Abstract
Background
Cerebrovascular risk is increased in people living with HIV infection compared with age-matched uninfected individuals. Cerebrovascular endothelial dysfunction related to antiretroviral therapy and inflammation may contribute to higher stroke risk in HIV infection.
Methods
We compared cerebral vasoreactivity—a measure of cerebrovascular endothelial function assessed by the breath holding index (BHI) using transcranial Doppler ultrasound—between virologically suppressed Chinese HIV-infected individuals followed in an HIV clinic in Beijing, China and uninfected controls. We constructed mixed effects models to evaluate the association of HIV, antiretroviral therapy and inflammatory markers with cerebral vasoreactivity.
Results
In an unadjusted model, HIV infection was associated with a trend toward lower cerebral vasoreactivity (BHI 1.08 versus 1.26, p=0.079). In multivariable analyses, cholesterol modified the association between HIV infection and cerebral vasoreactivity (p=0.015 for interaction). At a lower total cholesterol of 4.15 mmol/L, HIV was associated with lower cerebral vasoreactivity (BHI −0.28, p=0.019), whereas at a cholesterol of 5.15 mmol/L, the reduction in cerebral vasoreactivity associated with HIV was no longer statistically significant (BHI −0.05, p=0.64). Among HIV-infected individuals, use of lopinavir/ritonavir compared with efavirenz was associated with lower cerebral vasoreactivity (BHI −0.24, p=0.040). We did not find a significant association between inflammatory markers and cerebral vasoreactivity.
Conclusion
Cerebrovascular endothelial dysfunction associated with HIV infection may be most relevant for individuals with less traditional vascular risk, such as those with lower cholesterol. Further study of the impact of ART on cerebrovascular endothelial function is warranted to aid with ART selection in individuals at high cerebrovascular risk.
Keywords: HIV and stroke, cerebral vasoreactivity, cerebrovascular endothelial function, transcranial Doppler ultrasound, China
Introduction
An estimated 800,000 individuals are living with HIV in China[1] with the number of newly diagnosed cases, including in older individuals, on the rise[2]. In response to the HIV/AIDS epidemic, China has made major strides in access to treatment, implementing the National Free Antiretroviral Therapy (ART) program which covers 28 provinces across China[3,4]. As accessibility to ART improves and life expectancy of HIV-infected individuals increases, new challenges, many of which are more typical of an aging population, have emerged[5,6] including in low- and middle-income countries (LMIC) where the burden of chronic disease is growing[7]. Of these, the association between HIV and cerebrovascular disease[8–10] is of particular concern in China, where stroke occurs at younger ages[11] and is the leading cause of vascular mortality and third leading cause of death overall[12].
Cerebral vasoreactivity, a measure of cerebrovascular endothelial function[13] that can be evaluated non-invasively using transcranial Doppler (TCD) ultrasound, is associated with cerebral small vessel disease and severe large artery atherosclerosis[14–16]—two common stroke mechanisms in HIV infection[17]. A vasodilatory response to the rise in carbon dioxide (CO2) that occurs with breath holding, indicated by an increase in mean blood flow velocity of the cerebral arteries, is one method of assessing cerebral vasoreactivity and associated cerebrovascular risk that can be readily implemented in diverse settings. We recently demonstrated in our UNderstanding Cerebral VasoReactivity in HIV infection (UNCoVeR) study that treated, virologically suppressed HIV infection is an independent risk factor for lower cerebral vasoreactivity[18]. In that study of a predominantly white male cohort in the U.S., we were unable to compare the impact of specific ART medications on cerebral vasoreactivity. In addition, we did not have access to inflammatory markers for the cohort, which can remain elevated in treated HIV infection[19] and predict incident cardiovascular events and mortality[20,21], to examine their association with cerebrovascular endothelial function. Ongoing inflammation despite virologic suppression on ART may explain, in part, the observed association between treated HIV infection and lower cerebrovascular endothelial function.
The primary and secondary goals of the UNderstanding Cerebral VasoReactivity in HIV infection in Beijing (UNCoVeR Beijing) were to 1) assess the effect of treated HIV infection on cerebral vasoreactivity in Chinese HIV-infected and uninfected individuals, 2) compare the impact of specific ART medications on cerebral vasoreactivity among HIV-infected individuals and 3) evaluate the association between markers of inflammation and cerebral vasoreactivity in HIV infection.
Methods
Study setting and population
We recruited a convenience sample of HIV-infected adults followed in the Peking Union Medical College Hospital (PUMCH) HIV clinic in Beijing, China as part of the China AIDS Clinical Trials Group (CACTG) study. The PUMCH clinic is one of multiple sites participating in the national CACTG study, which was originally designed to evaluate toxicities and adverse effects of ART in Chinese HIV-infected participants[22]. All HIV-infected individuals on a stable ART regimen for a minimum of 6 months with an undetectable plasma viral load were eligible to participate. Because the CACTG study does not include an HIV-uninfected control group, we separately recruited uninfected individuals through referrals of partners and friends of HIV-infected participants. Uninfected individuals underwent HIV testing to confirm their negative status prior to enrollment in the study.
