Abstract
Background
In the Guadeloupe and Saint Martin islands, Aedes aegypti mosquitoes are the only recognized vectors of dengue, chikungunya, and Zika viruses. For around 40 years, malathion was used as a mosquito adulticide and temephos as a larvicide. Since the European Union banned the use of these two insecticide molecules in the first decade of the 21st century, deltamethrin and Bacillus thuringiensis var. israelensis are the remaining adulticide and larvicide, respectively, used in Guadeloupe. In order to improve the management of vector control activities in Guadeloupe and Saint Martin, we investigated Ae. aegypti resistance to and mechanisms associated with deltamethrin, malathion, and temephos.
Methods
Ae. aegypti mosquitoes were collected from six different localities of Guadeloupe and Saint Martin. Larvae were used for malathion and temephos bioassays, and adult mosquitoes for deltamethrin bioassays, following World Health Organization recommendations. Knockdown resistance (Kdr) genotyping for V1016I and F1534C mutations, and expression levels of eight enzymes involved in detoxification mechanisms were examined in comparison with the susceptible reference Bora Bora strain.
Results
Resistance ratios (RR50) calculated for Ae. aegypti larvae showed high resistance levels to temephos (from 8.9 to 33.1-fold) and low resistance levels to malathion (from 1.7 to 4.4-fold). Adult females displayed moderate resistance levels to deltamethrin regarding the time necessary to affect 50% of individuals, varying from 8.0 to 28.1-fold. Molecular investigations on adult mosquitoes showed high resistant allele frequencies for V1016I and F1534C (from 85 to 96% and from 90 to 98%, respectively), as well as an overexpression of the glutathione S-transferase gene, GSTe2, the carboxylesterase CCEae3a, and the cytochrome genes 014614, CYP6BB2, CYP6M11, and CYP9J23.
Conclusions
Ae. aegypti populations from Guadeloupe and Saint Martin exhibit multiple resistance to organophosphates (temephos and malathion), and pyrethroids (deltamethrin). The mechanisms associated with these resistance patterns show strong frequencies of F1534C and V1016I Kdr mutations, and an over-expression of CCEae3a, GSTe2, and four cytochrome P450 genes (014614, CYP9J23, CYP6M11, CYP6BB2). These results will form the baseline for a deeper understanding of the insecticide resistance levels and associated mechanisms of Ae. aegypti populations and will be used to improve vector control strategies in Guadeloupe and Saint Martin.
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-017-0254-x) contains supplementary material, which is available to authorized users.
Keywords: Aedes aegypti, Mosquitoes, Insecticide resistance, Deltamethrin, Malathion, Temephos, Guadeloupe, Saint Martin
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the six official working languages of the United Nations.
Background
Recently, from December 2013 to January 2015, the chikungunya virus (CHIKV) intensely hit the Guadeloupe islands of the French West Indies, which resulted in a severe outbreak in which an estimated 40% (160,000 people) of the population became infected [1, 2]. Most of the Latin American countries and Caribbean islands were still reporting CHIKV outbreaks when a new threat was reported from Brazil—the arrival of the Zika virus (ZIKV) [3]. This arbovirus, new for the American region, was first considered as a mild disease until the discovery of Guillain-Barré and microcephaly syndromes associated with ZIKV infections [4]. Since then, the number of severe cases has drastically increased in Brazil and in most of the Latin American countries and Caribbean islands where ZIKV has been reported, and the first severe neurological cases associated with the ZIKV in the French West Indies were reported from Martinique in early 2016 [5]. In the French West Indies, both CHIKV and ZIKV are transmitted by the mosquito species Aedes aegypti, the vector of the dengue viruses that cause epidemics in the region every 2 to 3 years [6].
Since no specific treatment and/or vaccines are commonly available against CHIKV and ZIKV, and since the dengue vaccine has been deployed only very recently in a small number of experimental areas [7], the control of vectors and personal protection against mosquito bites remain the only available tools for preventing and controlling these emerging arboviral diseases [8, 9].
Historically, vector control activities in Guadeloupe have been carried out by a Vector Control Agency from the French Ministry of Health local delegation (Agence Régionale de Santé, ARS) and by ensuring larval reduction through the elimination of breeding sites and larvicide treatments, as well as the spraying of adulticide outdoors and indoors [10]. Biological larval control has also been tested using larvivorous fish in bigger tanks (J. Gustave, personal communication). The insecticides commonly used in the past have been temephos as a larvicide, and malathion and deltamethrin as adulticides [11]. However, since 2009, temephos has been withdrawn from the list of vector control insecticides, following European Union recommendations, and has been replaced by biological products using Bacillus thuringiensis var. israelensis (Bti) [12]. In 2010, the adulticide malathion was also withdrawn, consequently, the vector control activities in Guadeloupe were reorganized and now comprise routine elimination of breeding sites, the use of Bti as a larvicide, and the use of deltamethrin as an indoor adulticide.
In the French overseas departments of Guadeloupe, French Guiana, and Martinique, the surveillance of arbovirus epidemics is organized around a specific system called the dengue epidemics surveillance, alert and management program (PSAGE). Surveillance and control measures increase if necessary, according to the epidemiological situation, which is monitored all year round through a network of physicians in each territory who report weekly numbers of clinical cases for dengue, CHIKV, and now ZIKV. The PSAGE system proposes five operational situations [http://opac.invs.sante.fr/doc_num.php?explnum_id=3517]: phase 1) inter-epidemic situation with sporadic transmission; phase 2) some clusters of cases, seasonal increase, outdoor spraying of deltamethrin in and around clusters; phase 3) pre-epidemic alert with a significant increase in the number of clinical cases, outdoor spraying of deltamethrin in selected areas, reinforcement of entomological surveillance; phase 4) outbreak alert, intensification of outdoor spraying of deltamethrin in all places, communicating messages adapted to the epidemic context (including personal protection and reinforcement of personal elimination of breeding sites) to populations; and the final phase 5) end of the epidemic and evaluation of the lessons learned.
The large deployment of insecticides to control mosquito vectors has allowed the development of resistance worldwide [13]. For the Guadeloupe islands, very few studies have been done on the resistance levels of the mosquito Ae. aegypti [10], and resistance mechanisms have not been investigated. The resistance of mosquitoes to chemical insecticides can be attributed to two main mechanisms [14]. The first mechanism is the modification of the molecular site targeted by the chemical, and the second is an increased metabolism of the chemical through mutations and/or over-expression of detoxifying enzymes.
First report of Ae. aegypti insecticide resistance in Guadeloupe and Saint Martin are almost 20 years old, and resistance levels have probably significantly evolved with the increasing insecticide dosages. Thus, an update of Ae. aegypti resistance levels is urgently needed to improve the management of vector control activities in the Guadeloupe and Saint-Martin islands. Therefore, in this study, we investigated insecticide resistance levels and their associated molecular mechanisms for six Ae. aegypti populations collected in Guadeloupe and Saint Martin islands between January 2014 and October 2015.
Methods
Mosquito collection and rearing
Ae. aegypti mosquitoes were collected as larvae or pupae in urban, suburban, and rural areas from six different locations of Guadeloupe and Saint Martin (see Table 1 and Fig. 1). Mosquitoes were collected around private houses and in public areas, and were chosen randomly. Larvae and pupae were brought back to the insectarium facilities and reared in containers with around 150 to 200 mosquitoes per liter of dechlorinated tap water and supplemented with one yeast tablet at a constant temperature of 27° ± 1 °C, 80% of humidity, and a 12-h light/12-h dark cycle. Emerged adults were kept in cages and fed with a 10% sucrose solution. In order to produce the first generation (F1) of mosquitoes, the females were fed with fresh human blood using a Hemotek feeding system (Hemotek Ltd. Great Britain, United Kingdom). Human blood was chosen because the natural Ae. aegypti populations of Guadeloupe are highly anthropophilic and do not easily feed on other blood sources. Samples were taken from the investigators in the medical laboratory of the Pasteur Institute of Guadeloupe. The first laboratory generation(F1) mosquitoes were used for all experiments and molecular biology investigations, except for mosquitoes from Baie-Mahault and Deshaies for which second laboratory generation (F2) mosquitoes were used. Bora-Bora susceptible mosquitoes were provided by the Martinique mosquito control Agency at the egg stage and reared under the same conditions. The Bora-Bora strain is considered as a reference for insecticide susceptibility tests as previously used in Marcombe et al. 2009 [15]. All of the mosquito collections were done between January 2014 and October 2015 (see Table 1).
Table 1.
