Abstract
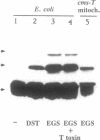
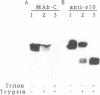
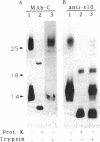
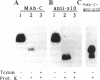
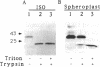
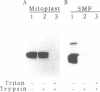
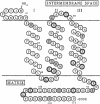
URF13, an inner mitochondrial membrane protein of the maize Texas male-sterile cytoplasm (cms-T), has one orientation in the inner membrane of maize mitochondria but two topological orientations in the plasma membrane when expressed in Escherichia coli. Antibodies specific for the carboxyl terminus of URF13 and for an amino-terminal tag fused to URF13 in E. coli were used to determine the location of each end of the protein following protease treatments of right-side-out and inside-out vesicles derived from cms-T mitochondria and the E. coli plasma membrane. Cross-linking studies indicate that a portion of the URF13 population in mitochondria and E. coli exists in membranes in an oligomeric state and, in combination with proteolysis studies, show that individual subunits within a given multimer have the same orientation. A three-membrane-spanning helical model for URF13 topology is presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Siedow J. N., Levings C. S., 3rd Fungal toxins bind to the URF13 protein in maize mitochondria and Escherichia coli. Plant Cell. 1990 Feb;2(2):153–161. doi: 10.1105/tpc.2.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J., Siedow J. N., Williams M. E., Levings C. S., 3rd Mutations in the maize mitochondrial T-urf13 gene eliminate sensitivity to a fungal pathotoxin. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4435–4439. doi: 10.1073/pnas.86.12.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Beegle K. H., Gebhard D. H. Monoclonal antibodies. Clinical uses and potential. Vet Clin North Am Small Anim Pract. 1986 Nov;16(6):1171–1179. doi: 10.1016/s0195-5616(86)50135-2. [DOI] [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A., Cohen F. S. Studies on the mechanism of action of channel-forming colicins using artificial membranes. J Membr Biol. 1984;79(2):105–118. doi: 10.1007/BF01872115. [DOI] [PubMed] [Google Scholar]
- Decker G. L., Greenawalt J. W. Ultrastructural and biochemical studies of mitoplasts and outer membranes derived from French-pressed mitochondria. Advances in mitochondrial subfractionation. J Ultrastruct Res. 1977 Apr;59(1):44–56. doi: 10.1016/s0022-5320(77)80027-0. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Siedow J. N., Timothy D. H., Levings C. S., 3rd A 13-kilodalton maize mitochondrial protein in E. coli confers sensitivity to Bipolaris maydis toxin. Science. 1988 Jan 15;239(4837):293–295. doi: 10.1126/science.3276005. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Glab N., Wise R. P., Pring D. R., Jacq C., Slonimski P. Expression in Saccharomyces cerevisiae of a gene associated with cytoplasmic male sterility from maize: respiratory dysfunction and uncoupling of yeast mitochondria. Mol Gen Genet. 1990 Aug;223(1):24–32. doi: 10.1007/BF00315793. [DOI] [PubMed] [Google Scholar]
- Hack E., Lin C., Yang H., Horner H. T. T-URF 13 Protein from Mitochondria of Texas Male-Sterile Maize (Zea mays L.) : Its Purification and Submitochondrial Localization, and Immunogold Labeling in Anther Tapetum during Microsporogenesis. Plant Physiol. 1991 Mar;95(3):861–870. doi: 10.1104/pp.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986 Nov;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Lee S. H., Lin C., Medici R., Hack E., Myers A. M. Expression in yeast of the T-urf13 protein from Texas male-sterile maize mitochondria confers sensitivity to methomyl and to Texas-cytoplasm-specific fungal toxins. EMBO J. 1990 Feb;9(2):339–347. doi: 10.1002/j.1460-2075.1990.tb08116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science. 1990 Nov 16;250(4983):942–947. doi: 10.1126/science.250.4983.942. [DOI] [PubMed] [Google Scholar]
- Nałecz M. J., Casey R. P., Azzi A. Use of N,N'-dicyclohexylcarbodiimide to study membrane-bound enzymes. Methods Enzymol. 1986;125:86–108. doi: 10.1016/s0076-6879(86)25009-0. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Nilsson I., von Heijne G. Fine-tuning the topology of a polytopic membrane protein: role of positively and negatively charged amino acids. Cell. 1990 Sep 21;62(6):1135–1141. doi: 10.1016/0092-8674(90)90390-z. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Hull J. D., Lamb R. A. Transposition of domains between the M2 and HN viral membrane proteins results in polypeptides which can adopt more than one membrane orientation. J Cell Biol. 1989 Nov;109(5):2023–2032. doi: 10.1083/jcb.109.5.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Lamb R. A. Topology of eukaryotic type II membrane proteins: importance of N-terminal positively charged residues flanking the hydrophobic domain. Cell. 1991 Feb 22;64(4):777–787. doi: 10.1016/0092-8674(91)90507-u. [DOI] [PubMed] [Google Scholar]
- Reenstra W. W., Patel L., Rottenberg H., Kaback H. R. Electrochemical proton gradient in inverted membrane vesicles from Escherichia coli. Biochemistry. 1980 Jan 8;19(1):1–9. doi: 10.1021/bi00542a001. [DOI] [PubMed] [Google Scholar]
- Smith W. P. Cotranslational secretion of diphtheria toxin and alkaline phosphatase in vitro: involvement of membrane protein(s). J Bacteriol. 1980 Mar;141(3):1142–1147. doi: 10.1128/jb.141.3.1142-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegink S. J., Siedow J. N. Binding of Butyl Gallate to Plant Mitochondria : II. Relationship to the Presence or Absence of the Alternative Pathway. Plant Physiol. 1986 Jan;80(1):196–201. doi: 10.1104/pp.80.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]