Exclusion criteria for both HIV-infected and uninfected participants included: history of stroke, transient ischemic attack, central nervous system (CNS) infection or new unexplained neurologic deficit in the past 3 months, serious illness requiring systemic treatment (e.g., antibiotics) or hospitalization in the past month, or use of immune-modulatory therapy (e.g., corticosteroids) in the past month. The PUMCH Ethics Committee and University of California, San Francisco Committee on Human Research approved the study.
Study measurements
HIV-infected participants and uninfected controls were seen for a one-time visit during which all study measurements were obtained. We assessed cerebral vasoreactivity using the breath holding challenge[15], measuring the change in mean flow velocities (MFV) of the bilateral middle cerebral arteries (MCAs) in response to a 30-second period of breath holding. Results from two trials were averaged. A single vascular technologist blinded to HIV status with TCD expertise and training in the breath holding challenge performed all studies to minimize interrater variability. A Pioneer TC8080 TCD machine (Nicolet Vascular Inc., Viasys Healthcare, Madison, Wisconsin, USA) was used for all studies. The primary cerebral vasoreactivity outcome was the breath holding index (BHI), defined as the percentage change in MFV from baseline to the conclusion of breath holding per second of breath holding. A lower BHI indicates worse cerebrovascular endothelial function.
We administered a questionnaire to participants to obtain information that might affect cerebral vasoreactivity and stroke risk, including history of vascular risk factors (defined by self-report of prior diagnosis by a medical provider with or without current use of anti-hypertensive, anti-diabetic or lipid-lowering therapy); anti-platelet, anticoagulant and statin use; current smoking, alcohol and substance use; and a history of migraines, traumatic brain injury and sleep apnea. HIV-specific characteristics, including duration of HIV infection, current and prior ART use, most recent and nadir CD4 count, were obtained from the CACTG database. Routine laboratory test results were available through the CACTG study for HIV-infected participants, in addition to fasting lipids, interleukin-6 (IL-6) and high sensitivity C-reactive protein (hsCRP) obtained expressly for our study for both the HIV-infected and uninfected individuals.
Statistical analysis
We compared baseline characteristics between HIV-infected and uninfected participants using Student’s t, Wilcoxon rank-sum, Chi-square or Fisher’s exact test, as appropriate. We used mixed effects linear regression models to determine the association of HIV with cerebral vasoreactivity. We constructed a separate model restricted to HIV-infected participants to assess the association of specific ART medications, IL-6 and hsCRP with cerebral vasoreactivity. Because cerebral vasoreactivity is measured for the left and right MCA, we included a random person effect to account for within-person correlation. All models were adjusted for age and the change in mean arterial pressure with breath holding. We then considered clinically relevant covariates of a priori interest as candidate covariates, with the ultimate multivariable model constructed using forward stepwise selection. We evaluated whether any vascular risk factors modified the effect of HIV on cerebral vasoreactivity by entering 2-way statistical interaction terms into the final multivariable model. To assess the stability of our final multivariable model, we constructed more parsimonious three-predictor models to compare coefficients for our main findings. Finally, given the modest number of HIV-uninfected controls, we investigated the impact of removing potential influential control points on the main effects of the model. P values were 2-sided with ≤0.05 considered statistically significant. Statistical analyses were performed using Stata (StataCorp 2012. Stata Statistical Software: Release 12; Stata Corporation, College Station, TX).
Results
Demographics
We enrolled 78 HIV-infected individuals and 18 uninfected controls over a 6-month study period. Three HIV-infected individuals, 2 of whom were women, were excluded due to absent bilateral temporal windows. One control was excluded when the screening HIV test returned positive. Another control participant was excluded due to the presence of a focal intracranial stenosis of the MCA. Demographic and clinical characteristics of the remaining 91 participants (75 HIV-infected, 16 HIV-uninfected) are in Table 1. The mean age of the HIV-infected group was 41 years and of the uninfected controls was 39 years. There were more female uninfected controls than HIV-infected participants (44% versus 19%, p=0.031). The majority of participants were Han Chinese.
Table 1.