Ae. aegypti mosquito populations used in the study
| Mosquito population | Collection site | GPS coordinates | Breeding sites: type (number) | Date of collection | Conducted tests |
|---|---|---|---|---|---|
| ABY | Les Abymes | 16°14'09.7"N 61°30'20.9"W | tires (multiple) | 14 Jan 2014 | Larval test |
| ABY | Les Abymes | 16°14'09.7"N 61°30'20.9"W | tires (multiple); casks (4); buckets (2) | 23 Jan 2015 | Adult test |
| ABY | Les Abymes | 16°17'57.5"N 61°29'25.6"W | casks (1) | 30 Jan 2015 | Adult test |
| ABY | Les Abymes | 16°16'03.5"N 61°30'09.5"W | casks (2); bucket (1) | 30 Jan 2015 | Adult test |
| SF | Saint-François | 16°15'N 61°16'W | aquatic plants (2); plant cutting (1) | 16 Jan 2014 | Larval test |
| SF | Saint-François | 16°16'N 61°16'W | plant cutting (2) | 16 Jan 2014 | Larval test |
| SF | Saint-François | 16°17'N 61°17'W | buckets (9); casks (3); small wastes (4); pot dish (1) | 24 Sep 2015 | Adult test |
| BM | Baie-Mahault | 16°14'00.2"N 61°36'36.8"W | plant cutting (1); bucket (1) | 22 Jan 2014 | Larval test |
| BM | Baie-Mahault | 16°15'30.9"N 61° 35'14.6"W | watering can (1) | 22 Jan 2014 | Larval test |
| BM | Baie-Mahault | 16°15'09.0"N 61°35'54.9"W | plant cutting (1); bucket (1) | 13 Feb 2015 | Adult test |
| BM | Baie-Mahault | 16°14'22.8"N 61°36'03.7"W | Cask (1) | 13 Feb 2015 | Adult test |
| SXM East | Saint Martin East French part | 18°03'N 63°01'W | abandoned boat (1); abandoned jacuzzi (1); tires (multiple); casks (2); discarded small containers (1) | 04–05 Feb 2014 | Larval test |
| SXM West | Saint-Martin West French part | 18°03'N 63°06'W | tires (2); casks (3); small waste (1); plant cutting (1); pot dish (1); buckets (3) | 04–05 Feb 2014 | Larval test |
| SXM | Saint-Martin | 18°04'N 63°03'W | abandoned boat (1); tires (multiple); abandoned jet ski (1); discarded small containers (1) | 25–26 Nov 2014 | Adult test |
| AB | Anse-Bertrand | 16°26'N 61°28'W | pot dish (2); flower vase (1); casks (4); watering can (1); bucket (2); tires (5) | 07 Oct 2015 | Larval and Adult test |
| DH | Deshaies | 16°18'N 61°47'W | casks (2); bucket (1); flower pot (1); watering can (1); tires (multiple) | 06 Oct 2015 | Larval and Adult test |
ABY Les Abymes, SF Saint-François, BM Baie-Mahault, SXM Saint Martin, AB Anse-Bertrand, DH Deshaies
Fig. 1.

Geographical distribution of Ae. aegypti sampling sites in Guadeloupe and Saint Martin islands. In Guadeloupe, the sampling sites were located in Grande-Terre island (Anse-Bertrand, Saint-François, Les Abymes), and Basse-Terre island (Baie-Mahault and Deshaies)
Larval bioassays
Larval bioassays were performed following WHO recommendations [16]. The late third- and early fourth-instar larvae were used for each mosquito population. Four replicates per concentration and five concentrations were tested with 25 larvae per replicate and per concentration. The insecticide alcohol dilutions were provided by the WHO Malaysia manufacturer (Vector Control Research Unit, School of Biological Sciences, Universiti Sains Malaysia). The insecticide solutions received were temephos (156.25 μg/ml and 31.25 μg/ml) and malathion (156.25 μg/ml and 31.25 μg/ml). To test the mosquito field samples and the Bora- Bora strain, concentrations were adjusted to include different percentages of survival/mortality, varying between 0 and 100%. Larvae were not fed during the 24 h of insecticide exposure. The concentrations tested for field samples were 1.5, 0.3, 0.15, 0.05, 0.015 μg/ml, and 1, 0.4, 0.2, 0.1, 0.04 μg/ml, for temephos and malathion, respectively (from insecticide solutions at 156.25 μg/ml); and 0.009, 0.0075, 0.006, 0.004, 0.0025 μg/ml (temephos), and 0.1, 0.075, 0.05, 0.04, 0.025 μg/ml (malathion) for the reference Bora Bora strain (from insecticide solutions at 31.25 μg/ml). The results were analyzed with XLSTAT-Biomed software (XLSTAT 2015 France) to determine the lethal concentration for 50% (LC50) and 95% (LC95) of the populations. Resistance ratios (RR50 and RR95) were calculated using LC50 and LC95 rates from Ae. aegypti-field-sampled populations compared with the LC50 and LC95 rates of the susceptible Bora Bora strain. The resistance levels were ranked into three categories: low resistance (RR50 < 5), medium or moderate resistance (5 ≤ RR50 ≤ 10), and high resistance (RR50 > 10) [17, 18].
Adult bioassays
Adult bioassays were performed following WHO recommendations adapted for this study [19, 20]. Deltamethrin-impregnated papers were used at three different concentrations: 0.05, 0.06, and 0.08%. For each mosquito population and each concentration, four replicates and two negative controls of 25 females each were tested. The 0.05% deltamethrin-impregnated papers were ordered from the WHO Malaysia provider, and the 0.06 and 0.08% deltamethrin papers were impregnated in the laboratory using the deltamethrin, PESTANAL® (Sigma-Aldrich, Inc. Missouri, USA) in a solution of two-thirds acetone (Sigma-Aldrich, Inc.) and one-third silicone (VWR® Pennsylvanie, USA) with Whatman® Grade 1 Qualitative Filtration Paper (Sigma-Aldrich, Inc. Missouri, USA). The impregnation was done following WHO procedures [19] using 2 ml of insecticide solution to impregnate one 12 × 15 cm paper that was dried for one night and stored at 4 °C. Adult mosquitoes were exposed by tarsal contact for 1 h to the 0.06 and 0.08% deltamethrin-impregnated papers. The knockdown (KD) (after 1 h of exposure) and mortality rates (after 24 h) were determined. A kinetic of KD rate during 2 h (by accounting the number of KD mosquitoes every 5 min) was also conducted for each population using 0.05% impregnated papers to estimate the KDT50 (Time necessary to affect 50% of mosquitoes) using XLSTAT software (dose-effect option). The KRR50 is the ratio between KDT50 of mosquitoes from the field and KDT50 of mosquitoes from the Bora Bora strain. KRR50 was scaled as follows: KRR50<1 = susceptible, 1≤KRR50<10 = low resistance, 10≤KRR50≤30 = moderate resistance, 30<KRR50<100 = high resistance, and KRR50≥100 = very high resistance [21].
Knockdown resistance (Kdr) genotyping
Total DNA of 24 single female mosquitoes per Ae. aegypti population was extracted using the QIAamp® DNA Mini Kit (Qiagen, Redwood city, CA, USA), following the manufacturer’s instructions. The region of the gene encoding sodium channel was amplified using the primers summarized in Table 2. A guanine-adenine transition in the first position of 1016 encodes a valine/isoleucine replacement (V1016I), while a thymine-guanine transition in the second position of 1534 encodes a phenylalanine/cysteine replacement (F1534C) [22–24].
Table 2.
| Kdr mutation | Primer name | Primer sequence (5' – 3') |
|---|---|---|
| V1016I | Val1016-f | GCGGGCAGGGCGGCGGGGGCGGGGCCACAAATTGTTTCCCACCCGCACCGG |
| Iso1016-f | GCGGGCACAAATTGTTTCCCACCCGCACTGA | |
| Iso1011-r | GGATGAACCSAAATTGGACAAAAGC | |
| F1534C | C1534-f | GCGGGCAGGGCGGCGGGGGCGGGGCCTCTACTTTGTGTTCTTCATCATGTG |
| F1534-f | GCGGGCTCTACTTTGTGTTCTTCATCATATT | |
| CP-r | TCTGCTCGTTGAAGTTGTCGAT |
The allele-specific real-time quantitative polymerase chain reaction (PCR) using SYBR® Green dye (Applied Biosystems®, Californie, USA) was done on an Applied Biosystems® 7500 thermal cycler (Californie, USA). The amplification consisted of a 95 °C 3 min holding stage, 40 cycles at 95 °C for 15 s, a 60 °C 31 s cycling stage, and a melt curve stage. For the PCR, a 15 μl solution comprising 7.5 μl of SYBR® Green PCR Master Mix, 0.4 μl of 10 μM reverse primer, 0.2 μl of 10 μM of both forward primers (C1534-f and F1534-f, see Table 2), 3.7 μl of H2O, and 3 μl of genomic DNA was made.
