Abstract
Fenretinide, a synthetic retinoid, induces apoptotic cell death in B-cell non-Hodgkin lymphoma (B-NHL) and acts synergistically with rituximab in preclinical models. We report results from a phase I–II study of fenretinide with rituximab for B-NHLs.
Eligible diagnoses included indolent B-NHL or mantle cell lymphoma. The phase I design de-escalated from fenretinide at 900 mg/m2 PO BID for days 1–5 of a 7-day cycle. The phase II portion added 375 mg/m2 IV rituximab weekly on weeks 5–9 then every 3 months. Fenretinide was continued until progression or intolerance.
Thirty-two patients were treated: 7 in phase I, and 25 in phase II of the trial. No dose-limiting toxicities were observed. The phase II component utilized fenretinide 900 mg/m2 twice daily with rituximab. The most common treatment-related adverse events of grade 3 or higher were rash (n = 3) and neutropenia (n = 3). Responses were seen in 6 (24%) patients on the phase II study, with a median duration of response of 47 months (95% confidence interval, 2 – 56).
The combination of fenretinide and rituximab was well tolerated, yielded a modest overall response rate, but with prolonged remission durations. Further study should focus on identifying the responsive subset of B-NHL.
Keywords: fenretinide, 4HPR, retinoids, B cell lymphoma, indolent lymphoma
Background
Indolent B-cell non-Hodgkin lymphoma (B-NHL), chronic lymphocytic leukaemia (CLL) and mantle cell lymphoma (MCL) are together among the most common types of incurable B-cell malignancies. Rituximab, a chimeric mouse anti-human CD20 monoclonal antibody, is an essential component of treatment for CD20-expressing B-NHLs, and has a diverse repertoire of actions, including induction of apoptosis, activation of antibody-dependent cellular cytotoxicity, and complement-dependent cytotoxicity (Keating, 2010). Retinoids are a class of compounds both synthetic and natural which are analogues of Vitamin A. The retinoid acid and retinoid X receptors are two classes of receptors that these compounds act through (Barna et al., 2005). N-(4-hydroxyphenyl) retinamide (4-HPR), also known as fenretinide, is a retinoid that has shown both anti-proliferative and pro-apoptotic in pre-clinical studies (Faderl et al., 2003, Lovat et al., 2000, Pergolizzi et al., 1999, Shan et al., 2001).
Our group has demonstrated that fenretinide alone, at concentrations as low as 2µM, could induce apoptosis in B-NHL cell lines and generate synergistic killing of malignant B-cells in combination with rituximab (Shan et al., 2001). These data were corroborated by human xenograft models demonstrating supra-additive tumour control and improved survival with concurrent rituximab and fenretinide over the individual single agents and that this effect was mediated by caspase induction and apoptosis (Gopal et al., 2004). Prior studies have used a dose of 900 mg/m2 twice daily in oral formulation, which has proven to be tolerable and results in potentially efficacious peak serum levels (Cheung et al., 2009, William et al., 2009). In this translational study, we dosed fenretinide at 900 mg/m2 twice daily for 5 days per week, with a 2-day drug holiday, for 4 weeks mirroring the regimen used in the in-vivo preclinical studies. We also hypothesized that the 2-day drug holiday would allow endogenous retinol levels to partially recover, potentially reducing nyctalopia. We then combined this regimen with rituximab to evaluate its safety and potential efficacy for treating patients with B-NHLs. We tested this hypothesis in a phase I/II clinical trial of oral fenretinide plus rituximab.
Methods
Patient Eligibility
Inclusion criteria for this study were a diagnosis of a CD20+ B-NHL, either newly diagnosed, or previously treated. Other eligibility criteria were age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, and a pre-treatment serum bilirubin and creatinine < 2 times the upper limit of normal. Measureable disease was required, defined as lesions that can be measured in two dimensions by conventional imaging and a greatest transverse diameter of ≥ 1 cm.
Study Design and Treatment
This open-label, phase I/II study was conducted at the Fred Hutchinson Cancer Research Center, the University of Washington, and Seattle Cancer Care Alliance, Seattle, WA (ClinicalTrials.gov identifier: NCT00288067). Patients were recruited from October 2005 until October 2009. Primary data analysis was performed in June 2015. The study was approved by the institutional review board at Fred Hutchinson Cancer Research Center. Written informed consent was obtained in accordance with the Declaration of Helsinki.