Demographic and clinical characteristics of HIV-infected individuals and uninfected controls
| HIV-infected individuals (n=75) |
Uninfected controls (n=16) |
P value | |
|---|---|---|---|
| Demographics, n (% of total) unless indicated | |||
|
| |||
| Age (years), mean (SD) | 41 (11) | 39 (10) | 0.62 |
|
| |||
| Female sex | 14 (19) | 7 (44) | 0.031 |
|
| |||
| Non-Han ethnicity | 1 (1) | 0 (0) | 1.00 |
|
| |||
| Vascular risk factors and other medical conditions, n (% of total) unless indicated | |||
|
| |||
| Hypertension | 8 (11) | 2 (13) | 1.00 |
|
| |||
| Anti-hypertensive medication use | 9 (12) | 2 (13) | 1.00 |
|
| |||
| Dyslipidemia | 17 (23) | 2 (13) | 0.51 |
|
| |||
| Statin use overall | 7 (9) | 0 (0) | 0.35 |
| Statin use among individuals with dyslipidemia | 7 (41) | 0 (0) | 0.25 |
|
| |||
| Coronary heart disease/MI | 3 (4) | 1 (6) | 0.55 |
|
| |||
| Antiplatelet or anticoagulation use | 2 (3) | 2 (13) | 0.14 |
|
| |||
| Diabetes mellitus | 5 (7) | 1 (6) | 1.00 |
|
| |||
| Stroke or cerebral vessel abnormality | 2 (3) | 1 (6) | 0.44 |
|
| |||
| Positive hepatitis C antibody | 4 (5) | NA | — |
|
| |||
| Current substance use, n (% of total) unless indicated | |||
|
| |||
| Alcohol use | 20 (27) | 1 (6) | 0.11 |
|
| |||
| Tobacco use | 21 (28) | 4 (25) | 1.00 |
|
| |||
| Substance use | 1 (1) | 0 (0) | 1.00 |
|
| |||
| Laboratories (mmol/L), mean (SD) unless indicated | |||
|
| |||
| Total cholesterola | 4.78 (1.02) | 4.86 (1.02) | 0.75 |
|
| |||
| Low-density lipoprotein | 2.64 (0.74) | 2.83 (0.90) | 0.18 |
|
| |||
| High-density lipoprotein | 1.18 (0.33) | 1.23 (0.17) | 0.61 |
|
| |||
| Triglycerides | 1.74 (1.66) | 1.45 (1.36) | 0.51 |
|
| |||
| hsCRP (mg/L), median (Q1, Q3) | 1.06 (0.54, 2.13) | 0.48 (0.20, 0.89) | 0.003 |
|
| |||
| IL-6 (pg/mL), median (Q1, Q3)b | 2.0 (2.0, 2.2) | 2.0 (2.0, 2.0) | 0.023 |
|
| |||
| HIV-specific factors (cell/mm3), mean (SD) unless indicated | |||
|
| |||
| CD4 count | 454 (229) | — | — |
|
| |||
| Nadir CD4 count | 193 (145) | — | — |
|
| |||
| CD8 count | 738 (377) | — | — |
|
| |||
| CD4 to CD8 ratio | 0.70 (0.36) | — | — |
|
| |||
| Duration of HIV infection (years), median (Q1, Q3) | 4.8 (2.3, 6.3) | — | — |
|
| |||
| Duration of ART use (years), median (Q1, Q3) | 4.0 (2.0, 6.0) | — | — |
|
| |||
| Current ART regimen, n (% of total): | |||
| 3TC + TDF or AZT + EFZ | 37 (49) | — | — |
| 3TC + TDF or AZT + NVP | 26 (35) | — | — |
| 3TC + TDF or AZT + LPV/r | 8 (11) | — | — |
| 3TC + TDF + LPV/r + RAL | 2 (3) | — | — |
| 3TC + ABC + NVP or LPV/r | 2 (3) | — | — |
Abbreviations: SD, standard deviation; NA, not available; 3TC, lamivudine; TDF, tenofovir; EFZ, efavirenz; AZT, zidovudine; NVP, nevirapine; LPV/r, lopinavir/ritonavir; RAL, raltegravir; ABC, abacavir
Total cholesterol among HIV-infected individuals 45 years of age and older was higher compared with younger HIV-infected individuals (5.07 versus 4.60 mmol/L, p=0.05). See discussion for additional context.
26% of HIV-infected individuals had an IL-6 >2 pg/mL (lowest limit of reliable detection) compared with 0% for uninfected controls, p=0.021.
Prevalence of vascular risk factors
We did not observe any statistically significant differences in prevalence of vascular risk factors by HIV status (Table 1). The most common vascular risk factor among HIV-infected participants was dyslipidemia (23%), followed by hypertension (11%) and diabetes mellitus (7%). Of the HIV-infected individuals with dyslipidemia, 41% were taking a statin (Table 1).
HIV-related characteristics
The mean CD4 count among HIV-infected participants was 454 cells/mm3. Median duration of HIV infection was 4.8 years. The majority of HIV-infected participants were on a nucleoside reverse transcriptase inhibitor backbone of lamivudine plus tenofovir or zidovudine in combination with efavirenz (49%), nevirapine (35%) and lopinavir/ritonavir (11%). Only two individuals were on abacavir (Table 1).