A Ile1016/Ile1016 homozygote (resistant) has a single peak at 76 °C; a Val1016/Val1016 homozygote (susceptible) has double peaks at 79 °C and 83 °C; and a Val1016/Ile1016 heterozygote has triple peaks at 76 °C, 79 °C, and 83 °C. A Cys1534/Cys1534 homozygote (resistant) has a single peak at 82 °C; a Phe1534/Phe1534 (susceptible) homozygote has a single peak at 78 °C; and a Phe1534/Cys1534 heterozygote has double peaks at 78 °C and 82 °C. Lastly, the distribution of the three genotypes for both mutations and allele frequencies were calculated.
Gene expression study
RNA was extracted from three pools of 25 female mosquitoes each using the TRIzol®/chloroform (Invitrogen, Carlsbad, CA, USA) method, and cDNA was synthetized with SuperScript VILO Master Mix (Invitrogen) after a DNAse I treatment with DNAse I Amplification Grade (Invitrogen). A 15 ng/μl of cDNA was used for relative quantification PCR. For PCR, a 15 μl solution comprising 7.5 μl of SYBR® Green PCR Master Mix, 0.45 μl of 10 μM of each primer, 3.6 μl of H2O, and 3 μl of cDNA was made. The thermocycling conditions were the same as for Kdr genotyping. The differential expressions of eight candidate genes (CCEae3a, CCEae6a, 014614, CYP6BB2, CYP6M11, CYP9J23, CYP9J28, and GSTe2; All primer sequences were designed by “Pollution, Environment, Ecological toxicology, Ecological remediation unit of Alpine Ecological Laboratory of Grenoble, summarized in Additional file 2) for the studied six populations were calculated using the ∆∆Ct method, taking into account PCR efficiency (see Table 3). Results show genes relative quantification for field mosquito populations compared to Bora Bora strain and using both RpS7 and RpL8 housekeeping genes (DataAssist™ v3.01 software).
Table 3.
The 12 genes used in relative quantitative PCR [59]
| Enzyme type | Accession number | Name (VectorBase) | Name in this study |
|---|---|---|---|
| Carboxyl/cholinesterase alpha esterase | AAEL005112 | CCEae3a | CCEae3a |
| Carboxyl/cholinesterase alpha esterase | AAEL005122 | CCEae6a | CCEae6a |
| Cytochrome P450 | AAEL014614 | ND | 014614 |
| Cytochrome P450 | AAEL014893 | CYP6BB2 | CYP6BB2 |
| Cytochrome P450 | AAEL009127 | CYP6M11 | CYP6M11 |
| Cytochrome P450 | AAEL014615 | CYP9J23 | CYP9J23 |
| Cytochrome P450 | AAEL014617 | CYP9J28 | CYP9J28 |
| Cytochrome P450 | AAEL007808 | CYP4D39 | 007808 |
| Glutathione transferase | AAEL007951 | GSTe2 | GSTe2 |
| 60S ribosomal protein L8 | AAEL000987 | RpL8 | RpL8 |
| 40S ribosomal protein S7 | AAEL009496 | RpS7 | RpS7 |
| Chloride channel protein 2 | AAEL005950 | ND | 005950 |
Gene amplification
The same DNA that was used for Kdr PCR was also used for the gene amplification study. Relative quantitative PCR was performed for five of the eight genes, i.e. CCEae3a, 014614, CYP6M11, CYP9J23, and GSTe2. The PCR was done as previously described in the Gene expression study section above. Results show genes relative quantification for field mosquito populations compared to Bora Bora strain and using both 007808 and 005950 housekeeping genes (DataAssist™ v3.01 software).
Statistical analysis
Statistical analyses were performed with STATISTICA 8 software (Statsoft Inc., Oklahoma, USA) and XLSTAT 2015 software. STATISTICA was used to perform ANOVA Kruskal-Wallis tests (to compare adult KD/mortality rates regarding deltamethrin and amplification gene ratios); Kruskal-Wallis Z tests (to compare larval mortality rates regarding temephos and malathion, adult mortality rates regarding deltamethrin, and expression and amplification genes ratios); and Mann–Whitney U tests (to compare gene expression ratios of Bora Bora strain and each population). XLSTAT was used to perform log-probit logistical regression to investigate the dose-effect relation regarding insecticide larval tests data, as well as to estimate KDT values using adult kinetic data. XLSTAT was also used to perform Spearman’s rank correlation tests (between expression and amplification ratios) and the principal component analysis (PCA).
Results
Larval bioassays
Ae. aegypti larvae of mosquito populations from the Guadeloupe and Saint Martin islands are highly resistant to temephos (see Table 4), with RR50 ranging from 8.9 (7.75–10.13) for Saint Martin West to 33.1 (29.63–37.75) for Les Abymes. The RR95 varied from 11.4 (10.21–13.07) for Saint Martin West to 39.8 (31.43–54.50) for Anse-Bertrand. The mosquito populations from Les Abymes and Baie-Mahault had the greatest resistance ratios, suggesting that these are the most resistant to temephos, followed by populations from Saint-François and Saint Martin East with intermediate levels of resistance, while mosquitoes from Saint Martin West had the lowest resistance ratio and were thus the most susceptible to temephos.
Table 4.
Resistance status of Ae. aegypti larvae from Guadeloupe and Saint Martin, compared to the reference Bora Bora strain, to malathion and temephos
| Population | Temephos | Malathion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (mg/L) | LC50 95% CI | RR50 | LC95 (mg/L) | LC95 95% CI | RR95 | LC50 (mg/L) | LC50 95% CI | RR50 | LC95 (mg/L) | LC95 95% CI | RR95 | |
| ABY | 0.265 | 0.237–0.302 | 33.125 | 0.529 | 0.461–0.632 | 37.786 | 0.285 | 0.265–0.307 | 4.385 | 0.464 | 0.429–0.510 | 3.899 |
| SF | 0.123 | 0.111–0.136 | 15.375 | 0.233 | 0.211–0.262 | 16.643 | 0.168 | 0.155–0.183 | 2.585 | 0.278 | 0.253–0.314 | 2.336 |
| BM | 0.233 | 0.211–0.259 | 29.125 | 0.450 | 0.402–0.519 | 32.143 | 0.277 | 0.256–0.299 | 4.262 | 0.465 | 0.428–0.515 | 3.908 |
| SXM (West) | 0.071 | 0.062–0.081 | 8.875 | 0.160 | 0.143–0.183 | 11.429 | 0.203 | 0.187–0.221 | 3.123 | 0.354 | 0.324–0.395 | 2.975 |
| SXM (East) | 0.128 | 0.112–0.144 | 16.000 | 0.303 | 0.273–0.345 | 21.643 | 0.239 | 0.219–0.262 | 3.677 | 0.449 | 0.409–0.502 | 3.773 |
| AB | 0.132 | 0.115–0.152 | 16.500 | 0.557 | 0.440–0.763 | 39.786 | 0.111 | 0.101–0.122 | 1.708 | 0.260 | 0.227–0.313 | 2.185 |
| DH | 0.151 | 0.132–0.172 | 18.875 | 0.555 | 0.442–0.758 | 39.643 | 0.109 | 0.099–0.119 | 1.677 | 0.238 | 0.208–0.284 | 2.000 |
| Bora Bora | 0.008 | 0.008–0.009 | 1.000 | 0.014 | 0.012–0.017 | 1.000 | 0.065 | 0.061–0.068 | 1.000 | 0.119 | 0.108–0.135 | 1.000 |
LC 50 lethal concentration for 50% of individuals, RR 50 resistance ratio between field samples and the Bora Bora strain, LC 95 lethal concentration to 95% of individuals, RR 95 resistance ratio between field samples and Bora Bora strain, ABY Les Abymes, SF Saint-François, BM Baie-Mahault, SXM Saint-Martin, AB Anse-Bertrand, DH Deshaies
The differences in the levels of resistance observed between Ae. aegypti populations for temephos were tested for statistical significance using the Kruskal-Wallis Z test, which highlighted significantly higher resistance levels among mosquitoes from Les Abymes and Baie-Mahault, compared to Saint-François and Saint Martin (P < 0.001). In addition, significantly higher resistance levels were also observed in the three mosquito populations from Anse-Bertrand, Saint-François, and Deshaies, compared to Saint Martin West (P = 0.000 for Anse-Bertrand, P = 0.027 for Saint-François, and P = 0.000 for Deshaies). Finally, the mosquitoes from Saint Martin West had a significantly higher mortality rate than all mosquito populations from Guadeloupe.