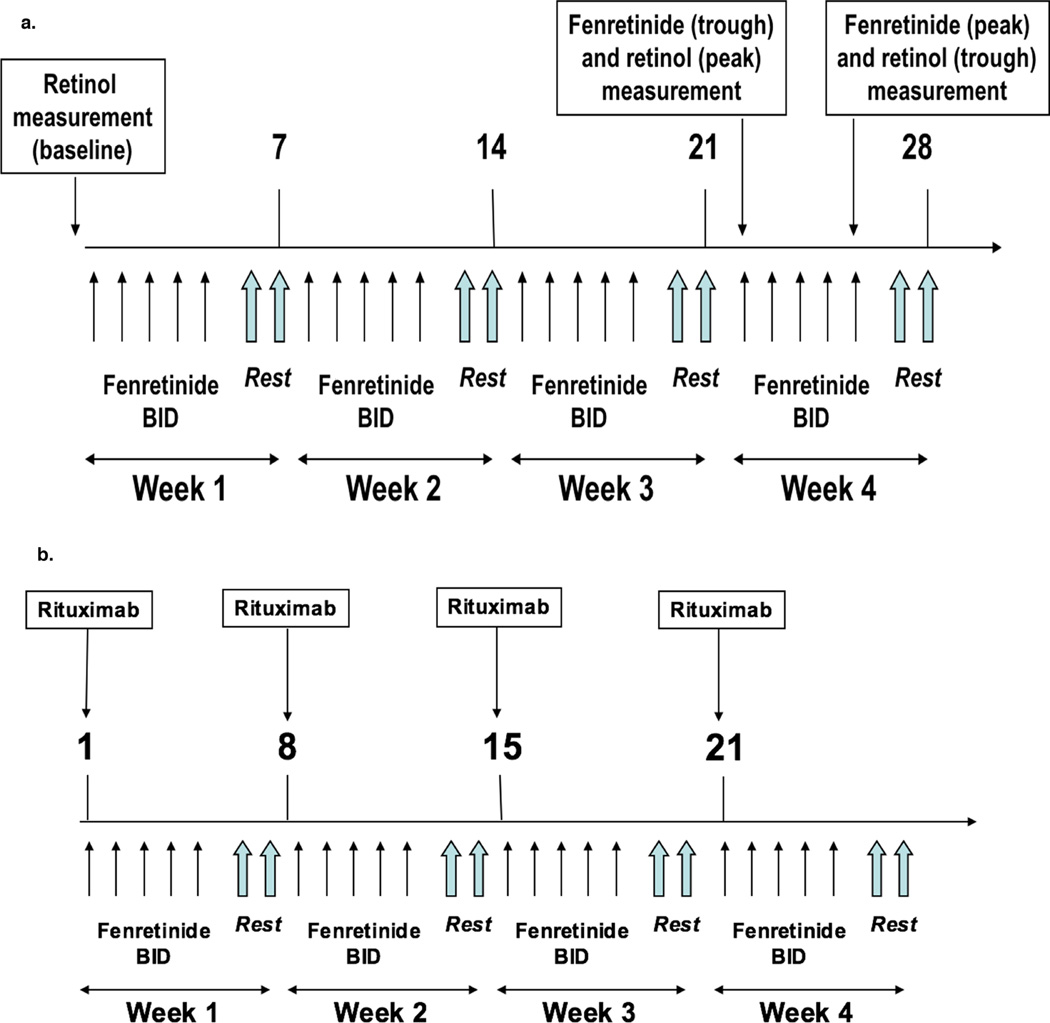
The phase I component utilized a dose de-escalation design in cohorts of 6 patients starting at the maximal dose of 900 mg/m2 by mouth twice daily, on days 1 to 5 followed by a 2-day drug holiday (Figure 1a). Reduction in the dose level to 700 mg/m2 was required in the next cohort if 2 or more of the 6 patients experienced a dose limiting toxicity (DLT) within the first 4 weeks of therapy. A DLT was defined as any related toxicity of grade 4 or 5 on or before the completion of 4 weeks of therapy. Patients without DLTs or disease progression were allowed to continue beyond 4 weeks until disease progression, toxicity or voluntary withdrawal.
Figure 1. Study schema for phase I portion (1a), and phase II portion (1b).
Patients in the phase II cohort expansion portion were treated at the dose level derived from phase I with the identical 5 of 7-day schedule. In addition, rituximab was administered at a dose of 375 mg/m2 intravenously, weekly for 4 weeks, starting at week 5 followed by a single maintenance infusion of 375 mg/m2 rituximab every 3 months (Figure 1b). Treatment was continued until unacceptable toxicity, disease progression, voluntary withdrawal or cessation of drug supply.