Association of HIV with cerebral vasoreactivity
In an unadjusted model, HIV infection was associated with a trend toward lower cerebral vasoreactivity (mean BHI 1.08 for HIV-infected versus 1.26 for uninfected group, p=0.079). In a multivariable model adjusted for age, sex, statin use, total cholesterol, coronary heart disease/myocardial infarction (CHD/MI), use of an antiplatelet or anticoagulant and smoking, we observed an interaction between HIV infection and total cholesterol on the BHI (p=0.015 for the interaction)(Figure 1). Among individuals with low total cholesterol, HIV infection was associated with lower cerebral vasoreactivity. However, in individuals with a moderate cholesterol level, HIV had no statistically significant effect on cerebral vasoreactivity. For example, at a total cholesterol of 4.15 mmol/L (160 mg/dL), HIV infection was associated with a reduction in BHI of −0.28 (95% CI −0.52 to −0.05, p=0.019). At a total cholesterol level of 4.50 mmol/L (174 mg/dL), the magnitude of the effect of HIV infection on BHI lessened (−0.20, 95% CI −0.41 to −0.001, p=0.049), while at a total cholesterol of 5.15 mmol/L (199 mg/dL), the lower BHI associated with HIV infection was no longer statistically significant (−0.05, 95% CI −0.23 to +0.14, p=0.64)(Figure 1, Table 2).
Figure 1. Total cholesterol modifies effect of HIV infection on cerebral vasoreactivity.
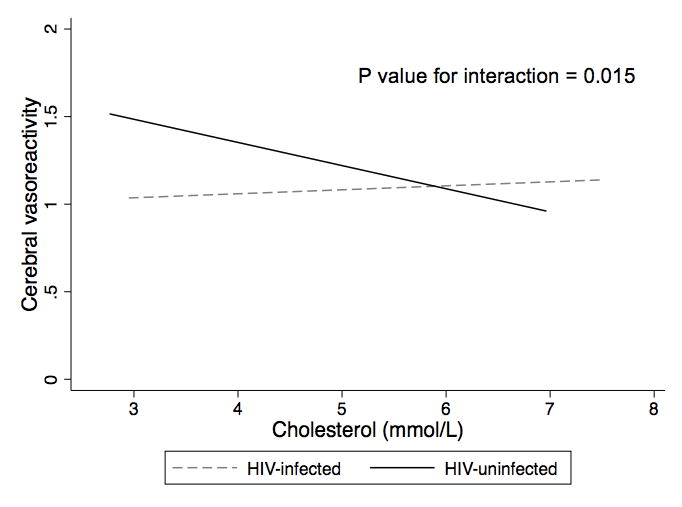
At lower cholesterol levels, HIV infection was associated with worse cerebral vasoreactivity (measured with breath holding index), while at higher cholesterol levels, this association was not observed.
Table 2.
Adjusted difference in cerebral vasoreactivity (measured with breath holding index) by HIV infection and other risk factors among all participants (total observations, left + right sides, N=168)a
| Difference in breath holding index (% change in mean flow velocity per second) (95% CI) |
P value | |
|---|---|---|
| HIV infection | At total cholesterol of 5.15 mmol/Lb: −0.05 (−0.23 to +0.14) |
0.64 |
| At total cholesterol of 4.15 mmol/Lb: −0.28 (−0.52 to −0.05) |
0.019 | |
|
| ||
| Demographics | ||
|
| ||
| Age (per 10 years) | −0.07 (−0.15 to +0.001) | 0.055 |
|
| ||
| Female sex | −0.04 (−0.24 to +0.16) | 0.70 |
|
| ||
| Vascular and other risk factors | ||
|
| ||
| Statin use | +0.23 (−0.04 to +0.51) | 0.096 |
|
| ||
| Coronary heart disease/myocardial infarction | −0.33 (−0.69 to +0.04) | 0.080 |
|
| ||
| Antiplatelet or anticoagulation use | +0.26 (−0.11 to +0.63) | 0.11 |
|
| ||
| Tobacco use | +0.15 (−0.02 to +0.32) | 0.078 |
|
| ||
| Laboratories | ||
|
| ||
| Total cholesterol (per 1 mmol/L) | −0.22 (−0.40 to −0.05) | 0.011 |
Adjusted for factors listed in the column in addition to the change in mean arterial pressure between baseline and breath holding
P value for interaction term between HIV and cholesterol level, p=0.015
To investigate if the interaction between HIV infection and cholesterol on cerebral vasoreactivity was driven by statin use, which has an established positive impact on cerebral vasoreactivity,[23,24] we repeated the analysis after exclusion of statin users, all of whom were HIV-infected. A statistically significant interaction (p=0.006) was still present between HIV infection and total cholesterol on BHI, with a negative effect of HIV on cerebral vasoreactivity among individuals with lower total cholesterol levels. In more parsimonious models that included the interaction between HIV infection and total cholesterol and each of the covariates from the multivariable model individually, we found that the main effects of HIV, cholesterol and their interaction remained statistically significant in these models without substantial impact on effect sizes. Finally, we removed potentially influential controls from the analysis, including uninfected individuals with the highest and lowest BHI, and found that the relationship between HIV and cholesterol on cerebral vasoreactivity was still present.