For malathion, the results for all field mosquito populations show some resistance in comparison with the susceptible Bora Bora strain (see Table 4), with the RR50 ranging from 1.7 for Anse-Bertrand and Deshaies, to 4.4 for Les Abymes. The RR95 varied from 2.0 for Deshaies to 3.9 for Les Abymes and Baie-Mahault.
The differences in the levels of resistance between Ae. aegypti populations were tested for statistical significance using the Kruskal-Wallis Z test. The results show higher mortality rates suggesting lower resistance for the mosquito populations of Anse-Bertrand and Deshaies compared to all other field populations (P < 0.01) except for Saint-François (P = 0.325 for Anse-Bertrand and P = 0.227 for Deshaies). The mosquito population of Saint-François showed a higher mortality rate than those from Les Abymes (P = 0.001) and Baie-Mahault (P = 0.002), which suggests that these two latter populations have a higher resistance to malathion.
Adult bioassays
The KD rates, mortality rates, and resistance ratios (KRR50) were estimated for adult mosquitoes and are summarized in Table 5. The KD rates after 1 h of exposure varied from 0.93 (Deshaies) to 1.0 (Saint Martin), and 0.96 (Anse-Bertrand) to 1.0 (Saint-François, Saint Martin, Baie-Mahault, and Deshaies), for the 0.06 and 0.08% concentrations, respectively. The differences between the KD rates were significant between the mosquito populations of Anse-Bertrand (0.96) and Les Abymes (0.99), which had the lowest KD rates suggesting higher resistance to the 0.08% deltamethrin concentration (ANOVA Kruskal-Wallis: P = 0.011). Meanwhile, Deshaies (0.93) and Anse-Bertrand (0.94) mosquito populations had the lowest KD rates for the 0.06% deltamethrin concentration (ANOVA Kruskal-Wallis: P = 0.000).
Table 5.
Resistance status of Ae. aegypti females from Guadeloupe and Saint Martin to deltamethrin
| Deltamethrin concentration/time of exposure | Assessed parameters | DH | AB | SF | SXM | ABY | BM |
|---|---|---|---|---|---|---|---|
| 0.06%/1 h | KD rate | 0.93 | 0.94 | 0.98 | 1 | 0.96 | 0.99 |
| Mortality rate | 0.83 | 0.64 | 0.79 | 0.88 | 0.79 | 0.90 | |
| 0.08%/1 h | KD rate | 1 | 0.96 | 1 | 1 | 0.99 | 1 |
| Mortality rate | 0.94 | 0.93 | 0.81 | 0.98 | 0.85 | 0.99 | |
| 0.05%/2 h | KRR50 (ci 95%) | 17.7 (16.7–20.5) | 28.1 (24.2–34.9) | 13.7 (13.0–14.4) | 12.4 (12.0–12.8) | 8.0 (7.8–8.3) | 13.6 (13.1–14.2) |
KD rate: rate of knockdown mosquitoes after one hour of exposition, KDT50: time (min) necessary to knock down 50% of mosquitoes
KDT50 were determined using papers impregnated with 0.05 g/100 ml deltamethrin following WHO insecticide testing recommendations, KRR50: ratio between field samples KDT50 and susceptible Bora Bora strain KDT50, DH Deshaies, AB Anse-Bertrand, SF Saint-François, SXM Saint-Martin, ABY Les Abymes, BM Baie-Mahault
The mortality rates after 24 h following a 1-h exposure to a 0.06% deltamethrin concentration were significantly different between mosquito populations from Anse-Bertrand (0.64) and Baie-Mahault (0.90) (Kruskal-Wallis Z test, P = 0.040). The mortality rates after 24 h following a 1-h exposure to a 0.08% deltamethrin concentration were significantly different, with mosquito populations from Saint-François and Les Abymes exhibiting the lowest mortality rates, thus suggesting higher resistance levels (ANOVA Kruskal-Wallis: P < 0.0001).
KRR50 values were variable, ranging from 8.0 (Les Abymes) to 28.1 (Anse-Bertrand), indicating a low to moderate resistance level for deltamethrin when compared to the susceptible Bora Bora strain. Two levels of resistance were detected in the six field mosquito populations. The mosquito population of Les Abymes had the lowest KRR50 (8.0), suggesting a low resistance level to deltamethrin. All others mosquito populations had a KRR50 of between 12 and 28, and are considered moderately resistant.
Kdr genotyping
Real-time PCR revealed high frequency of Kdr mutations V1016I and F1534C with high allelic frequencies (see Table 6). For the V1016I mutation, the frequency of the mutant and resistant allele (ƒ[I]) ranged from 0.85 (Saint-François) to 0.96 (Deshaies). For the F1534C mutation, the frequency of the mutant and resistant allele (ƒ[C]) ranged from 0.90 (Saint-François) to 0.98 (Anse-Bertrand and Baie-Mahault). No Kdr mutation was found in the susceptible Bora Bora strain. All mosquito populations exhibited more than 70% of the resistant homozygote genotype for both F1534C and V1016I mutations simultaneously (see Fig. 2). The F1534C Kdr mutation was more frequent in Ae. aegypti mosquitoes than the V1016I one because in five of the populations (Les Abymes, Baie-Mahault, Saint Martin, Anse-Bertrand, Deshaies), more than 80% of mosquitoes displayed the resistant homozygote genotype C/C of the F1534C mutation, while for the V1016I mutation, only three mosquito populations (Baie-Mahault, Saint-Martin, Deshaies) displayed the same frequency of resistant homozygote genotype I/I.
Table 6.
Allele frequencies for the V1016I and F1534C Kdr mutations for each Ae. aegypti population
| Population | Mutation V1016I ƒ[I] | Mutation V1016I ƒ[V] | Mutation F1534C ƒ[C] | Mutation F1534C ƒ[F] |
|---|---|---|---|---|
| ABY | 0.86 | 0.14 | 0.92 | 0.08 |
| SF | 0.85 | 0.15 | 0.90 | 0.10 |
| BM | 0.90 | 0.10 | 0.98 | 0.02 |
| SXM | 0.91 | 0.09 | 0.94 | 0.06 |
| AB | 0.89 | 0.11 | 0.98 | 0.02 |
| DH | 0.96 | 0.04 | 0.96 | 0.04 |
| Bora Bora | 0.00 | 1.00 | 0.00 | 1.00 |
ABY Les Abymes, SF Saint-François, BM Baie-Mahault, SXM Saint Martin, AB Anse-Bertrand, DH Deshaies, f[I] allele frequency of the mutant allele I for Kdr mutation V1016I, f[V] allele frequency of the wild allele V for Kdr mutation V1016I, f[C] allele frequency of the mutant allele I for Kdr mutation F1534C, f[F] allele frequency of the wild allele V for Kdr mutation F1534C
Fig. 2.

Histogram of genotype proportions for the six populations studied regarding V1016I (a) and F1534C (b) Kdr mutations. V/V: V1016I wild homozygote genotype; I/V: V1016I heterozygote genotype; I/I: V1016I mutant homozygote; F/F: F1534C wild homozygote genotype; F/C: F1534C heterozygote genotype; C/C: F1534C mutant homozygote; ABY Les Abymes, SF Saint-François, BM Baie-Mahault, SXM Saint Martin, AB Anse-Bertrand, DH Deshaies, BORA Bora Bora susceptible strain
Detoxification enzyme levels
The transcription profiles of the eight candidate detoxification genes potentially involved in metabolic resistance to insecticides were compared for the adults of the six mosquito populations of Guadeloupe and the susceptible Bora Bora strain (see Fig. 3). Genes with transcription ratio ≥2 and P-value < 0.05 (according to the Mann–Whitney U test done for each mosquito population and the Bora Bora strain) were considered significantly over-transcribed.
Fig. 3.
