Abstract
Background
Clinical and pharmacological studies of obsessive-compulsive disorder (OCD) have suggested that the serotonergic systems are involved in the pathogenesis, while structural imaging studies have found some neuroanatomical abnormalities in OCD patients. In the etiopathogenesis of OCD, few studies have performed concurrent assessment of genetic and neuroanatomical variables.
Methods
We carried out a two-way ANOVA between a variable number of tandem repeat polymorphisms (5-HTTLPR) in the serotonin transporter gene and gray matter (GM) volumes in 40 OCD patients and 40 healthy controls (HCs).
Results
We found that relative to the HCs, the OCD patients showed significant decreased GM volume in the right hippocampus, and increased GM volume in the left precentral gyrus. 5-HTTLPR polymorphism in OCD patients had a statistical tendency of stronger effects on the right frontal pole than those in HCs.
Conclusions
Our results showed that the neuroanatomical changes of specific GM regions could be endophenotypes of 5-HTTLPR polymorphism in OCD.
Keywords: Hippocampus, Precentral gyrus, Frontal pole, Imaging genetics, Obsessive-compulsive disorder (OCD), Serotonin transporter gene, 5-HTTLPR, Voxel-based morphometry (VBM)
Background
Obsessive-compulsive disorder (OCD) was made a disease independent of anxiety disorder in DSM-5. One of the reasons for this separation is that the biological bases of OCD and anxiety disorder are different [1].
Structural imaging studies have found neuroanatomical abnormalities in the cortico–striatal–thalamo–cortical (CSTC) circuits in OCD patients [2]. A recent voxel-based morphometry (VBM) systematic review suggested that widespread structural abnormalities may contribute to neurobiological vulnerability to OCD [3]. We previously found the presence of regional gray matter (GM) and white matter (WM) volume abnormalities in OCD patients [4].
Furthermore, positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have revealed abnormal activities in different nodes of the CSTC circuits in OCD patients compared with healthy controls (HCs) [2, 5]. In our previous fMRI study, we found decreased activations in several brain regions including the orbitofrontal cortex (OFC) [6] and a specific relationship between fMRI activation and symptom subtypes [7].
Meanwhile, family and twin studies have provided evidence for the involvement of a genetic factor in OCD. However, many linkage, association, and genome-wide association studies have failed to identify responsible genes [8, 9].
Molecular genetic studies have focused on some structures, including receptor and transporter proteins, in the serotonergic and dopaminergic system.
Based on transporter imaging findings, a PET study [10] found a decrease of serotonin transporter binding in the insular cortex in OCD patients. They suggested that dysfunction of the serotonergic system in the limbic area might be involved in the pathophysiology of OCD.
It is possible to hypothesize that a polymorphism in the transcriptional control region upstream of the 5-hydroxytryptamine (serotonin) transporter (5-HTT) coding sequence could be an important factor in conferring susceptibility to OCD [8, 11, 12]. The 5-HTTLPR consists of a 44-bp deletion/insertion yielding a 14-repeat allele (short; S) and a 16-repeat allele (long; L). The S allele reduces the transcriptional efficiency of the 5-HTT gene promotor, resulting in decreased 5-HTT expression and availability. Bloch et al. [11]. suggested the possibility that the L allele is associated with specific OCD subgroups such as childhood-onset OCD. In contrast, Lin et al. [12] found that OCD was associated with the SS homozygous genotype. Some researchers suggested that this L allele could be subdivided further to LA and LG alleles [13]. The LG allele, which is the L allele with an A→G substitution (rs25531), is thought to be similar to the S allele in terms of reuptake efficiency [14]. Rocha et al. [15] found that the LA allele was associated to OCD. Hu et al. [14] found that the LALA genotype was approximately twice as common in 169 whites with OCD than in 253 ethnically matched controls, and the LA allele was twofold overtransmitted to the patients with OCD.
Despite the genetic and neuroanatomical importance, few studies of the etiopathogenesis of OCD have concurrently assessed genetic and neuroanatomical variables. We hypothesized that the widespread structural brain changes in OCD indicate the endophenotype of the 5-HTTLPR polymorphism. Therefore, the aim of this study was to investigate the association of genetic variations of the 5-HTTLPR with neuroanatomical changes in OCD.