Impact of ART and other HIV-related factors on cerebral vasoreactivity
In the HIV only cohort, use of lopinavir/ritonavir in combination with a backbone of lamivudine and either tenofovir or zidovudine was associated with lower BHI compared with those on the same backbone with efavirenz (Figure 2, Table 3). Because individuals on efavirenz tended to have more recently diagnosed HIV infection and shorter duration of ART, we added time since HIV diagnosis (as a proxy for duration of infection) and duration of ART use to the multivariable model. Addition of these two variables did not attenuate the negative effect of lopinavir/ritonavir use on cerebral vasoreactivity. We did not observe a significant association in unadjusted models between abacavir use, current CD4 count, nadir CD4 count, or CD4 to CD8 ratio and the BHI, and none of these variables remained in the final adjusted model.
Figure 2. Adjusted cerebral vasoreactivity by type of antiretroviral therapy (ART)a.
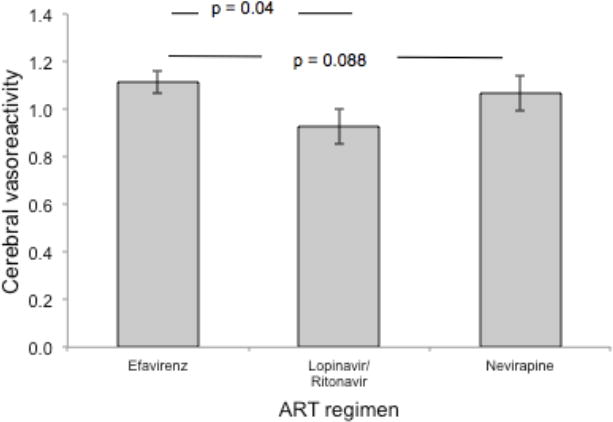
Use of lopinavir/ritonavir was associated with lower mean cerebral vasoreactivity (measured with breath holding index) compared with use of efavirenz, independent of the duration of HIV infection or use of antiretroviral therapy.
aAdjusted for age, statin use, coronary heart disease/myocardial infarction, duration of HIV infection and duration of antiretroviral therapy use.
Table 3.
Adjusted difference in cerebral vasoreactivity (measured with breath holding index) by HIV-related and other risk factors among HIV-infected participants (total observations, left + right sides, N=133)a
| Difference in breath holding index (% change in mean flow velocity per second) (95% CI) |
P value | |
|---|---|---|
| Demographics | ||
|
| ||
| Age (per 10 years) | −0.08 (−0.15 to −0.01) | 0.036 |
|
| ||
| Vascular and other risk factors | ||
|
| ||
| Statin use | +0.25 (−0.05 to +0.54) | 0.097 |
|
| ||
| Coronary heart disease/MI | −0.44 (−0.78 to −0.11) | 0.009 |
|
| ||
| HIV-related factors | ||
|
| ||
| Duration of HIV infection (per year) | −0.06 (−0.13 to +0.01) | 0.078 |
|
| ||
| Duration of antiretroviral therapy (per year) | +0.08 (+0.001 to +0.16) | 0.048 |
|
| ||
| Current antiretroviral therapy: | ||
| Efavirenz | Reference | — |
| Nevirapine | −0.13 (−0.28 to +0.02) | 0.088 |
| Lopinavir/ritonavir | −0.24 (−0.47 to −0.01) | 0.040 |
Adjusted for factors listed in the column in addition to the change in mean arterial pressure between baseline and breath holding
Inflammation and cerebral vasoreactivity
Both IL-6 and hsCRP were higher among HIV-infected individuals compared with uninfected controls (Table 1). However, we did not find a statistically significant association between IL-6 or hsCRP and cerebral vasoreactivity. In exploratory analyses, HIV-infected women had a higher median IL-6 compared with HIV-infected men (2.25 vs 2.0 pg/mL, p=0.016). In age-adjusted models, IL-6 >2 pg/mL was associated with a lower BHI among all women (−0.08, 95% CI −0.15 to +0.0005, p=0.052) and among HIV-infected women (−0.09, 95% CI −0.17 to −0.01, p=0.022) but not HIV-infected men (+0.005, 95% CI −0.07 to +0.08, p=0.90).
Discussion
Association between HIV and cerebral vasoreactivity varies by cholesterol level
In this study, ART-treated, virologically suppressed HIV-infected individuals with lower total cholesterol had worse cerebral vasoreactivity compared with HIV-uninfected controls. However, with higher total cholesterol levels, the negative effect of HIV infection on cerebral vasoreactivity weakened and eventually disappeared. This pattern suggests that the contribution of HIV to cerebrovascular endothelial dysfunction and stroke risk may be most pronounced in individuals with lower traditional vascular risk.