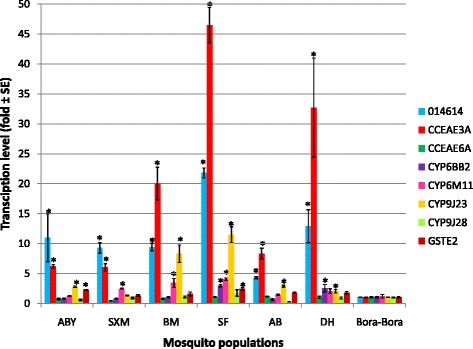
Adult transcription levels of five cytochrome P450 monooxygenases (014614, CYP6BB2, CYP6M11, CYP9J23, CYP9J28), two carboxyl/cholinesterases (CCEae3a, CCEae6a), and one glutathione S-transferase (GSTe2) for the six Ae. aegypti populations of Guadeloupe and Saint Martin, as compared to the susceptible Bora Bora strain. The transcription ratios obtained from real-time quantitative PCR were normalized with the two housekeeping genes RpL8 and RpS7 and shown as mean value (±SE) for three independent biological replicates. Genes significantly over-transcribed with transcription ratio ≥2 and P-value <0.05) are indicated by asterisks. ABY Les Abymes, SXM Saint Martin, BM Baie-Mahault, SF Saint-François, AB: Anse-Bertrand, DH Deshaies
The cytochrome P450 monooxygenase, 014614, was overexpressed in the six mosquito populations compared to the susceptible Bora Bora strain, and ranged from 4.3 for Anse-Bertrand to 21.8 for Saint-François, with significant differences in expression ratios between these two locations (Kruskal-Wallis Z test: P = 0.011). The cytochrome P450 monooxygenase, CYP6BB2, was found to be overexpressed only in the Saint-François and Deshaies populations with similar ratios of 3.0 and 2.5, respectively (Mann–Whitney U test: P = 0.512). The cytochrome P450 monooxygenase, CYP6M11, was over-transcribed for mosquito populations of Saint Martin, Baie-Mahault, and Saint-François, with ratios of 2.5, 3.4, and 4.0, respectively, and similar transcription levels (ANOVA Kruskal-Wallis: P = 0.201). The cytochrome P450 monooxygenase, CYP9J23, was significantly overexpressed for all mosquito populations with the exception of Saint Martin, with ratios ranging from 2.0 (Deshaies) to 11.5 (Saint-François). The transcription levels were significantly higher for Saint-François than for Deshaies (Kruskal-Wallis Z test: P = 0.026).
The carboxyl/cholinesterase 3A (CCEea3a) was over-transcribed in the six mosquito populations, with expression ratios ranging from 6.02 for Saint Martin to 46.5 for Saint-François. Mosquito populations of Saint-François (46.5-fold), Deshaies (32.7-fold), and Baie-Mahault (20.0-fold) exhibited higher levels of over-transcription than the rest of the populations (ANOVA Kruskal-Wallis: P = 0.011).
The glutathione S-transferase 2 (GSTe2) was over-transcribed only in the mosquito populations of Les Abymes and Saint-François with similar ratios of 2.3 and 2.4, respectively (Mann–Whitney U test: P = 0.218).
CCEae6a and the cytochrome P450 monooxygenase, CYP9J28, were not found to be over-transcribed in any of the populations when compared to the susceptible Bora Bora strain.
Gene amplification study
The gene amplification profiles of five previously studied genes (014614, CYP6M11, CYP9J23, CCEae3a, and GSTe2) were compared between the susceptible Bora Bora strain and the six populations of Guadeloupe and Saint Martin (see Fig. 4).
Fig. 4.
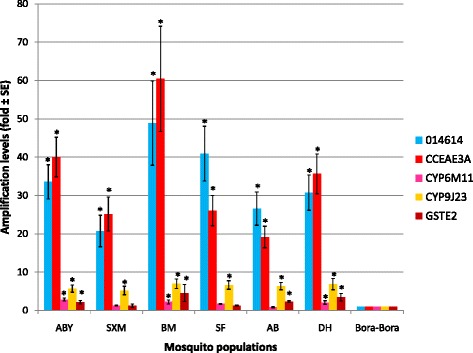
Adult gene amplification levels for three cytochrome P450 monooxygenases (014614, CYP6M11, CYP9J23), one carboxyl/cholinesterase (CCEae3a), and one glutathione S-transferase (GSTe2) for the six Ae. aegypti populations of Guadeloupe and Saint Martin compared to the susceptible Bora Bora strain. The gene amplification levels obtained from real-time quantitative PCR were normalized with both 007808 and 005950 housekeeping genes and shown as mean value (±SE) over 22 to 24 individual DNA. The genes significantly amplified (ratio ≥2) are indicated by asterisks. ABY Les Abymes, SXM Saint Martin, BM Baie-Mahault, SF Saint-François, AB Anse-Bertrand, DH Deshaies
The cytochrome P450 monooxygenase 014614 gene was amplified in all Ae. aegypti populations, with ratios ranging from 20.7 for Saint-Martin to 48.9 for Baie-Mahault. Significant differences in 014614 gene amplification were found between Saint Martin and both Baie-Mahault (Kruskal-Wallis Z test: P = 0.001) and Saint-François populations (Kruskal-Wallis Z test: P = 0.005). The cytochrome P450 monooxygenase, CYP6M11, was significantly amplified in the mosquito populations of Les Abymes, Baie-Mahault, and Deshaies, with ratios of 2.8, 2.2, and 2.0 respectively (ANOVA Kruskal-Wallis test: P = 0.036). The cytochrome P450 monooxygenase, CYP9J23, was amplified similarly in all Ae. aegypti populations with ratios of 5.2 to 7.0 (ANOVA Kruskal-Wallis test: P = 0.329). CCEae3a was amplified with ratios of >19 in all Ae. aegypti populations. Significant differences in amplification levels were observed between mosquito populations of Anse-Bertrand with a ratio of 19.2 and both Baie-Mahault and Les Abymes populations with ratios of 60.4 (Kruskal-Wallis Z test: P = 0.003) and 40.0 (Kruskal-Wallis Z test: P = 0.013), respectively. GSTe2 was similarly amplified in mosquito populations from Baie-Mahault (4.5-fold), Deshaies (3.4-fold), Anse-Bertrand (2.3-fold), and Les Abymes (2.1-fold) (ANOVA Kruskal-Wallis test: P = 0.115).
Correlation between overexpression and gene amplification of detoxification genes
Correlations between mean values of the expression and amplification ratios were investigated using the Spearman’s rank correlation test in XLSTAT software for each gene and all mosquito populations studied (see Additional file 3), however, no significant correlation was found. Nevertheless, the highest levels of association between gene amplification and expression ratios were found for the cytochrome P450 monooxygenases CYP9J23 and 014614 (r = 0.543; P = 0.297), followed by CCEae3a (r = 0.200; P = 0.714). Finally, the cytochrome CYP6M11 and GSTe2 showed the weakest associations between gene amplification and expression levels (r = −0.143; P = 0.803).
Association analysis between phenotypes, Kdr mutations, and detoxification enzymes regarding insecticide resistance
A PCA was performed for the six Ae. aegypti populations and 18 variables, including: (i) larval resistance levels regarding temephos and malathion, (ii) adult resistance levels regarding deltamethrin, (iii) adult transcription and amplification ratios of detoxification enzymes, and (iv) adult resistant Kdr allele frequencies (see Additional file 4). The PCA results are shown in Fig. 5.
Fig. 5.
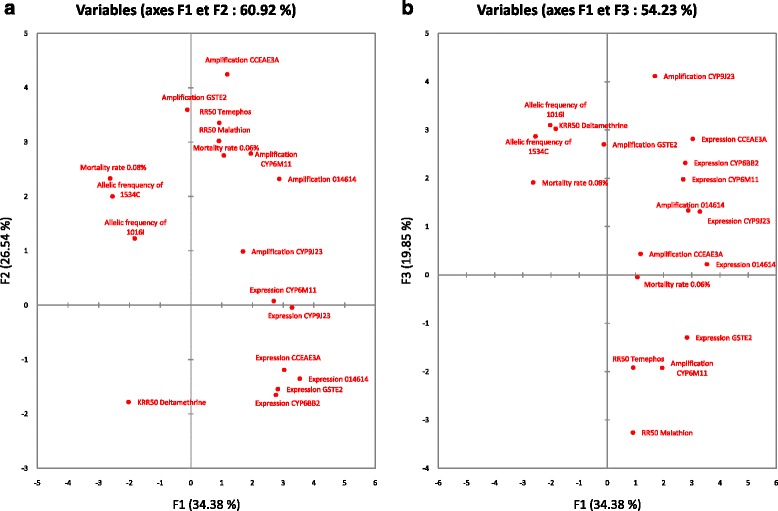
PCA for 18 variables including larval resistance levels regarding temephos and malathion, adult resistance levels regarding deltamethrin, adult transcription and amplification ratios of detoxification enzymes, and adult resistant Kdr allele frequencies, for the six mosquito populations tested. Information represented by axis 1-2 (a) and axis 1-3 (b) are shown
The first three PCA axes allow to represent 76.77% of the starting information with 34.38, 26.54, and 19.85%, respectively. The expression of the 014614 gene was mainly represented on the first PCA axe (Cos2 = 0.811) and was found to be significantly correlated to those of CYP6BB2 and CCEae3a genes (r = 0.870 and 0.853, respectively). The amplification ratio of CCEae3a was mainly represented on the second PCA axe (Cos2 = 0.901) and principally correlated with the GSTe2 amplification ratio (r = 0.808) and RR50 for temephos (r = 0.769). The CYP9J23 amplification ratio was mainly represented on the third axe (Cos2 = 0.633), and was found to be positively correlated with the 014614 and GSTe2 amplification ratios (r = 0.712 and r = 0.680, respectively). Furthermore, the expression ratios of CYP9J23 and CYP6M11 genes were significantly correlated (r = 0.850). The allele frequencies of resistant Kdr 1534C and 1016I mutations were positively correlated (r = 0.611), but only the 1534C resistant mutant allele was correlated with deltamethrin resistance KRR50 (r = 0.611). Temephos resistance was significantly correlated with the amplification ratio of the CYP6M11 gene (r = 0.849). Finally, the mortality rate after 24 h following a 1-h exposure to 0.08% deltamethrin was significantly and negatively correlated with the expression ratio of the GSTe2 gene (r = −0.960).