Study Assessments
Baseline evaluations included history and physical examination, complete blood count with differential, comprehensive metabolic panel, peripheral blood flow cytometry, molecular studies for immunoglobulin heavy chain clonality, and bone marrow aspirate and biopsy. Positron emission tomography-computed tomography (PET-CT) and contrast-enhanced CT were obtained, followed by PET-CT and contrast-enhanced CT scans at week 5, month 3, and every 6 months thereafter. Clinical response was determined by the principal investigators of the study per the International Response Criteria for Non-Hodgkin Lymphomas and the National Cancer Institute-Sponsored Working Group Guidelines for CLL (Cheson et al., 1996, Cheson et al., 1999). Correlative studies included apoptosis markers from blood and bone marrow.
Assessments of apoptosis markers were obtained at weeks 1, 2, 3, 4/5, and months 2 and 3, and then every 3 months thereafter. Samples were screened for residual disease using a routine clinical flow cytometric assay; this allowed identification of informative reagents for gating of the neoplastic population, typically CD19, CD20, CD5 and/or CD10. These gating reagents were combined with Annexin V and Caspase 3 to assess early and late apoptosis of the neoplastic cells using standard flow cytometric methods. In addition, the ratio of the pro-apoptotic and anti-apoptotic molecules BAX and BCL2 were assessed on the neoplastic cells using a standard fixation and permeabilization procedure (Fix & Perm; Invitrogen, Carlsbad, CA, USA).
High performance liquid chromatography measurements of plasma retinol and fenretinide were obtained at baseline and before and after the 2-day weekly drug holiday to reflect peak and trough levels. Long-term pharmacokinetic data, collected at 3-month intervals post-month 3, were also obtained.
Statistical Analysis
The primary endpoint of the phase I study was to evaluate the safety of fenretinide delivered in a 5 of 7-day regimen. Secondary endpoints included pharmacokinetic studies, determination of the intratumoural concentrations of fenretinide, evaluation of the in vivo mechanism of action of fenretinide, identification of predictors of response to fenretinide and estimation of response rates.
The primary endpoint of the phase II study was to estimate the overall response rate (ORR) of fenretinide and rituximab in patients with B-NHL. Secondary endpoints included overall survival (OS), progression-free survival (PFS), duration of response, pharmacokinetic studies, intratumoural concentrations of fenretinide, identification of predictors of response and evaluation of the in vivo mechanism of action of fenretinide. PFS was defined as the time from the start of study treatment to progression, or to death as a result of any cause. OS was defined as the time from start of study treatment until date of death from any cause. Duration of response was defined as the time from the first documented objective response until progression or death.
The ORR per the independent review was calculated. Response-evaluable patients must have received at least 4 weeks of fenretinide for the phase I study, and 8 weeks for the phase II study, unless discontinuation was due to progressive disease. Median and two-sided 95% confidence intervals (CIs) were estimated for OS, PFS and duration of response. Kaplan-Meier curves were created for OS and PFS.
Results
Patients
Patient characteristics are summarized in Table 1. Thirty-two patients enrolled in the study; 7 patients enrolled in the phase I, and 25 patients enrolled in the phase II study. The median age of patients was 64 years (range, 40 to 78 years). The majority of the patients were men, 26 patients (81%), with 6 women (19%). Patients had received a median of 1 prior regimen and 69% had received prior rituximab with 8 suffering rituximab-refractory disease. The most common histology present was CLL/small lymphocytic lymphoma (SLL) in 13 patients (41%). Other histologies were follicular lymphoma (FL) in 10 patients (31%), MCL in 7 patients (22%), lymphoplasmacytic lymphoma in 1 patient (3%), and mantle zone lymphoma (MZL) in 1 patient (3%).
Table I.
Demographics and patient characteristics
| Characteristics | Patients, |
|---|---|
| Male (%) | 26 (81) |
| Median age (range) | 64 (40 – 78) |
| Median number of prior chemotherapy regimens (range) | 1 (0 – 1) |
| CD20 positive (%) | 31 (97) |
| Prior hematopoietic stem cell transplantation (%) | 4 (13) |
| Eastern Cooperative Oncology Group Performance Status (%) | |
| 0 | 26 (81) |
| 1 | 5 (16) |
| 2 | 1 (3) |
| Median number of extranodal sites (range) | 1 (1 – 3) |
| Prior rituximab (%) | 22 (69) |
| Histology (%) | |
| Chronic lymphocytic leukaemia/small lymphocytic lymphoma | 13 (41) |
| Follicular lymphoma | 10 (31) |
| Mantle cell lymphoma | 7 (22) |
| Lymphoplasmacytic lymphoma | 1 (3) |
| Marginal zone lymphoma | 1 (3) |
In the phase I study, the dose chosen was 900 mg/m2 twice daily; this dose was moved forward to the phase II study.