Methods
Subjects
We studied 40 OCD patients (20 females and 20 males) who met DSM-IV [16] criteria for OCD and had no DSM-IV Axis I disorders except OCD and major depressive disorder as screened by the Structured Clinical Interview for DSM-IV (SCID). Patients who displayed a comorbid axis I diagnosis, neurological disorder, head injury, serious medical condition, or history of drug/alcohol addiction were excluded. We determined psychiatric diagnoses by a consensus of at least two psychiatrists after screening by SCID. Patients were recruited from among outpatients and inpatients of the Department of Neuropsychiatry, Kyushu University Hospital, Japan. Severity of OCD symptoms was assessed with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) [17]. Patients were also screened for the presence of depressive symptoms through the administration of the 17-item Hamilton Depression Rating Scale (HDRS) [18]. Forty HCs (26 females and 14 males) who were matched to the patients in age and sex were recruited from the staff of Kyushu University Hospital and related agencies. They had no DSM-IV Axis I disorders as determined by the SCID. They also had no current medical problems, psychiatric histories, neurological disorders, or mental retardation. Handedness was determined according to the Edinburgh Handedness Inventory for both OCD patients and HCs [19].
The study was approved by the local ethics committee <22-111, 491-01>, and each participating patient provided written informed consent after receiving a complete description of the study, which was approved by the institutional review board.
MRI procedures
All imaging examinations were performed on a 3.0-T MRI scanner (Achieva TX, Philips Healthcare, Best, The Netherlands) with a standard head coil at the Department of Radiology, Kyushu University. T1-weighted images were acquired with a 3D T1-weighted turbo field echo sequence with the following parameters: repetition time (TR) = 8.2 ms, echo time (TE) = 3.8 ms, flip angle = 8°, matrix = 240 × 240, T1 inversion time = 1026 ms, field of view (FOV) = 240 × 240 mm, NSA = 1, slice thickness = 1 mm, number of slices = 190, and scan time = 320 s.
VBM data processing
Acquired images were first converted from DICOM to NifTI-1 format using dcm2nii software (http://www.mccauslandcenter.sc.edu/mricro/mricron/dcm2nii.html). Data processing and examinations were performed with SPM8 software (developed under the auspices of the Functional Imaging Laboratory, The Wellcome Trust Centre for Neuroimaging at the Institute of Neurology at University College London, UK, http://www.fil.ion.ucl.ac.uk/spm/) in the environment of MATLAB (2011b ver., http://www.mathworks.co.jp/products/matlab/). AC–PC orientation was conducted on all T1-weighted data by an automatic process. Then, we applied the VBM8 toolbox (http://dbm.neuro.uni-jena.de/467/) for preprocessing the structural images by the VBM procedure. This VBM8 algorithm involves image bias correction, tissue classification, and normalization to the standard Montreal Neurological Institute (MNI) space using linear (12-parameter affine) and non-linear transformations. High-dimension DARTEL normalization, which is rather unbiased in its segmentation process, was used as anatomical registration with the default template provided in the VBM8 toolbox. Gray matter (GM) and white matter (WM) segments were modulated only by non-linear components, which allowed comparing the absolute amount of tissue corrected for individual brain volume, that is, correction for total brain volume.
Finally, modulated images were smoothed with a Gaussian kernel of 8 mm full width at half maximum. Although we used the East Asian Brains template in the process of affine regularization instead of European Brains, the default parameters were used in all other steps. Finally, 40 OCD patients and 40 HCs were assessed by structural MRI examinations with a 3.0-T MRI scanner.
Genotyping
A 10-ml venous blood sample was collected in EDTA vacuum tubes. Samples were immediately frozen at −80 °C until extraction of genomic DNA from nucleated white blood cells. Genomic DNA was extracted from peripheral blood leukocytes using a Promega DNA Purification Kit (Promega, Madison, WI, USA).
The polymerase chain reaction (PCR) was used to amplify 5-HTTLPR polymorphism. Forward (5′-GGCGTTGCCGCTCTGAATGC-3′) and reverse (5′-GAGGGACTGAGCTGGACAACCAC-3′) primers were used to amplify a fragment including 5-HTTLPR [20]. These primers amplify a 529-bp fragment for the S allele and a 575-bp fragment for the L allele.
PCR amplification was carried out in a final volume of 15 μl consisting of 50–100 ng genomic DNA, 2.5 mM deoxyribonucleotides, 0.2 μM of forward and reverse primers, PCR buffer (2× GC Buffer I, Takara Bio Inc., Shiga, Japan), and 1.25 U of DNA polymerase (TaKaRa LA Taq, Takara Bio Inc.). Denaturation was carried out at 94 °C for 30 s, annealing at 64 °C for 30 s, and extension at 72 °C for 3 min for 40 cycles.