Discussion
The larval tests carried out on Ae. aegypti mosquito populations from Guadeloupe and Saint Martin showed that these mosquitoes have high resistance to temephos and low resistance to malathion. Tests done with adult mosquitoes showed moderate resistance levels to deltamethrin for KRR50s.
The chemical larvicide temephos was used for about 40 years, from the late 1960s to 2010, and resistance was reported as early as 1984 (Pasteur Institute archives). For adult vector control, malathion was used from the late 1960s to 2009, and deltamethrin from the early 1980s until the present day (Pasteur Institute archives). The mosquito populations of Guadeloupe and Saint Martin are reported to be resistant (weakly) to malathion for the first time in Guadeloupe (in this study), but are known to be resistant to deltamethrin since the late 1990s (Pasteur Institute and ARS archives). The long duration of use of temephos as a larvicide in Guadeloupe and Saint Martin, and the rapid increase of deltamethrin resistance may be explained by cross-resistance between these two molecules [25].
For temephos, the results of our study (i.e. RR50 between 8.9 and 33) are similar to those obtained for Ae. aegypti populations from the region, including in Martinique [26], Cuba, Venezuela, Costa Rica, Panama, Nicaragua, and Jamaica [27]. The detoxification enzymes commonly associated with organophosphates resistance are carboxylesterases (COEs) [28–30]. The gene expression study performed on adult mosquitoes revealed an overexpression for CCEae3a. Furthermore, the PCA showed a positive correlation between RR50 for temephos and CCEae3a amplification (r = 0.769). These results agree with previous studies demonstrating the implication of CCEae3a upregulation on larval temephos resistance [30]. In 1999, the higher resistance levels found for Ae. aegypti mosquito populations [10] were likely a consequence of the strong selective pressure exerted by the use of temephos as the main larvicide at the time especially due to its low price, efficacy and remanence. Since 2010, only Bti has been used in Guadeloupe as a larvicide, which explains the decrease in resistance levels and the lower resistance ratios found for temephos in this study.
For malathion, the resistance levels observed on larvae are low, with the RR50 ranging from 1.7 to 4.4 for all mosquito populations tested, when compared to the Bora Bora strain. Although malathion has been used for more than 40 years to control adult mosquitoes in Guadeloupe, the mosquito larvae did not develop a strong resistance to this molecule. This observation can be explained by the alternate use of malathion and deltamethrin since the late 1980s/early 1990s in Guadeloupe (Pasteur Institute archives), which may have delayed the emergence of higher resistance levels. Furthermore, the use of malathion in Guadeloupe has been intensified mostly during epidemic outbreaks. Another factor that may have mitigated the resistance level for malathion in mosquito populations is the possible dependency on a specific carboxylesterase mechanism with no cross-resistance with temephos. Our results on resistance levels to malathion are in agreement with recent studies conducted on Ae. aegypti larvae from Martinique [31], Cuba, Jamaica, Panama, Costa Rica [27], and adult mosquitoes from Venezuela [32].
Deltamethrin has been used since the late 1980s/early 1990s and is currently the only adulticide authorized in European countries, including the French overseas territories of Guadeloupe and Saint Martin. The observed knockdown deltamethrin resistance ratios (8≤KRR50≤28) suggest that the Ae. aegypti populations of Guadeloupe and Saint Martin are more resistant than those of Martinique [26]. On the contrary, when compared to French Guiana [33], Ae. aegypti populations from Guadeloupe were found to be less resistant in terms of KD (93–100% for Guadeloupe versus 27–37% for French Guiana) and mortality rates (64–90% for Guadeloupe versus 14–30% for French Guiana) obtained after exposure to 0.06% deltamethrin. Although our results contrast with those obtained by Dusfour and colleagues in 2015 [34] (in which lower KD and mortality rates, 37 and 42%, respectively, were obtained for the Ae. aegypti populations from Baie-Mahault), our study and the study of Dusfour and colleagues in 2015 show that the Ae. aegypti populations from Guadeloupe have lower resistance levels to deltamethrin than those from French Guiana.
It has been previously reported that COEs, cytochrome P450 monooxygenases, and GSTs are involved in pyrethroids resistance and metabolization [35–39]. The adult gene expression study performed on eight detoxification enzymes revealed a significant overexpression of the genes GSTe2, CCEae3a, and the cytochrome P450 monooxygenases 014614, CYP9J23, CYP6M11, and CYP6BB2. The PCA revealed a significant and negative correlation between the GSTe2 expression ratio and the mortality rates obtained 24 h after a 1-h exposure to 0.08% deltamethrin. The overexpression of GSTe2 in Ae. aegypti resistant to DichloroDiphenylTrichloroethane (DDT) and permethrin had previously been reported in Thailand [40].
Kdr mutations are known to be strongly associated with organochloride and pyrethroid resistance for many mosquito species including Ae. aegypti [41, 42]. Kdr genotyping of the six mosquito populations studied revealed a highly resistant allele frequency of V1016I mutation ranging from 85 to 96%. These results are in agreement with those found for mosquito populations from Martinique and French Guiana, where frequencies ranged from 87 to 97% and from 65 to 92%, respectively [26, 33]. The mutant allele frequency of F1534C was also extremely high in populations from Guadeloupe, ranging from 90 to 98%. Similar frequencies of these mutations have been found in Mexico and Grand Cayman islands [43, 44]. A V1016I mutation has often been associated with permethrin resistance [45], while a F1534C mutation has been associated with a permethrin and DDT resistance [44]. The PCA revealed a positive correlation between the deltamethrin resistance ratio and the mutant allele frequency of F1534C (r = 0.611), but a low correlation with V1016I (r = 0.262). Similar observations have already been reported in 2013 for the mosquito populations from Martinique regarding the V1016I mutation [46].
In conclusion, our results highlight that deltamethrin resistance levels seem to be more linked to Kdr mutation 1534C and GSTe2 at high concentrations, rather than other detoxification enzyme mechanisms. In terms of temephos and malathion, resistance levels were found to be more related to detoxification mechanisms involving esterase (CCEae3a) and cytochrome (CYP6M11) enzymes rather than Kdr mutations.
The levels of resistance of Ae. aegypti populations of the Guadeloupe and Saint Martin islands to the different types of insecticides are likely a consequence of vector control and agricultural activities. In Guadeloupe, lindane (ɣHCH) and chlordecone, two organochlorine pesticides, have been used in banana cultures to eliminate weevils in 1965–1974 and 1972–1993, respectively [47]. Furthermore, the principal active products of herbicides used in local sugar cane cultures are generally organophosphates and carbamates [http://e-phy.agriculture.gouv.fr/], while certain insecticides used in horticulture contain pyrethroids such as deltamethrin. Today, the most abundant pesticide in water in the French overseas departments is organochlorine [http://www.statistiques.developpement-durable.gouv.fr/lessentiel/ar/246/211/contamination-globale-eaux-souterraines-pesticides.html]. The long history of pesticide use in agriculture in Guadeloupe with the same classes of molecules applied for vector control could thus have contributed to the type of insecticide resistance in Ae. aegypti populations. This phenomenon has been commonly described for malaria vectors [48–50].