A variable effect of HIV on vascular risk by age has been demonstrated in prior studies. In the Boston Partners HIV cohort, HIV was associated with a greater risk of hemorrhagic stroke, but its effect diminished with increasing age[9]. Similarly, in a study from Blantyre, Malawi, the proportion of stroke risk in individuals older than 45 years attributable to HIV was lower [population attributable fraction (PAF) 6%] than the risk attributable to hypertension (PAF 68%)[25]. In comparison, the PAF of stroke risk for younger HIV-infected individuals was 42% compared to 11% for hypertension. As expected, HIV-infected individuals 45 years and older in our study cohort had higher mean total cholesterol compared with younger individuals (Table 1). As traditional vascular risk factors such as dyslipidemia become more prevalent with increasing age among HIV-infected individuals, they may play a greater role in overall stroke risk and overshadow the impact of HIV infection. The corollary, that HIV-infected individuals without traditional vascular risk factors such as elevated total cholesterol may still be at increased cerebrovascular risk compared with uninfected controls, should be underscored.
Statin use associated with higher cerebral vasoreactivity
We observed a trend toward higher cerebral vasoreactivity among statin users, similar to our findings in UNCoVeR in the U.S.[18] Despite the established cardiovascular benefits of statins, including improvement in cerebrovascular endothelial function[23], more than half of HIV-infected individuals with dyslipidemia in this Chinese cohort were not taking a statin. Underutilization of statins for primary prevention of cardiovascular disease has been reported in HIV infection[26]. Understanding and addressing the root causes of lower rates of statin use in this high-risk population should be a focus of future work given the tremendous opportunity to impact vascular outcomes.
Lower cerebral vasoreactivity among HIV-infected individuals on lopinavir/ritonavir
A secondary goal of UNCoVeR Beijing was to assess the association between specific ART medications and cerebral vasoreactivity. Our current understanding of the effect of ART on cerebrovascular risk is limited and may be complicated by the heterogeneity of stroke types and mechanisms. Special attention has been paid to abacavir, which has been implicated as a risk factor for systemic endothelial dysfunction[27] and cardiovascular events including stroke[28,29] in HIV-infected individuals. We did not observe a significant association between abacavir use and cerebral vasoreactivity, although this finding should be interpreted with caution given the minimal use of abacavir among study participants. However, among individuals on lamivudine plus either tenofovir or zidovudine (95% of participants), use of lopinavir/ritonavir—the only protease inhibitors (PI) to which this cohort has been exposed—was associated with lower cerebral vasoreactivity when compared with efavirenz use. This effect persisted after adjusting for duration of HIV infection and of ART use.
Increased cardiovascular risk has been observed in patients with both recent and cumulative exposure to PIs, and specifically to lopinavir[30,31]. PI use is associated with various metabolic abnormalities, including dyslipidemia and insulin resistance, that promote endothelial dysfunction and cardiovascular disease[32]. A more atherogenic lipid profile and impaired systemic endothelial function have been observed in HIV-infected individuals treated with a PI-containing regimen compared with those on a PI-sparing regimen[33]. Our study provides evidence that endothelial dysfunction related to PI use may extend beyond the systemic vasculature to the brain and offers a potential mechanism to explain the observed higher risk of nonhemorrhagic stroke events[34] and cerebral small vessel disease pathology[35] associated with PI exposure in HIV-infected individuals.
The observed association between PI use and lower cerebral vasoreactivity in this study can only be generalized to lopinavir-containing ART regimens which, while rarely used in routine practice in high-income countries, remain first-line therapy in many LMIC worldwide with high prevalence of HIV. An important question is whether newer PIs, which have a less atherogenic profile and may not be associated with an increase in vascular events[36], have the same negative impact on cerebrovascular function as lopinavir. In one small clinical trial, HIV-infected patients treated with older PI-containing regimens, the majority of which included nelfinavir or lopinavir, randomized to switch to unboosted atazanavir (versus continuing on their current regimen) had improved lipid profiles but no corresponding improvement in systemic endothelial function[37]. Conversely, a larger predefined substudy of a prospective randomized trial of three modern ART regimens [AIDS Clinical Trials Group study A5257] found that individuals randomized to boosted atazanavir had slower progression of carotid intima-media thickness compared with boosted darunavir, with intermediate changes in those on raltegravir[38]. No significant effect of starting an ART regimen on systemic endothelial function was observed. Studies focused specifically on the effect of newer PIs on cerebrovascular endothelial function are needed.