To identify LA and LG alleles, a two-step protocol was performed. Step I: determination of the L or S allele, as described above; and step II: digestion of this amplicon with HapII (Takara Bio Inc.) restriction endonuclease. The assay was designed to include an invariant HapII digest site located 94 bp from the end of the amplicon to provide an internal control for digestion/partial digestion. Products were separated on a 4.0% agarose gel (Agarose-LE, Classic Type, Nacalai Tesque, Inc., Kyoto, Japan) supplemented with ethidium bromide (0.01%, Nacalai Tesque) and visualized under ultraviolet light. After separation of the digestion products by electrophoresis, the following restriction fragment allele sizes were obtained: LA (341, 126, 62 bp) and LG (174, 167, 126, 62 bp).
Statistical analysis
We conducted a two-sample t test, Chi square test, and Fisher’s exact test to test for differences in demographic variables between OCD patients and HCs as well as between different variants of the alleles of 5-HTTLPR in OCD patients.
The genotype frequencies of OCD patients and HCs were compared using Chi square test after checking the Hardy–Weinberg equilibrium.
We divided the patients into LA allele carriers (SLA, LALG, and LALA) and non-LA allele carriers (SS, SLG, and LGLG). Hu et al. [14] noted that the normalized (to SS genotype) expression value of the LA allele was approximately double the values of the S and LG alleles. Thus, we thought that expressions of genotypes including the LA allele were higher than those of other genotypes.
Statistical analysis was performed with SPM8, which implemented a general linear model. First, we performed a two-sample t test to detect the difference in GM volume between patients with OCD and HCs. The initial voxel threshold was set to P < 0.001 uncorrected. Clusters were considered significant that fell below a cluster-corrected family-wise error (FWE), P = 0.05. Next, we performed a two-way factorial analysis of variance between the 5-HTTLPR polymorphism and GM volumes in the OCD patients and HCs. A two-way ANOVA test was applied to assess the relationship between 5-HTTLPR polymorphism (LA or non-LA allele carriers) and GM brain volume changes in the OCD patients and HCs. If a statistical difference was present, a post hoc t test was performed to detect the inter-group difference of brain regions. Age and sex were set as covariates in the statistical analysis. We used a threshold of P < 0.05 cluster-corrected family-wise error (FWE) and P < 0.001 uncorrected with expected voxels per clusters. The P < 0.001 value is commonly used in VBM-based OCD studies [21–23].
Results
In demographic variables of age, gender, and handedness, OCD patients and HCs did not show any significant differences (Table 1). These variables also showed no significant difference between the genotypes of LA allele carriers or non-LA allele carriers in OCD patients (Table 2). OCD patients had significantly fewer years of education than HCs (Table 1). Non-LA allele carriers, furthermore, had significantly fewer years of education than those of LA allele carriers (Table 2). No significant differences were shown between the two genotypes in OCD regarding illness duration, age of onset, total Y-BOCS, or the 17-item HDRS (Table 2).
Table 1.
Clinical and demographic characteristics of OCD patients and HCs
| OCD patients (n = 40) | HCs (n = 40) | P value | |
|---|---|---|---|
| Age (years, mean ± SD)a | 35.40 ± 12.07 | 39.70 ± 12.97 | 0.129 |
| Gender (female/male)b | 20/20 | 26/14 | 0.175 |
| Handedness (right/left)b | 37/3 | 39/1 | 0.305 |
| Education (years, mean ± SD)a | 13.69 ± 2.43 | 15.15 ± 1.35 | 0.001 |
| Illness duration (years, mean ± SD) | 11.33 ± 10.10 | ||
| Age of onset (years, mean ± SD) | 23.98 ± 11.24 | ||
| Total Y-BOCS (total score, mean ± SD) | 21.95 ± 6.32 | ||
| HDRS (17 items)a | 6.08 ± 6.87 | 0.55 ± 0.88 | 0.000 |
| 5-HTTLPRa | |||
| 14/14 | 26 | 17 | 0.040 |
| 14/16 | 13 | 22 | |
| 16/16 | 1 | 1 | |
| LA allele carriers (SLA, LALG, LALA)b | 10 | 20 | 0.021 |
| Non-LA allele carriers (SS, SLG, LGLG)b | 30 | 20 |
We found a significant difference between the OCD patients and HCs in the distribution of LA allele carriers or non-LA allele carriers of 5-HTTLPR polymorphism
a T test
bChi square test
Table 2.