Treatment received
For the phase I component, patients were treated for a median of 0.9 months (range, 0.3 – 9.4 months). The median number of cycles administered was 1 (range, 1 – 4); 1 patient discontinued due to a drug eruption after 1 cycle of treatment. In the phase II study, patients were treated for a median of 6.8 months (range, 0.2 – 54.3 months) and received a median 3 cycles of treatment (range, 1 – 18). A total of 3 patients discontinued drug on the phase II study, due to photosensitivity in 1, drug rash in 1, and gastrointestinal distress in the final patient. Following completion of the study, 5 patients (16%) remained on rituximab on a maintenance schedule.
Safety Profile
The most common treatment-related adverse events of any grade were reversible nyctalopia (56%), other eye disorders (53%), rash (38%), decreased lymphocyte count (25%), photosensitivity (25%), diarrhoea (22%), decreased platelet count (22%) and decreased neutrophil count (19%) (Table II). A total of 3 patients (9%) on study discontinued treatment early due to toxicity from therapy. In 2 of these patients, discontinuation occurred during the first week of therapy due to development of a diffuse maculo-papular eruption; in both patients, the rash abated following cessation of therapy. One patient discontinued treatment on day 140 due to gastrointestinal symptoms, weakness and fatigue.
Table II.
Adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) v. 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf)
| Adverse Event | Events related to fenretinide and rituximab (any grade) |
Grade 3 Events | Grade 4 Events | |||
|---|---|---|---|---|---|---|
| Patients (n) | % | Patients (n) | % | Patients (n) | % | |
| Night blindness | 18 | 56 | 1 | 3 | 0 | 0 |
| Eye disorders, other * | 17 | 53 | 0 | 0 | 0 | 0 |
| Maculopapular rash | 12 | 38 | 3 | 9 | 0 | 0 |
| Lymphocyte count decreased | 8 | 25 | 1 | 3 | 0 | 0 |
| Photosensitivity | 8 | 25 | 0 | 0 | 0 | 0 |
| Diarrhoea | 7 | 22 | 1 | 3 | 0 | 0 |
| Platelet count decreased | 7 | 22 | 0 | 0 | 0 | 0 |
| Neutrophil count decreased | 6 | 19 | 1 | 3 | 2 | 6 |
| White blood cell decreased | 6 | 19 | 0 | 0 | 1 | 3 |
| Anaemia | 5 | 16 | 0 | 0 | 0 | 0 |
| Aspartate transaminase increased | 5 | 16 | 0 | 0 | 0 | 0 |
| Hypocellular bone marrow | 5 | 16 | 1 | 3 | 0 | 0 |
| Dry skin | 5 | 16 | 0 | 0 | 0 | 0 |
Yellowing/discoloration of lights, altered colour perception, peripheral vision changes, blind spot in visual field
Twenty-two patients (69%) experienced eye disorders of any grade. Of these patients, 18 experienced nyctalopia (56%). Of these 18 patients, 3 had severe enough night vision impairment to impact their activities of daily living and 7 required either dose reduction or brief discontinuation of treatment due to nyctalopia. All patients who required either dose reduction or brief treatment holidays had improvement in nyctalopia. Descriptions of night vision impairment ranged from mild changes, to more moderate difficulty with navigation at night.
Three patients (9%) on this study had Grade 3 related dermatological eruptions while on treatment. In all three patients, this was described as a morbilliform drug eruption. In 2 patients, the eruption appeared within 2 weeks of commencing treatment with fenretinide. In 1 patient, the reaction was severe enough to require systemic steroids and hospital admission.