Impact of IL-6 on cerebral vasoreactivity among HIV-infected women
The final aim of the study was to assess the association between inflammatory markers—IL-6 and hsCRP—and cerebral vasoreactivity. To our knowledge, this is the first study to explore the relationship between inflammation and cerebrovascular endothelial function in HIV infection. Prior studies have demonstrated a link between markers of inflammation, including IL-6 and hsCRP, and subclinical cardiovascular disease and incident cardiovascular events in HIV-infected individuals[20,39]. These data have bolstered the prevailing hypothesis that chronic inflammation and immune activation drive increased cardiovascular risk in HIV infection. Although HIV-infected individuals had higher markers of inflammation compared with uninfected controls, we did not observe a significant association between IL-6 or hsCRP and cerebral vasoreactivity in the overall cohort or in the HIV only group. In exploratory analyses adjusted for age and stratified by sex, higher IL-6 was associated with worse cerebral vasoreactivity in women, including HIV-infected women, but not in HIV-infected men. While these exploratory analyses were limited by the modest number of women and must be verified, the differential impact of greater inflammation on cerebrovascular function in women versus men in this cohort merits further study, especially in light of existing evidence indicating that the risk of stroke associated with HIV infection may differ by sex[8,9,40,41].
Strengths and limitations
Results from UNCoVeR Beijing should be interpreted cautiously alongside the study’s limitations. First, due to the modest sample size, particularly in terms of uninfected controls, our findings are preliminary and need to be verified in a larger, independent study. As with our U.S.-based UNCoVeR study, UNCoVeR Beijing was not designed to assess the impact of HIV on changes in cerebrovascular function over time. Unmatched uninfected controls were recruited separately from HIV-infected participants, which may have introduced selection bias. While demographic and clinical characteristics of the two groups were largely similar, some differences were present that did not reach statistical significance. In addition, we lacked information on immune activation markers, which have been a primary focus of studies aimed at understanding the pathophysiology of increased cardiovascular risk in HIV, although we were able to investigate the relationship between non-specific inflammatory markers and cerebral vasoreactivity. We also did not have data on menopause status or exogenous hormone use, which may have been relevant in our exploratory analyses of HIV-infected individuals stratified by sex. Strengths of our study included successful enrollment of nearly 20% HIV-infected women, in contrast to UNCoVeR, which was 95% men. While still a modest proportion of women, we were able to perform preliminary analyses to explore differences in the association between inflammation and cerebral vasoreactivity by sex, which should be replicated in a larger study. Furthermore, the lack of substance use in the cohort removed a major potential confounder in the association between HIV and cerebrovascular endothelial function. Lastly, because few ART regimens are available through the Chinese National ART program, we had a relatively clean sample in which to investigate the impact of specific ART medications on cerebrovascular risk.
Conclusion
In summary, we found that ART-treated HIV infection is associated with worse cerebrovascular endothelial function among individuals with lower total cholesterol, while the negative effect of HIV diminished as total cholesterol increased. We propose that the additional contribution of HIV infection to cerebrovascular risk may be most relevant for individuals at lower traditional vascular risk. We also demonstrated an association between lopinavir use and lower cerebrovascular endothelial function, which, if confirmed, suggests that lopinavir/ritonavir should be avoided in high cerebrovascular risk patients from LMIC where this PI combination is still widely used. These findings expand our understanding of the potential impact of treated, virologically suppressed HIV infection on cerebrovascular risk. Furthermore, we demonstrated the feasibility of applying an economical and accessible technology to evaluate cereobrovascular endothelial function in a LMIC. Future studies should focus on the pathogenetic mechanisms underlying cerebrovascular disease in HIV infection, including specifically in HIV-infected individuals from LMIC, in order to identify and evaluate strategies to prevent and treat stroke in this at-risk population.
Acknowledgments
Sources of funding: This work was supported by National Institutes of Health (NIH) Research Training Grant R25TW009343 funded by the Fogarty International Center, the NIH Office of the Director Office of AIDS Research, the NIH Office of the Director Office of Research on Women’s Health, the NIH Office of the Director Office of Behavioral and Social Science Research, the National Institute of Mental Health, and the National Institute on Drug Abuse, as well as the University of California Global Health Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the University of California Global Health Institute. Support for statistical analysis provided through the UCSF Clinical and Translational Science Institute, UL1TR000004.
Footnotes
Author contributions
F.C.C. participated in the study concept, design and data acquisition, performed the data analysis and interpretation and drafted the manuscript. Y.L. and Y.H. contributed to study design and data acquisition. J.C. contributed to data analysis and interpretation. H.W. and W.X. contributed to study concept and design and data interpretation. R.W.P. contributed to study design and data interpretation. F.A.S. contributed to study design and data interpretation. T.L. contributed to study concept and design and data interpretation. All authors critically revised the manuscript for important intellectual content and approved the final version of the paper.
Conflicts of interest: No authors report any relevant conflicts of interest.
References
- 1.World Health Organization, WHO Representative Office China News Release. China urged to “close the gap” on World AIDS Day. Available at: http://www.wpro.who.int/china/mediacentre/releases/2014/2014112801/en. Accessed April 4, 2016.