Clinical and demographic characteristics of non-LA allele carriers and LA allele carriers in OCD patients
| Non-LA allele carriers (n = 30) (SS, SLG, LGLG) |
LA allele carriers (n = 10) (SLA, LALG, LALA) |
P value | |
|---|---|---|---|
| Age (years, mean ± SD)a | 36.77 ± 12.50 | 31.30 ± 10.15 | 0.219 |
| Gender (female/male)b | 15/15 | 5/5 | 1.000 |
| Handedness (right/left)b | 27/3 | 10/0 | 0.411 |
| Education (years, mean ± SD)a | 13.23 ± 2.46 | 15.00 ± 1.70 | 0.042 |
| Illness duration (years, mean ± SD)a | 12.27 ± 10.75 | 8.50 ± 7.61 | 0.313 |
| Age of onset (years, mean ± SD)a | 24.53 ± 11.90 | 22.30 ± 9.30 | 0.593 |
| Total Y-BOCS (total score, mean ± SD)a | 22.75 ± 6.53 | 19.44 ± 5.15 | 0.176 |
| HDRS (17 items)a | 6.76 ± 7.61 | 4.10 ± 3.70 | 0.157 |
a T test
bChi square test
The genotype frequencies of our samples did not deviate significantly from the values predicted by the Hardy–Weinberg equation.
As for the genotypic distribution, 1/40 OCD patients (2.5%) and 1/40 HCs (2.5%) were LL homozygotes, 13/40 OCD patients (32.5%) and 22/40 HCs (55.0%) were LS heterozygotes, 26/40 OCD patients (65%) and 17/40 HCs (42.5%) were SS homozygotes, and 10/40 OCD patients (25.0%) and 20/40 HCs (50.0%) were LA allele carriers. We found a significant difference between the OCD patients and HCs in the distribution of LA allele carriers or non-LA allele carriers of 5-HTTLPR polymorphism (χ 2=5.333, 1 df, P = 0.021; Table 1).
In morphological changes in OCD, compared to the HCs, the OCD patients showed significant decreased GM volumes in the right hippocampus (extent threshold; k = 763 voxels, P < 0.05, FWE; Table 3; Fig. 1a) and increased GM volume in the left precentral gyrus (extent threshold; k = 797 voxels, P < 0.05, FWE; Table 3; Fig. 1b). In morphological changes associated with the 5-HTTLPR polymorphism, compared to LA allele carriers, non-LA allele carriers showed no significant GM volume difference.
Table 3.
VBM analysis including association of variance between 5-HTTLPR polymorphisms and GM volumes in OCD patients and HCs
| Regions | Brodmann area | Cluster size | Z | Talairach coordinates |
|---|---|---|---|---|
| x, y, z (mm2) | ||||
| Main effects | ||||
| Diagnosis effects (P < 0.05, FWE, 〈k〉 = 77.666) | ||||
| R hippocampus (OCD patients < HCs) | 763 | 5.08 | 33, −16, −18 | |
| L precentral gyrus (OCD patients > HCs) | 4 | 797 | 4.88 | −28, −27, 64 |
| Genotype effects | ||||
| No suprathreshold clusters | ||||
| Genotype-diagnosis interaction effects (P < 0.001, uncorrected, 〈k〉 = 63.146) | ||||
| R frontal pole | 10 | 112 | 4.35 | 26, 50, −6 |
R right, L left, FWE family-wise error, 〈k〉 expected voxels per clusters
Fig. 1.