Efficacy
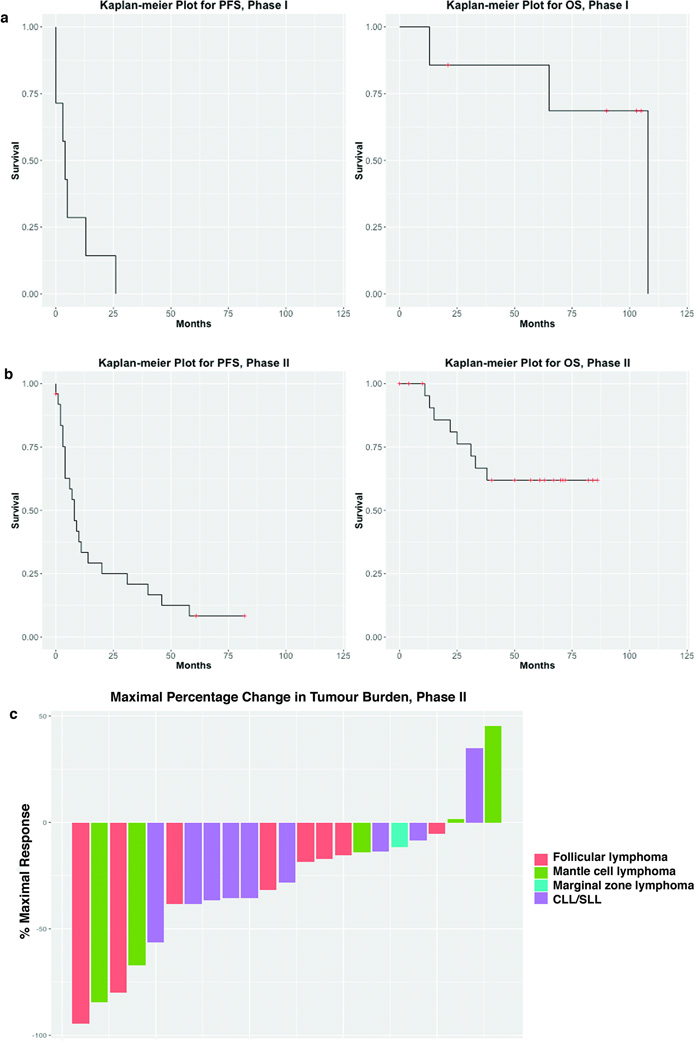
The overall disease control rate (complete response + partial response + stable disease for ≥ 6 months) for all patients on study was 56%, with a median duration of disease control of 15 months (range, 6– 58 months). Documented tumour reductions occurred in 91.7% of patients on the phase II study (Figure 2c).
Figure 2. Kaplan-Meier plots for PFS and OS for phase I patients (2a) and phase II patients (2b). Waterfall plot for phase II patients (2c).
PFS, progression-free survival; OS, overall survival; CLL/SLL, Chronic lymphocytic leukaemia/small lymphocytic lymphoma
No patient had a response in the phase I study, with a median PFS of 4 months (95% CI, 0 to not reached), and a median OS was 108 months (95% CI, 65 to not reached) (Figure 2a). Two patients in the phase I portion had MCL; one patient experienced stable disease (SD) lasting 35 months (having had a 6-month prior remission to rituximab monotherapy), and the other had SD lasting 25 months, with a 37.3% tumour reduction, the most marked reduction of the group (prior receipt of 4 lines of therapy; most recent being bortezomib, with a 5-month response duration). Of the 3 patients with CLL/SLL, 1 had SD lasting 12 months, and 2 either did not respond or had SD lasting less than 1 month. Two patients had FL; 1 discontinued drug early due to toxicity, and the other had early progression after less than 1 month of treatment.
In the phase II component, 6 (24%) patient’s disease responded (Figure 2b), with 2 (8%) achieving complete response (CR) (1 FL, 1 MCL) and 4 (16%) achieving partial response (PR) (2 CLL/SLL, 1 FL, 1 MCL). In addition, 16 (64%) patients had SD. The median PFS was 8 months (95% CI, 4 – 31 months), and the median OS was not reached (95% CI, 33 months to not reached) (Figure 2b). Median duration of response was 47 months (95% CI, 2 – 56). The median PFS for MCL (n = 5) was 8 months, for FL (n = 8) was 17 months, for MZL (n = 1) was 7 months, and for CLL/SLL (n = 10) was 4 months. The median PFS for the 2 patients who achieved a CR was 59.5 months.
This study closed at the end of 2012 when the National Cancer Institute ended provision of fenretinide. At that time, 4 patients, all of whom had an objective response, were on continuous fenretinide and rituximab without disease progression, and had to stop therapy on study. Of these patients, one had MCL, with a 56-month duration of response after a CR, who continued on maintenance rituximab alone with no disease progression to date. Another patient with FL, who also had a CR, had a 50-month duration of response, continued on rituximab maintenance after discontinuation of fenretinide, but experienced disease progression 12 months later and started chlorambucil and rituximab. After a 58-month duration of response, a patient with FL who had a PR completed 3 additional cycles of maintenance rituximab and remains progression-free to date. A final patient with MCL with a PR, had a 44-month duration of response and continued on maintenance rituximab after discontinuation of fenretinide. He progressed 2 years later and has started ibrutinib.
Two other patients had an objective response. One patient with CLL/SLL who had a PR, sustained their response for 38 months before developing progressive disease and receiving subsequent therapy. Another patient with CLL/SLL, who had an initial PR to treatment, developed progressive disease within 2 months and went on to receive subsequent treatment with bendamustine, mitoxantrone and rituximab.
The majority of patients received more than 1 prior line of therapy. Only 1 patient was previously treated with rituximab monotherapy. This patient had FL, and had a 3-year response to rituximab as a single agent; in contrast, there was only a 12-month duration of response with fenretinide and rituximab on study.
