review of the literature. Int Surg. 2005;90:236–240. [PubMed] [Google Scholar]
- 18.Fiorino AS. Intrauterine contraceptive device-associated actinomycotic abscess and Actinomyces detection on cervical smear. Obstet Gynecol. 1996;87:142–149. doi: 10.1016/0029-7844(95)00350-9. [DOI] [PubMed] [Google Scholar]
- 19.Sung HY, Lee IS, Kim SI, et al. Clinical features of abdominal actinomycosis: a 15-year experience of a single institute. J Korean Med Sci. 2011;26:932–937. doi: 10.3346/jkms.2011.26.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cato EP, Moore WEC, Nygaard G, Holdeman LV. Actinomyces meyeri sp. nov. Specific Epithet rev. Int J Syst Bacteriol. 1984;34:487–489. [Google Scholar]
- 21.Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183–197. doi: 10.2147/IDR.S39601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28:419–442. doi: 10.1128/CMR.00100-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett AH, Rivera AL, Krishnamurthy R, Baker CJ. Thoracic actinomycosis in children: case report and review of the literature. Pediatr Infect Dis J. 2008;27:165–169. doi: 10.1097/INF.0b013e3181598353. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics. Red Book: 2015 Report of the Committee on Infectious Diseases. 28th. Elk Grove Village, IL: American Academy of Pediatrics; 2015. [Google Scholar]