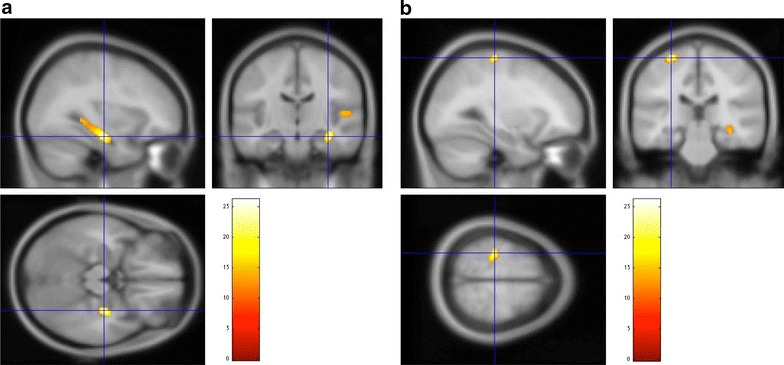
a OCD patients showed decreased GM volume in the right hippocampus compared to HCs. b OCD patients showed increased GM volume in the left precentral gyrus compared to HCs [P < 0.005, cluster-corrected family-wise error (FWE), 〈k〉 = 77.666]
As of genotype–diagnosis interaction, although no voxels survived multiple comparison, we observed a tendency that 5-HTTLPR polymorphism in OCD patients had stronger effects on the right frontal pole than those in HCs (P < 0.001, uncorrected; Table 3; Fig. 2). The OCD patients with the LA allele carriers of 5-HTTLPR polymorphism exhibited a statistical tendency of reduction of GM volumes in the right frontal pole compared to the HCs with the LA allele carriers.
Fig. 2.
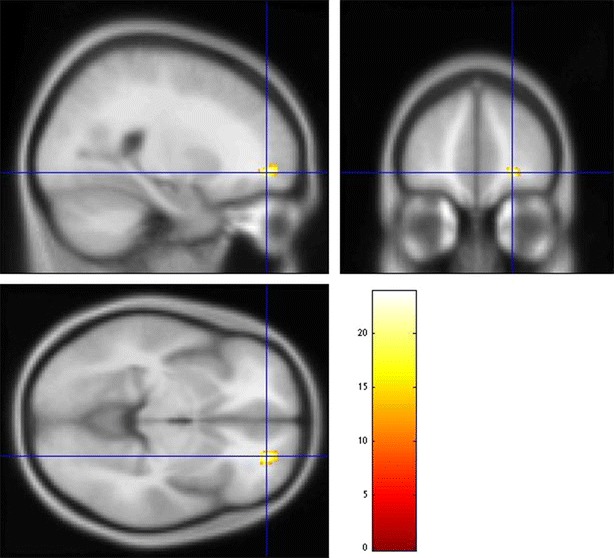
Results of genotype–diagnosis interaction effects on brain morphology. The stronger effects of 5-HTTLPR polymorphism on brain morphology in OCD patients than those in HCs were noted in the right frontal pole (P < 0.001, uncorrected, 〈k〉 = 63.146)
Discussion
In the present study, we found that the OCD patients showed significant decreased GM volume in the right hippocampus and increased GM volume in the left precentral gyrus. Moreover, our study suggested that LA allele carriers of the 5-HTTLPR polymorphism in OCD patients are associated with decreased GM volume in the right frontal pole.
Functional neuroimaging studies have been suggested that hippocampus might have an important role in the pathophysiology of OCD [24, 25]. On the other hand, structural imaging studies have been suggested that hippocampal alteration may play an important role in the pathophysiology of OCD [26, 27].
The precentral gyrus is a prominent structure on the surface of the posterior frontal lobe. It is the site of the primary motor cortex (Brodmann area 4). Several researchers have suggested that the precentral gyrus may be involved in the pathophysiology of OCD [28, 29]. Russo et al. [30] suggested that OCD might be considered as a sensory motor disorder where a dysfunction of sensory–motor integration might play an important role in the release of motor compulsions. Our results also showed that the precentral gyrus might be involved in the pathophysiology of OCD.
The frontal pole comprises the most anterior part of the frontal lobe that approximately covers BA10. During human evolution, the functions in this area resulted in its expansion relative to the rest of the brain [31]. Specifically, the functions include multi-tasking [32], cognitive branching [33], prospective memory [34], conflict resolution [35], and selection of sub-goals [36]. It is suggested that such a highly advanced cognitive function is affected in OCD [37, 38].
In the field of imaging genetics, many researchers reported [39–42] an association between the serotonin transporter gene and brain structure. Regarding OCD, Atmaca et al. [43] found a significant genotype-by-side interaction for the OFC.
In contrast to the previous result reported by Atmaca et al. [43], our result suggested that a liability in development of the central nervous system might have occurred in OCD patients who are LA allele carriers. Frodl et al. [44] suggested that the high-activity LA allele with its increased number of 5-HTT transporter proteins, concomitant decrease in serotonin levels, and reduced effects on neuroplastic processes might cause structural changes during major depression. With similar mechanism, the volume decrease in the right frontal pole might have occurred in OCD patients who are LA allele carriers.