Finally, gene amplification is commonly associated with an overexpression of detoxification enzymes [13, 51, 52]. In our study, no significant correlation was found between expression and amplification ratios for any of the five genes tested, however, the strongest associations were recorded for CYP9J23 and 014614 (r = 0.543).
Conclusion
In the present study, the populations of Ae. aegypti mosquitoes—the main vector of dengue, CHIKV, and ZIKV—from the Guadeloupe and Saint Martin islands were found to have different resistance levels to several insecticides, with a high resistance to the former larvicide temephos, moderate resistance to the adulticide deltamethrin, and low resistance to the former adulticide malathion. The resistance levels are associated with strong frequencies of F1534C and V1016I Kdr mutations, as well as the over-transcription of CCEae3a, GSTe2, and four cytochromes P450 (014614, CYP9J23, CYP6M11, CYP6BB2) genes.
Mosquito resistance to deltamethrin is a serious challenge for vector control authorities since this product is the only adulticide authorized in the French overseas territories, in particular during epidemics. In addition, deltamethrin has also been used in agriculture for decades. In this context, there is an urgent need to carefully choose the molecules used for vector control and agricultural activities, as well as reduce their utilization in order to limit the chance of resistance. It is also urgent to identify alternative molecules that are effective and respectful of the environment that could be used in a vector control strategy based on a rotation and/or combination of insecticides (which leads to resistance development slowing down), as recommended by the WHO [53].
Our knowledge of the molecular basis of Ae. aegypti insecticide resistance has contributed to real progress in the past few years, but it is still not developed enough either to be used as a diagnostic tool for identifying resistance without the necessity to develop heavy testing procedures or understanding how the resistance mechanisms are developing. The present study elucidated that it could be interesting to: i) assess the activity of some families of detoxification enzymes using biochemical/enzymatic assays; ii) use synergists such as Triphenyl phosphate (COEs inhibitor), piperonyl butoxide (P450s inhibitor), or ethacrynic acid or diethyl maleate (GSTs inhibitor) in order to specify enzyme families and mechanisms involved in resistance [54]; iii) segregate tested mosquitoes and conduct quantitative PCR to understand induced gene expression and thus to specify the role of some genes individually; iv) add more detoxification genes in the analysis (i.e. GSTe7, CYP6Z) [26]; and v) investigate more Kdr mutations as I1011V which has already been associated to pyrethroid resistance in Cuba and Mexico [45], as well as I1011M and G923V in Brazil [55]. A better understanding of the resistant genes and their roles is needed in order to improve vector control strategies, mitigate insecticide resistance levels, and prevent and control the emergence and spread of Ae. aegypti-borne diseases.
Acknowledgements
We thank the regional health agency of Guadeloupe for its collaboration regarding mosquito sampling. We thank the vector control center of Martinique for graciously offering the Bora-Bora susceptible reference strain and for the training of DG on insecticide resistance tests. We thank the Pasteur Institute of French Guiana for graciously offering Kdr PCR controls and the International Division of Pasteur Institute for supporting DG’s training in Alpine ecology laboratory (LECA Grenoble, France).
Funding
This study was financially supported by the Fonds Européen de Développement Economique et Régional project FED-1-1.4 32932 and by the ARS through the collaboration convention no° 2014-140116. DG was supported by La Région Guadeloupe, Le Fond Social Européen, and the University of Antilles.
Availability of data and materials
Not applicable.
Authors’ contributions
DG, AVR, and FF conceived the study. DG, CD, AG and AVR collected biological materials, performed the experiments, and analyzed the data. DG, AVR and FF wrote the paper. CR and JG provided technical support for biological material collection. JPD, FFC and TG provided PCR primers, protocols and technical support for the screening of insecticide resistance mechanisms. All authors read and approved the final paper.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Informed consent was obtained from the owners of the houses that were investigated for mosquito collections. This consent was managed by the Vector Control Agency of Guadeloupe, through the Ethical Committee of the French Ministry of Health, because all mosquito collections were done in collaboration with their agents.
Abbreviations
- ARS
Agence Régionale de Santé
- Bti
Bacillus thuringiensis var. israelensis
- CCEae
Carboxyl/cholinesterase
- CHIKV
Chikungunya virus
- COE
Carboxylesterase
- F1
First laboratory generation mosquitoes
- F2
Second laboratory generation mosquitoes
- GST
Glutathione s-transferase
- KD
Knockdown
- KD
Knock-down
- Kdr
Knockdown resistance
- KDT
Knock-down time
- LC
Lethal concentration
- PCA
Principal component analysis
- PCR
Polymerase chain reaction
- PSAGE
Programme de Surveillance, d’Alerte et de Gestion des epidémies de dengue
- RR
Resistance ratio
- WHO
World Health Organization
- ZIKV
Zika virus
Additional files
Abstracts in the six official working languages of the United Nations. (PDF 760 kb)
Set of primers used for the expression and amplification of detoxification enzymes q-PCR. (DOCX 14 kb)
Mean expression and amplification ratios used for Spearman’s rank correlation tests. (XLSX 11 kb)
PCA matrix. (XLSX 11 kb)
Contributor Information
Daniella Goindin, Email: daniella.goindin@gmail.com.
Christelle Delannay, Email: cdollin@pasteur-guadeloupe.fr.
Andric Gelasse, Email: andric.gelasse971@gmail.com.
Cédric Ramdini, Email: cedric.ramdini@ars.sante.fr.
Thierry Gaude, Email: thierry.gaude@univ-grenoble-alpes.fr.
Frédéric Faucon, Email: frederic.faucon4@orange.fr.
Jean-Philippe David, Email: jean-philippe.david@univ-grenoble-alpes.fr.
Joël Gustave, Email: joel.gustave@ars.sante.fr.
Anubis Vega-Rua, Email: avega@pasteur.fr.
Florence Fouque, Email: fouquef@who.int.
References
- 1.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 2.Rollé A, Schepers K, Cassadou S, Curlier E, Madeux B, Hermann-storck C, et al. Severe sepsis and septic shock associated with Chikungunya virus infection, Guadeloupe, 2014. Emerg Infect Dis. 2016;22:891–894. doi: 10.3201/eid2205.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zanluca C, de Melo VCA, Mosimann ALP, dos Santos GIV, dos Santos CND, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 5.CIRE ANTILLES-GUYANE. Emergence du virus Zika aux Antilles Guyane. Situation épidémiologique. INVS, Point épidémiologique du 28 avril 2016: 16/2016.
- 6.Larrieu S, Cassadou S, Rosine J, Chappert JL, Blateau A, Ledrans M, et al. Lessons raised by the major 2010 dengue epidemics in the French West Indies. Acta Trop Elsevier BV. 2014;131:37–40. doi: 10.1016/j.actatropica.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Gottschamel J, Lössl A, Ruf S, Wang Y, Skaugen M, Bock R, et al. Production of dengue virus envelope protein domain III-based antigens in tobacco chloroplasts using inducible and constitutive expression systems. Plant Mol Biol. 2016;91(4–5):497–512. doi: 10.1007/s11103-016-0484-5. [DOI] [PubMed] [Google Scholar]
- 8.Weaver S, Costa F, Garcia-Blanco M, Ko A, Ribeiro G, Saade G, et al. Zika virus: history, emergence, biology, and prospects for control. Antivir Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debboun M, Strickman D. Insect repellents and associated personal protection for a reduction in human disease. Med Vet Entomol. 2013;27:1–9. doi: 10.1111/j.1365-2915.2012.01020.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosine J. Résistance d’Aedes aegypti et de Culex pipiens quinquefasciatus aux insecticides organophospohorés, biologiques et aux pyréthrinoïdes en Martinique et en Guadeloupe. Diplôme d’Etudes Approfondies. Univ. Pierre et Marie Curie (Paris VI) 51p. 1999.
- 11.Settimi L, Orford R, Davanzo F, Hague C, Desel H, Pelclova D, et al. Development of a new categorization system for pesticides exposure to support harmonized reporting between EU Member States. Env Int. 2016;91:332–340. doi: 10.1016/j.envint.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 12.ANSES. Recherche d’insecticides potentiellement utilisables en lutte anti-vectorielle. Avis de l’Anses. Rapp. d’expertise Collect. Ed. Sci. Saisine n°2009-SA-0338. ISBN 978-2-11-128697-9. Anses Ed. 2011;158p.
- 13.Faucon F, Dusfour I, Gaude T, Navratil V, Boyer F, Chandre F, et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res. 2015;25:1347–1359. doi: 10.1101/gr.189225.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, Strode C, et al. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies) BMC Genomics. 2009;10:494. doi: 10.1186/1471-2164-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides, Geneva. 2005 (WHO/CDS/WHOPES/GCDPP/2005.13).