Pharmacokinetic Studies
The pharmacokinetic studies are depicted in Table III. The median peak (pre-drug holiday) and trough (post-drug holiday) plasma fenretinide values were 12.9 µM (range, 0.7 to 33.5 µM) and 2.6 µM (range, 0.0 to 6.9 µM), respectively. Unlike other retinoids, there did not appear to be induced catabolism of fenretinide as concentrations did not appreciably change over time. Fenretinide concentrations within the bone marrow consistently exceeded that of the plasma at the same time points. We evaluated plasma retinol levels as a potential pharmacodynamic marker of toxicity. The median baseline plasma retinol was 5.5 µM (range, 1.7 – 15.3 µM) and predictably dropped to a median of 0.5 µM (range, 0.0 to 2.3 µM) at the first measured pre-drug holiday time point. Plasma retinol levels partially recovered following the 2-day drug holiday to a median of 1.3 µM (0.0 – 3.1µM).
Table III.
Pharmacokinetic Studies
| Sample* | Pre-treatment | Month 1 | Month 2 | Month 5 | Month 8 | Month 12 |
|---|---|---|---|---|---|---|
| Retinol | ||||||
| Peak | 1.4 (0.5 – 4.7), n = 21 |
1.3 (0.0 – 3.1), n = 17 |
1.2 (0.4 – 2.9), n = 10 |
1.4 (0.6 – 2.3), n = 7 |
1.2 (0.0 – 15.6), n = 5 |
|
| Trough | 0.5 (0.0 – 2.3), n = 18 |
0.5 (0.0 – 2.0), n = 21 |
||||
| Plasma fenretinide | ||||||
| Peak | 12.9 (0.7 – 33.5), n = 18 |
14.9 (0.3 – 26.1), n = 21 |
||||
| Trough | 2.6 (0.0 – 6.9), n = 7 |
3.4 (0.3 – 10.5), n = 17 |
3.4 (0.6 – 7.7), n = 10 |
3.9 (0.3 – 9.5), n = 7 |
3.3 (1.0 – 26.4), n = 6 |
|
| Trough bone marrow fenretinide |
5.2 (0.0 – 14.9), n = 14 |
4.8 (2.0 – 12.6), n = 9 |
2.5 (1.7 – 15.4), n = 7 |
6.2 (1.3 – 21.5), n = 5 |
All samples are the median value in µM, (range), with n denoting the number of subjects with available testing
Apoptosis studies
In patients who had evidence of circulating tumour cells (n = 13), we assessed the impact of fenretinide and rituximab on flow cytometrically quantified BCL2 and BAX expression, as well as annexin V as a measure of apoptosis. There was no correlation between BCL2 expression, BAX expression, BAX to BCL2 ratios or annexin V expression and clinical response or toxicity.
Discussion
This phase I/II study was the first to evaluate the combination of fenretinide and rituximab and demonstrated low response rates in B-NHLs; it was well tolerated with predictable, reversible toxicities with up to 4.5 years of continuous therapy. In contrast to the most widely used therapeutic retinoid in oncology, all-trans retinoic acid (ATRA), we have shown that fenretinide in an oral formulation has a stable pharmacokinetic profile. Early studies of ATRA in patients with acute promyelocytic leukaemia demonstrated diminishing plasma concentrations of drug within 1–2 weeks of commencing continuous oral therapy (Adamson, 1994, Adamson et al., 1995, Muindi et al., 1992). In our study, the results of pharmacokinetic studies for up to 12 months post-initiation of treatment document consistent plasma concentrations of fenretinide with oral dosing. We were also able to show higher concentrations of fenretinide within bone marrow as compared to the plasma, potentially explained by the high-lipid content of the marrow space.
In keeping with prior studies, the toxicity profile of fenretinide was tolerable and reversible. The principal toxicity associated with fenretinide, nyctalopia, has been reported in prior early-phase studies of fenretinide (Cheung et al., 2009, Maurer et al., 2013, Moore et al., 2010, Vaishampayan et al., 2005). As with previous observations of nyctalopia from fenretinide, all cases in our study were reversible, and none discontinued therapy permanently due to night blindness. The nyctalopia which results from fenretinide has been hypothesized to be secondary to reduced retinol levels in the blood, which can result in decreased visual pigments in the retina which are responsible for night vision (Formelli et al., 1989). Our strategy of a drug holiday demonstrated a partial recovery of retinol levels without significantly compromising fenretinide concentrations.