There are some limitations to this study. First, we divided the patients into LA allele carriers (SLA, LALG, and LALA) and non-LA allele carriers (SS, SLG, and LGLG). In the view of expression activity, it might be better to divide samples into LALA and others. Although we could not employ this division because our study included few LALA genotypes, the difference between LALA and other genotypes should be explored with larger samples in the future. In addition, our sample size was too small to identify the difference between the effects of LA and non-LA alleles on the brains of OCD patients and HCs. Thus, these findings should be considered preliminary until replicated in a larger sample.
The OCD patients had significantly fewer years of education than HCs, and non-LA allele carriers had significantly fewer than LA allele carriers. Education years might affect the difference in GM volumes if education years were proportional to high intelligence. In this study, we did not measure the intelligence quotient (IQ). Larger gray matter volumes are associated with higher IQ [45]. Ideally, the IQ should be measured and set as a covariate in the statistical analysis.
Although we examined 5-HTTLPR polymorphism as the sole candidate gene in this study, many other polymorphisms such as glutamate system genes and dopamine system genes [46, 47] may affect the brain morphology of OCD patients. We hope to explore an association between more candidate genetic polymorphisms and brain morphology in the future.
Moreover, the OCD patients were concurrently on medication. Our study was not designed to investigate medication effects. Thus, analyses of the effects of different medication types on the hippocampus, precentral gyrus, and frontal pole volumes did not reveal a significant difference. Further studies are necessary to explore possible effects of medication.
Finally, the uncorrected threshold used in the present study may not fully protect against results due to chance and the results may include false positives. Therefore, the significant clusters found in the present study need to be validated further.
Conclusions
We found that relative to the HCs, the OCD patients showed significant decreased GM volume in the right hippocampus, and increased GM volume in the left precentral gyrus. The OCD patients with the LA allele carriers of 5-HTTLPR polymorphism exhibited a statistical tendency of reduction of GM volumes in the right frontal pole compared to the HCs with the LA allele carriers. Our preliminary findings suggest that a variation of the 5-HTTLPR polymorphism might affect brain morphology differently in OCD patients and HCs in the right frontal pole volumes.
Authors’ contributions
Study conception and design: TN, HS. Acquisition of data and clinical assessments: KM, SH, KO, KI, MK, TY, OT. Analysis and interpretation of data: LG, HM, MT. Drafting of manuscript: SH. Supervisors of this study: TN, HM, HK, SK. Critical revision: TN. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank OCD patients and HCs who agreed to participate in this study. This study was supported by a Grant-in-Aid for Scientific Research (C) (22591262) (25461732) (16K10253) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by the SENSHIN Medical Research Foundation. We were supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan in terms of the analysis technique, especially Dr. Kiyotaka Nemoto, who assisted in the VBM analysis technique. Katherine Ono provided assistance with language.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are available in the Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University repository.
Consent for publication
All the authors have read the manuscript and have approved this submission.
Ethics approval and consent to participate
The study was approved by the local ethics committee <22-111, 491-01>, and each participating patient provided written informed consent after receiving a complete description of the study, which was approved by the institutional review board.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (C) (22591262) (25461732) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by the SENSHIN Medical Research Foundation.
Abbreviations
- OCD
obsessive-compulsive disorder
- CSTC
cortico–striatal–thalamo–cortical
- VNTR
a variable number of tandem repeat
- 5-HTTLPR
serotonin transporter polymorphism
- GM
gray matter
- HCs
healthy controls
- VBM
voxel-based morphometry
- OFC
orbitofrontal cortex
- DLPFC
dorsolateral prefrontal cortex
- FEF
frontal eye fields
- ACC
anterior cingulate cortex
- PET
positron emission tomography
- fMRI
functional magnetic resonance imaging
- SCID
structured clinical interview for DSM-IV
- Y-BOCS
Yale-Brown Obsessive Compulsive Scale
- HDRS
Hamilton Depression Rating Scale
- MNI
Montreal Neurological Institute
- WM
white matter
- PCR
polymerase chain reaction
- FWE
family-wise error
- IQ
intelligence quotient
Contributor Information
Shinichi Honda, Phone: +81 92-642-5623, Email: shinhonn@npsych.med.kyushu-u.ac.jp.
Tomohiro Nakao, Phone: +81 92-642-5623, Email: tomona@npsych.med.kyushu-u.ac.jp.
Hiroshi Mitsuyasu, Email: hm_ku@npsych.med.kyushu-u.ac.jp.
Kayo Okada, Email: kayook@npsych.med.kyushu-u.ac.jp.