- 17.Bisset JA, Rodríguez MM, Ricardo Y, Ranson H, Pérez O, Moya M, et al. Temephos resistance and esterase activity in the mosquito Aedes aegypti in Havana, Cuba increased dramatically between 2006 and 2008. Med Vet Entomol. 2011;25:233–239. doi: 10.1111/j.1365-2915.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- 18.Mazzarri M, Georghiou G. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J Am Mosq Control Assoc. 1995;11:315–322. [PubMed] [Google Scholar]
- 19.World Health Organization. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets, Geneva. 2006 (WHO/CDS/NTD/WHOPES/GCDPP/2006.3).
- 20.World Health Organization. Test procedures: for insecticide resistance monitoring in malaria vector mosquitoes, Geneva. 2013.
- 21.Khan HAA, Akram W, Shehzad K, Shaalan EA. First report of field evolved resistance to agrochemicals in dengue mosquito, Aedes albopictus (Diptera: Culicidae), from Pakistan. Parasit Vectors BioMed Central Ltd. 2011;4:146. doi: 10.1186/1756-3305-4-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanola J, Somboon P, Prapanthadara L. A novel point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. 2nd Int. Conf. Dengue Dengue Haemorhagic Fever, Oct. 2008. p. 15–7
- 23.Yanola J, Somboon P, Walton C, Nachaiwieng W, Prapanthadara L. A novel F1552/C1552 point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. Pestic. Biochem. Physiol. Elsevier Inc.; 2010
- 24.Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P, Prapanthadara L. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop Med Int Health. 2011;16:501–509. doi: 10.1111/j.1365-3156.2011.02725.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez MM, Bisset J, Ruiz M, Soca A. Cross-resistance to pyrethroid and organophosphorus insecticides induced by selection with temephos in Aedes aegypti (Ditera:Culicidae) from Cuba. J Med Entomol. 2002;39:882–888. doi: 10.1603/0022-2585-39.6.882. [DOI] [PubMed] [Google Scholar]
- 26.Marcombe S, Mathieu RB, Pocquet N, Riaz MA, Poupardin R, Sélior S, et al. Insecticide resistance in the dengue vector Aedes aegypti from martinique: distribution, mechanisms and relations with environmental factors. PLoS One. 2012;7:e30989. doi: 10.1371/journal.pone.0030989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez MM, Bisset JA, Fernandez D. Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 2007;23:420–429. doi: 10.2987/5588.1. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra-Rodriguez K, Strode C, Flores AE, Garcia-Luna S, Reyes-Solis G, Ranson H, et al. Differential transcription profiles in Aedes aegypti detoxification genes following temephos selection. Insect Mol Biol. 2014;23:199–215. doi: 10.1111/imb.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paiva MHS, Lovin DD, Mori A, Melo-santos MAV, Severson DW, Ayres FJ. Identification of a major Quantitative Trait Locus determining resistance to the organophosphate temephos in the dengue vector mosquito Aedes aegypti. Genomics Elsevier BV. 2015;107:40–48. doi: 10.1016/j.ygeno.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Poupardin R, Srisukontarat W, Yunta C, Ranson H. Identification of carboxylesterase genes implicated in temephos resistance in the dengue vector Aedes aegypti. PLoS Negl Trop Dis. 2014;8:e2743. doi: 10.1371/journal.pntd.0002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etienne M. Etude de la bioécologie d’Aedes aegypti à la Martinique en relation avec l’épidémiologie de la dengue. 2006
- 32.Alvarez LC, Ponce G, Oviedo M, Lopez B, Flores AE. Resistance to malathion and deltamethrin in Aedes aegypti (Diptera : Culicidae) from western Venezuela. J Med Entomol. 2013;50:1031–1039. doi: 10.1603/ME12254. [DOI] [PubMed] [Google Scholar]
- 33.Dusfour I, Thalmensy V, Gaborit P, Issaly J, Carinci R, Girod R. Multiple insecticide resistance in Aedes aegypti (Diptera: Culicidae) populations compromises the effectiveness of dengue vector control in French Guiana. Mem Inst Oswaldo Cruz. 2011;106:346–352. doi: 10.1590/S0074-02762011000300015. [DOI] [PubMed] [Google Scholar]
- 34.Dusfour I, Zorrilla P, Guidez A, Issaly J, Girod R. Deltamethrin resistance mechanisms in Aedes aegypti populations from three French overseas territories worldwide. PLoS Negl Trop Dis. 2015;9:1–17. doi: 10.1371/journal.pntd.0004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somwang P, Yanola J, Suwan W, Walton C, Lumjuan N, Prapanthadara P, Somboon LA. Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol Res. 2011;109:531–537. doi: 10.1007/s00436-011-2280-0. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson BJ, Pignatelli P, Nikou D, Paine MJI. Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: developing new tools to combat insecticide resistance. PLoS Negl Trop Dis. 2012;6:e1595. doi: 10.1371/journal.pntd.0001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandor-Proust A, Bibby J, Régent-Kloeckner M, Roux J, Guittard-Crilat E, Poupardin R, et al. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. Biochem J. 2013;455:75–85. doi: 10.1042/BJ20130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David J-P, Ismail HM, Chandor-proust A, Paine MJI. Role of cytochrome P450s in insecticide resistance : impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos Trans R Soc Lond B Biol Sci. 2013;368:1–12. doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maia RT, Nadvorny D. Molecular docking of Anopheles gambiae and Aedes aegypti glutathione S-Transferases Epsilon 2 (GSTE2) against usnic acid: an evidence of glutathione conjugation. Brazilian Arch Biol Technol. 2014;57:689–694. doi: 10.1590/S1516-8913201402234. [DOI] [Google Scholar]
- 40.Lumjuan N, McCarroll L, Prapanthadara LA, Hemingway J, Ranson H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:861–871. doi: 10.1016/j.ibmb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Donnelly MJ, Corbel V, Weetman D, Wilding CS, Williamson MS, Black WC. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Saavedra-Rodriguez K, Strode C, Flores Suarez A, Fernandez Salas I, Ranson H, Hemingway J, et al. Quantitative trait loci mapping of genome regions controlling permethrin resistance in the mosquito Aedes aegypti. Genetics. 2008;180:1137–1152. doi: 10.1534/genetics.108.087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black WC., IV Coevolution of the Ile1,016 and Cys1,534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2015;9:e0004263. doi: 10.1371/journal.pntd.0004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris AF, Rajatileka S, Ranson H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg. 2010;83:277–284. doi: 10.4269/ajtmh.2010.09-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I, et al. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007;16:785–798. doi: 10.1111/j.1365-2583.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 46.Marcombe S, Paris M, Paupy C, Bringuier C, Yebakima A, Chandre F, et al. Insecticide-driven patterns of genetic variation in the dengue vector Aedes aegypti in Martinique Island. PLoS One. 2013;8:e77857. doi: 10.1371/journal.pone.0077857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabidoche Y-M, Lesueur-Jannoyer M. Long term pollution of soils in French West Indies : how to manage chlordecone contamination? Innov Agron. 2011;16:117–133. [Google Scholar]
- 48.Philbert A, Lyantagaye SL, Nkwengulila G. A review of agricultural pesticides use and the selection for resistance to insecticides in malaria vectors. Adv Entomol. 2014;2:120–8. doi: 10.4236/ae.2014.23019. [DOI] [Google Scholar]
- 49.Diabaté A, Baldet T, Chandre F, Akogbéto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- 50.Nkya T, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, et al. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae : a multigenerational study in controlled conditions. Parasit Vectors. 2014;7:480. doi: 10.1186/s13071-014-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bariami V, Jones CM, Poupardin R, Vontas J, Ranson H. Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl Trop Dis. 2012;6:e1692. doi: 10.1371/journal.pntd.0001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Schuler M, Berenbaum M. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 53.WHO. The technical basis for coordinated action against insecticide resistance: preserving the effectiveness of modern malaria vector control. Glob. Malar. Program. WHO Headquarters. Draft Meet. Rep. 2010.
- 54.Bisset JA, Marin R, Rodriguez MM, Severson DW, Ricardo Y, French L, et al. Insecticide resistance in two Aedes aegypti (Diptera: Culicidae) strains from Costa Rica. J Med Entomol. 2013;50:352–361. doi: 10.1603/ME12064. [DOI] [PubMed] [Google Scholar]
- 55.Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, et al. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutatuions in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;17:87–94. doi: 10.1046/j.1365-2915.2003.00412.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