Despite promising results in one preventive trial in breast cancer using fenretinide, the majority of clinical trials evaluating fenretinide for cancer treatment have not shown a significant anti-tumour effect (Cheung et al., 2009, Puduvalli et al., 2004, Schneider et al., 2009, Vaishampayan et al., 2005). Given the high dose requirements necessary to achieve therapeutic levels of fenretinide, and the burden on the patient to take so many pills every day, one potential hypothesis for lack of efficacy might be therapeutic drug levels were not achieved. However, we performed correlative pharmacokinetic studies demonstrating that trough fenretinide levels from bone marrow and plasma that had previously been shown to be efficacious in induction of apoptosis in preclinical studies were present (Gopal et al., 2004, Shan et al., 2001). Induction of free radicals is another potential mechanism for the pro-apoptotic effect of fenretinide; potentially, the limited clinical effect in patients with B-NHL is the relative hypoxia of lymph nodes (Delia et al., 1997).
In summary, in this phase I/II study of fenretinide and rituximab in patients with B-NHLs, we found that this combination was tolerable for long-term use and yielded modest response rates. Many patients experienced prolonged disease control comparable to approved agents for indolent B-NHL, but the contribution of fenretinide beyond that provided by rituximab cannot be ascertained by this study design Future studies with fenretinide in lymphoma may require novel formulations, such as nanoparticulate or liposomal drug delivery systems, as well as identification of optimal combinations to further augment the proapoptotic effect of this agent (Pignatta et al., 2014, Sabnis et al., 2013).
Key Message.
Fenretinide is an orally bioavailable retinoid compound shown to act synergistically with rituximab in translational models. We conducted a phase I/II study of fenretinide and rituximab in patients with indolent B-cell non-Hodgkin lymphoma. The combination was well tolerated with predictable and reversible toxicities, and modest response rates. Further study should focus on identifying optimal drug combinations to augment these anti-tumour effects.
Acknowledgments
Research Support:
This work was supported by research funding from National Institutes of Health [grant numbers P01CA044991, 5T32CA009515-30 to A.J.C., K24CA184039, and R21CA119519]; the Cancer Therapy Evaluation Program Translational Research Initiative; a grant from CLL Topics; the Mary Wright Memorial Fund; and philanthropic gifts from Frank and Betty Vandermeer and Don and Debbie Hunkins.
A.J.C. analysed the data, and drafted the manuscript. P.S. analysed the data. A.K.G. designed the research study, conducted the clinical trial, and contributed to drafting of the manuscript. All the remaining authors contributed significantly to drafting of the manuscript.
Footnotes
Disclosure: The authors have declared no conflicts of interest.
References
- Adamson PC. Pharmacokinetics of all-trans-retinoic acid: clinical implications in acute promyelocytic leukemia. Semin Hematol. 1994;31:14–17. [PubMed] [Google Scholar]
- Adamson PC, Bailey J, Pluda J, Poplack DG, Bauza S, Murphy RF, Yarchoan R, Balis FM. Pharmacokinetics of all-trans-retinoic acid administered on an intermittent schedule. J Clin Oncol. 1995;13:1238–1241. doi: 10.1200/JCO.1995.13.5.1238. [DOI] [PubMed] [Google Scholar]
- Barna G, Sebestyen A, Weischede S, Petak I, Mihalik R, Formelli F, Kopper L. Different ways to induce apoptosis by fenretinide and all-trans-retinoic acid in human B lymphoma cells. Anticancer Res. 2005;25:4179–4185. [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Cheung E, Pinski J, Dorff T, Groshen S, Quinn DI, Reynolds CP, Maurer BJ, Lara PN, JR, Tsao-Wei DD, Twardowski P, Chatta G, Mcnamara M, Gandara DR. Oral fenretinide in biochemically recurrent prostate cancer: a California cancer consortium phase II trial. Clin Genitourin Cancer. 2009;7:43–50. doi: 10.3816/CGC.2009.n.008. [DOI] [PubMed] [Google Scholar]
- Delia D, Aiello A, Meroni L, Nicolini M, Reed JC, Pierotti MA. Role of antioxidants and intracellular free radicals in retinamide-induced cell death. Carcinogenesis. 1997;18:943–948. doi: 10.1093/carcin/18.5.943. [DOI] [PubMed] [Google Scholar]