Leo Gotoh, Email: gotohleo@hotmail.com.
Mayumi Tomita, Email: mayutomato@yahoo.co.jp.
Hirokuni Sanematsu, Email: sane@npsych.med.kyushu-u.ac.jp.
Keitaro Murayama, Email: keimura@npsych.med.kyushu-u.ac.jp.
Keisuke Ikari, Email: kesuikerikai1975@ybb.ne.jp.
Masumi Kuwano, Email: m.kogusu@gmail.com.
Takashi Yoshiura, Email: qqxn4ze9k@estate.ocn.ne.jp.
Hiroaki Kawasaki, Email: hkawasaki@fukuoka-u.ac.jp.
Shigenobu Kanba, Email: skanba@npsych.med.kyushu-u.ac.jp.
References
- 1.American Psychiatric Association . American Psychiatric Association: DSM-5 Task Force: Diagnostic and statistical manual of mental disorders: DSM-5. 5. Washington: American Psychiatric Association; 2013. [Google Scholar]
- 2.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico–striatal pathways. Trends Cogn Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piras F, Piras F, Chiapponi C, Girardi P, Caltagirone C, Spalletta G. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015;62:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Togao O, Yoshiura T, Nakao T, Nabeyama M, Sanematsu H, Nakagawa A, Noguchi T, Hiwatashi A, Yamashita K, Nagao E, et al. Regional gray and white matter volume abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Psychiatry Res. 2010;184(1):29–37. doi: 10.1016/j.pscychresns.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20(4):1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(8):901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Murayama K, Nakao T, Sanematsu H, Okada K, Yoshiura T, Tomita M, Masuda Y, Isomura K, Nakagawa A, Kanba S. Differential neural network of checking versus washing symptoms in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:160–166. doi: 10.1016/j.pnpbp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry. 2013;18(7):799–805. doi: 10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- 9.Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, Geller DA, Murphy DL, Knowles JA, Grados MA, et al. Genome-wide association study in obsessive-compulsive disorder: results from the OCGAS. Mol Psychiatry. 2014;20(3):337–344. doi: 10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto R, Ichise M, Ito H, Ando T, Takahashi H, Ikoma Y, Kosaka J, Arakawa R, Fujimura Y, Ota M, et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive-compulsive disorder: a [11C]DASB PET study. NeuroImage. 2010;49(1):121–126. doi: 10.1016/j.neuroimage.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 11.Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, Pittenger C, Leckman JF. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):850–858. doi: 10.1002/ajmg.b.30699. [DOI] [PubMed] [Google Scholar]
- 12.Lin PY. Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):683–689. doi: 10.1016/j.pnpbp.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 14.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha FF, Marco LA, Romano-Silva MA, Correa H. Obsessive-compulsive disorder and 5-HTTLPR. Revista brasileira de psiquiatria. 2009;31(3):287–288. doi: 10.1590/S1516-44462009000300021. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington: American Psychiatric Association; 1995. [Google Scholar]
- 17.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch General Psychiatry. 1989;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser R, Muller-Oerlinghausen B, Filler D, Tremblay PB, Berghofer A, Roots I, Brockmoller J. Correlation between serotonin uptake in human blood platelets with the 44-bp polymorphism and the 17-bp variable number of tandem repeat of the serotonin transporter. Am J Med Genet. 2002;114(3):323–328. doi: 10.1002/ajmg.10119. [DOI] [PubMed] [Google Scholar]
- 21.Valente AA, Jr, Miguel EC, Castro CC, Amaro E, Jr, Duran FL, Buchpiguel CA, Chitnis X, McGuire PK, Busatto GF. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005;58(6):479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Yoo SY, Roh MS, Choi JS, Kang DH, Ha TH, Lee JM, Kim IY, Kim SI, Kwon JS. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci. 2008;23(1):24–30. doi: 10.3346/jkms.2008.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 24.McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry. 1994;164(4):459–468. doi: 10.1192/bjp.164.4.459. [DOI] [PubMed] [Google Scholar]