- 2.Liu H, Lin X, Xu Y, Chen S, Shi J, Morisky D. Emerging HIV Epidemic Among Older Adults in Nanning, China. AIDS Patient Care STDS. 2012;26:565–567. doi: 10.1089/apc.2012.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han M, Chen Q, Hao Y, et al. Design and implementation of a China comprehensive AIDS response programme (China CARES), 2003–08. International Journal of Epidemiology. 2010;39:ii47–ii55. doi: 10.1093/ije/dyq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21(Suppl 8):S143–8. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 5.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and Aging in HIV-Infected Persons: The Swiss HIV Cohort Study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 6.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 7.Hirschhorn LR, Kaaya SF, Garrity PS, Chopyak E, Fawzi MCS. Cancer and the ‘other’ noncommunicable chronic diseases in older people living with HIV/AIDS in resource-limited settings. AIDS. 2012;26:S65–S75. doi: 10.1097/QAD.0b013e328355ab72. [DOI] [PubMed] [Google Scholar]
- 8.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of Ischemic Stroke Incidence in HIV-Infected and Non-HIV-Infected Patients in a US Health Care System. J Acquir Immune Defic Syndr. 2012;60:351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow FC, He W, Bacchetti P, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83:1705–1711. doi: 10.1212/WNL.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS. 2014;28:1911–1919. doi: 10.1097/QAD.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 11.Tsai C-F, Thomas B, Sudlow CLM. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 13.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–61. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 14.Molina C, Sabin JA, Montaner J, Rovira A, Abilleira S, Codina A. Impaired Cerebrovascular Reactivity as a Risk Marker for First-Ever Lacunar Infarction: A Case-Control Study. Stroke. 1999;30:2296–2301. doi: 10.1161/01.str.30.11.2296. [DOI] [PubMed] [Google Scholar]
- 15.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–467. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]
- 16.Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- 17.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and Clinical Features of Cerebrovascular Disease Among HIV-Infected Adults in the Southeastern United States. AIDS Res Hum Retroviruses. 2013;29:1068–1074. doi: 10.1089/aid.2012.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow FC, Boscardin WJ, Mills C, et al. Cerebral vasoreactivity is impaired in treated, virally suppressed HIV-infected individuals. AIDS. 2016;30:45–55. doi: 10.1097/QAD.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients with HIV Infection. Plos Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T, Dai Y, Kuang J, et al. Three generic nevirapine-based antiretroviral treatments in Chinese HIV/AIDS patients: multicentric observation cohort. PLoS ONE. 2008;3:e3918. doi: 10.1371/journal.pone.0003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forteza A, Romano JG, Campo-Bustillo I, et al. High-dose atorvastatin enhances impaired cerebral vasomotor reactivity. J Stroke Cerebrovasc Dis. 2012;21:487–492. doi: 10.1016/j.jstrokecerebrovasdis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Sterzer P, Meintzschel F, Rosler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small-vessel disease. Stroke. 2001;32:2817–2820. doi: 10.1161/hs1201.099663. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case-control study. Neurology. 2016;86:324–333. doi: 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park TE, Yusuff J, Sharma R. Use of aspirin and statins for the primary prevention of myocardial infarction and stroke in patients with human immunodeficiency virus infection. Int J STD AIDS. 2016;27:447–452. doi: 10.1177/0956462415585448. [DOI] [PubMed] [Google Scholar]
- 27.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS. 2009;23:2021–2027. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen LD, Engsig FN, Christensen H, et al. Risk of cerebrovascular events in persons with and without HIV. AIDS. 2011;25:1637–1646. doi: 10.1097/QAD.0b013e3283493fb0. [DOI] [PubMed] [Google Scholar]
- 29.Choi A, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25:1289–1298. doi: 10.1097/QAD.0b013e328347fa16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS ONE. 2013;8:e59551. doi: 10.1371/journal.pone.0059551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DAD study group. Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 32.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Stein JH, Klein MA, Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 34.Worm SW, Kamara DA, Reiss P, et al. Evaluation of HIV Protease Inhibitor Use and the Risk of Sudden Death or Nonhemorrhagic Stroke. Journal of Infectious Diseases. 2012;205:535–539. doi: 10.1093/infdis/jir788. [DOI] [PubMed] [Google Scholar]
- 35.Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28:1297–1306. doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monforte AD, Reiss P, Ryom L, et al. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS. 2013;27:407–415. doi: 10.1097/QAD.0b013e32835b2ef1. [DOI] [PubMed] [Google Scholar]
- 37.Flammer AJ, Vo NTT, Ledergerber B, et al. Effect of atazanavir versus other protease inhibitor-containing antiretroviral therapy on endothelial function in HIV-infected persons: randomised controlled trial. Heart. 2009;95:385–390. doi: 10.1136/hrt.2007.137646. [DOI] [PubMed] [Google Scholar]
- 38.Stein JH, Ribaudo HJ, Hodis HN, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS. 2015;29:1775–1783. doi: 10.1097/QAD.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow FC, Wilson MR, Wu Kunling, Ellis R, Bosch R, Linas B. Stroke incidence highest in women and black HIV-infected participants in ALLRT cohort. Presented at the 23nd Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2016. [Google Scholar]