- Faderl S, Lotan R, Kantarjian HM, Harris D, Van Q, Estrov Z. N-(4-Hydroxylphenyl)retinamide (fenretinide, 4-HPR), a retinoid compound with antileukemic and proapoptotic activity in acute lymphoblastic leukemia (ALL) Leuk Res. 2003;27:259–266. doi: 10.1016/s0145-2126(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Formelli F, Carsana R, Costa A, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M. Plasma retinol level reduction by the synthetic retinoid fenretinide: a one year follow-up study of breast cancer patients. Cancer Res. 1989;49:6149–6152. [PubMed] [Google Scholar]
- Gopal AK, Pagel JM, Hedin N, Press OW. Fenretinide enhances rituximab-induced cytotoxicity against B-cell lymphoma xenografts through a caspase-dependent mechanism. Blood. 2004;103:3516–3520. doi: 10.1182/blood-2003-08-2795. [DOI] [PubMed] [Google Scholar]
- Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70:1445–1476. doi: 10.2165/11201110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lovat PE, Ranalli M, Annichiarrico-Petruzzelli M, Bernassola F, Piacentini M, Malcolm AJ, Pearson AD, Melino G, Redfern CP. Effector mechanisms of fenretinide-induced apoptosis in neuroblastoma. Exp Cell Res. 2000;260:50–60. doi: 10.1006/excr.2000.4988. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Kang MH, Villablanca JG, Janeba J, Groshen S, Matthay KK, Sondel PM, Maris JM, Jackson HA, Goodarzian F, Shimada H, Czarnecki S, Hasenauer B, Reynolds CP, Marachelian A. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60:1801–1808. doi: 10.1002/pbc.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MM, Stockler M, Lim R, Mok TS, Millward M, Boyer MJ. A phase II study of fenretinide in patients with hormone refractory prostate cancer: a trial of the Cancer Therapeutics Research Group. Cancer Chemother Pharmacol. 2010;66:845–850. doi: 10.1007/s00280-009-1228-x. [DOI] [PubMed] [Google Scholar]
- Muindi JR, Frankel SR, Huselton C, Degrazia F, Garland WA, Young CW, Warrell RP., JR Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res. 1992;52:2138–2142. [PubMed] [Google Scholar]
- Pergolizzi R, Appierto V, Crosti M, Cavadini E, Cleris L, Guffanti A, Formelli F. Role of retinoic acid receptor overexpression in sensitivity to fenretinide and tumorigenicity of human ovarian carcinoma cells. Int J Cancer. 1999;81:829–834. doi: 10.1002/(sici)1097-0215(19990531)81:5<829::aid-ijc26>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pignatta S, Orienti I, Falconi M, Teti G, Arienti C, Medri L, Zanoni M, Carloni S, Zoli W, Amadori D, Tesei A. Albumin nanocapsules containing fenretinide: pre-clinical evaluation of cytotoxic activity in experimental models of human non-small cell lung cancer. Nanomedicine. 2014;11:263–273. doi: 10.1016/j.nano.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Puduvalli VK, Yung WK, Hess KR, Kuhn JG, Groves MD, Levin VA, Zwiebel J, Chang SM, Cloughesy TF, Junck L, Wen P, Lieberman F, Conrad CA, Gilbert MR, Meyers CA, Liu V, Mehta MP, Nicholas MK, Prados M. Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: A North American Brain Tumor Consortium study. J Clin Oncol. 2004;22:4282–4289. doi: 10.1200/JCO.2004.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis N, Pratap S, Akopova I, Bowman PW, Lacko AG. Pre-Clinical Evaluation of rHDL Encapsulated Retinoids for the Treatment of Neuroblastoma. Front Pediatr. 2013;1:6. doi: 10.3389/fped.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BJ, Worden FP, Gadgeel SM, Parchment RE, Hodges CM, Zwiebel J, Dunn RL, Wozniak AJ, Kraut MJ, Kalemkerian GP. Phase II trial of fenretinide (NSC 374551) in patients with recurrent small cell lung cancer. Invest New Drugs. 2009;27:571–578. doi: 10.1007/s10637-009-9228-6. [DOI] [PubMed] [Google Scholar]
- Shan D, Gopal AK, Press OW. Synergistic effects of the fenretinide (4-HPR) and anti-CD20 monoclonal antibodies on apoptosis induction of malignant human B cells. Clin Cancer Res. 2001;7:2490–2495. [PubMed] [Google Scholar]
- Vaishampayan U, Heilbrun LK, Parchment RE, Jain V, Zwiebel J, Boinpally RR, Lorusso P, Hussain M. Phase II trial of fenretinide in advanced renal carcinoma. Invest New Drugs. 2005;23:179–185. doi: 10.1007/s10637-005-5864-7. [DOI] [PubMed] [Google Scholar]
- William WN, JR, Lee JJ, Lippman SM, Martin JW, Chakravarti N, Tran HT, Sabichi AL, Kim ES, Feng L, Lotan R, Papadimitrakopoulou VA. High-dose fenretinide in oral leukoplakia. Cancer Prev Res (Phila) 2009;2:22–26. doi: 10.1158/1940-6207.CAPR-08-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]