- 25.Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34(4–5):317–324. doi: 10.1016/S0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 26.Atmaca M, Yildirim H, Ozdemir H, Ozler S, Kara B, Ozler Z, Kanmaz E, Mermi O, Tezcan E. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1283–1286. doi: 10.1016/j.pnpbp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Hong SB, Shin YW, Kim SH, Yoo SY, Lee JM, Kim IY, Kim SI, Kwon JS. Hippocampal shape deformity analysis in obsessive-compulsive disorder. Eur Arch Psychiatry Clin Neurosci. 2007;257(4):185–190. doi: 10.1007/s00406-006-0655-5. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto N, Nakaaki S, Kawaguchi A, Sato J, Kasai H, Nakamae T, Narumoto J, Miyata J, Furukawa TA, Mimura M. Brain structural abnormalities in behavior therapy-resistant obsessive-compulsive disorder revealed by voxel-based morphometry. Neuropsychiatr Disease Treat. 2014;10:1987–1996. doi: 10.2147/NDT.S69652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Silk T, Seal M, Dally K, Vance A. Widespread decreased grey and white matter in paediatric obsessive-compulsive disorder (OCD): a voxel-based morphometric MRI study. Psychiatry Res. 2013;213(1):11–17. doi: 10.1016/j.pscychresns.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Russo M, Naro A, Mastroeni C, Morgante F, Terranova C, Muscatello MR, Zoccali R, Calabro RS, Quartarone A. Obsessive-compulsive disorder: a “sensory–motor” problem? Int J Psychophysiol. 2014;92(2):74–78. doi: 10.1016/j.ijpsycho.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114(3):224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38(6):848–863. doi: 10.1016/S0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 33.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 34.Okuda J, Fujii T, Ohtake H, Tsukiura T, Yamadori A, Frith CD, Burgess PW. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int J Psychophysiol. 2007;64(3):233–246. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Netw. 2006;19(9):1422–1429. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 37.Cavedini P, Zorzi C, Piccinni M, Cavallini MC, Bellodi L. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry. 2010;67(12):1178–1184. doi: 10.1016/j.biopsych.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affect Disord. 2007;104(1–3):15–23. doi: 10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 39.Selvaraj S, Godlewska BR, Norbury R, Bose S, Turkheimer F, Stokes P, Rhodes R, Howes O, Cowen PJ. Decreased regional gray matter volume in S’ allele carriers of the 5-HTTLPR triallelic polymorphism. Mol Psychiatry. 2011;16(5):472–473. doi: 10.1038/mp.2010.112. [DOI] [PubMed] [Google Scholar]
- 40.Eker MC, Kitis O, Okur H, Eker OD, Ozan E, Isikli S, Akarsu N, Gonul AS. Smaller hippocampus volume is associated with short variant of 5-HTTLPR polymorphism in medication-free major depressive disorder patients. Neuropsychobiology. 2011;63(1):22–28. doi: 10.1159/000321834. [DOI] [PubMed] [Google Scholar]
- 41.Frodl T, Zill P, Baghai T, Schule C, Rupprecht R, Zetzsche T, Bondy B, Reiser M, Moller HJ, Meisenzahl EM. Reduced hippocampal volumes associated with the long variant of the tri- and diallelic serotonin transporter polymorphism in major depression. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1003–1007. doi: 10.1002/ajmg.b.30680. [DOI] [PubMed] [Google Scholar]
- 42.Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, Bottlender R, Schule C, Zwanzger P, Engel RR, et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch Gen Psychiatry. 2004;61(2):177–183. doi: 10.1001/archpsyc.61.2.177. [DOI] [PubMed] [Google Scholar]
- 43.Atmaca M, Onalan E, Yildirim H, Yuce H, Koc M, Korkmaz S, Mermi O. Serotonin transporter gene polymorphism implicates reduced orbito-frontal cortex in obsessive-compulsive disorder. J Anxiety Disord. 2011;25(5):680–685. doi: 10.1016/j.janxdis.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Frodl T, Koutsouleris N, Bottlender R, Born C, Jager M, Morgenthaler M, Scheuerecker J, Zill P, Baghai T, Schule C, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry. 2008;13(12):1093–1101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- 45.Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. NeuroImage. 2004;23(1):425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Wu K, Hanna GL, Easter P, Kennedy JL, Rosenberg DR, Arnold PD. Glutamate system genes and brain volume alterations in pediatric obsessive-compulsive disorder: a preliminary study. Psychiatry Res. 2013;211(3):214–220. doi: 10.1016/j.pscychresns.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olver JS, O’Keefe G, Jones GR, Burrows GD, Tochon-Danguy HJ, Ackermann U, Scott A, Norman TR. Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J Affect Disord. 2009;114(1–3):321–326. doi: 10.1016/j.jad.2008.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University repository.


