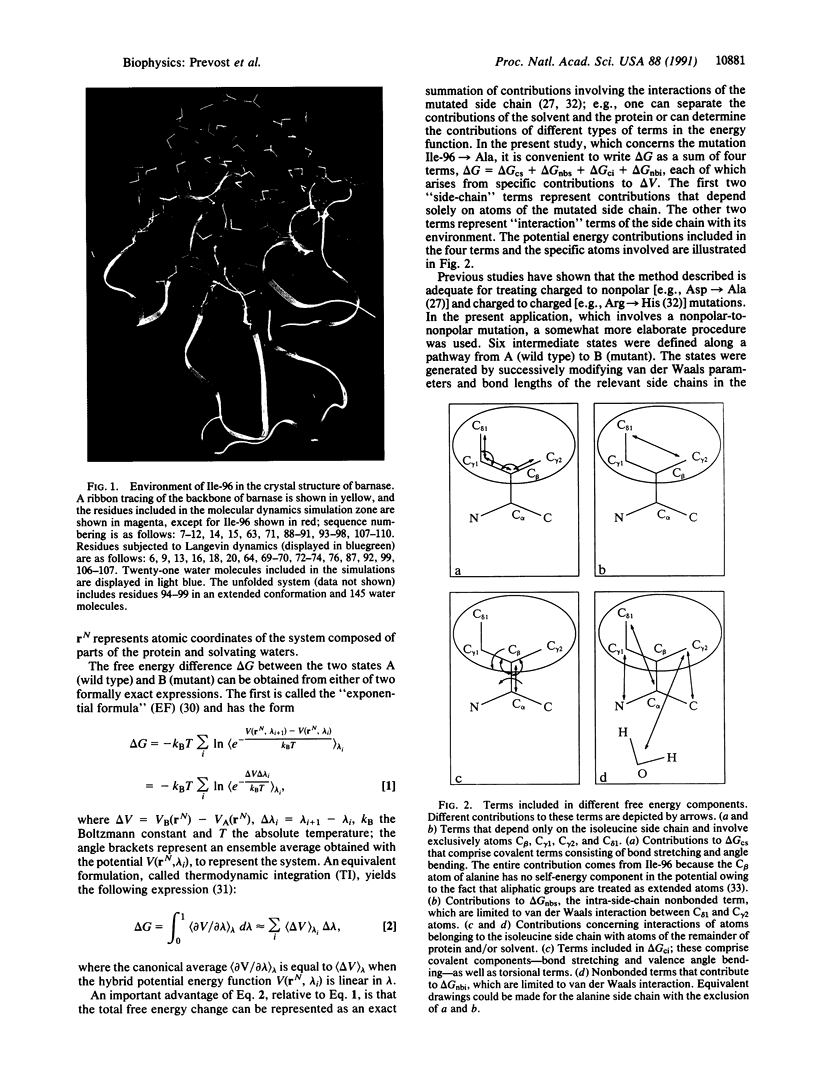
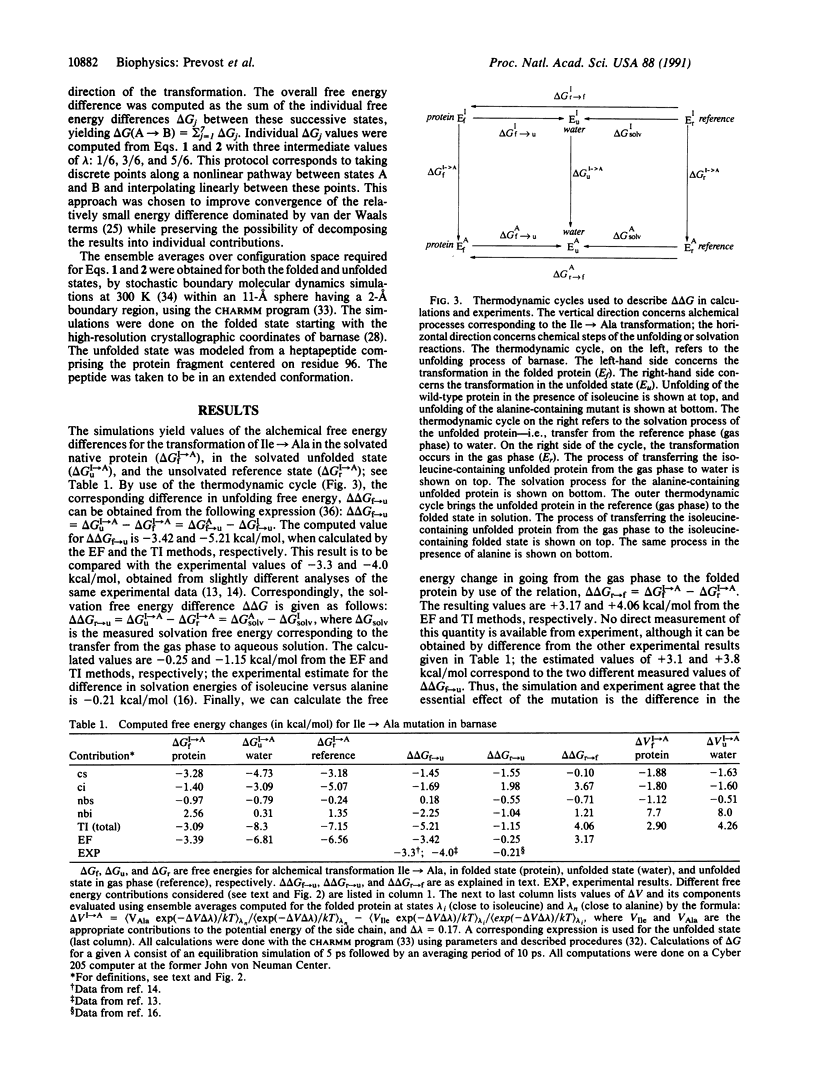
Abstract
Molecular dynamics simulations have been used to compute the difference in the unfolding free energy between wild-type barnase and the mutant in which Ile-96 is replaced by alanine. The simulations yield results (-3.42 and -5.21 kcal/mol) that compare favorably with experimental values (-3.3 and -4.0 kcal/mol). The major contributions to the free energy difference arise from bonding terms involving degrees of freedom of the mutated side chain and from nonbonded interactions of that side chain with its environment in the folded protein. By comparison with simulations of an extended peptide in the absence of solvent, used as a reference state, hydration effects are shown to play a minor role in the overall free energy balance for the Ile----Ala transformation. The implications of these results for our understanding of the hydrophobic effect and its contribution to protein stability are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T. Mutational effects on protein stability. Annu Rev Biochem. 1989;58:765–798. doi: 10.1146/annurev.bi.58.070189.004001. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge D. L., DiCapua F. M. Free energy via molecular simulation: applications to chemical and biomolecular systems. Annu Rev Biophys Biophys Chem. 1989;18:431–492. doi: 10.1146/annurev.bb.18.060189.002243. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Brooks C. L., 3rd, Karplus M. Active site dynamics of ribonuclease. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8458–8462. doi: 10.1073/pnas.82.24.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. J., Slater P., Reynolds G. P. Reduced high-affinity glutamate uptake sites in the brains of patients with Huntington's disease. Neurosci Lett. 1986 Jun 18;67(2):198–202. doi: 10.1016/0304-3940(86)90397-6. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Gao J., Kuczera K., Tidor B., Karplus M. Hidden thermodynamics of mutant proteins: a molecular dynamics analysis. Science. 1989 Jun 2;244(4908):1069–1072. doi: 10.1126/science.2727695. [DOI] [PubMed] [Google Scholar]
- Hartley R. W. A reversible thermal transition of the extracellular ribonuclease of Bacillus amyloliquefaciens. Biochemistry. 1968 Jun;7(6):2401–2408. doi: 10.1021/bi00846a050. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kellis J. T., Jr, Nyberg K., Fersht A. R. Energetics of complementary side-chain packing in a protein hydrophobic core. Biochemistry. 1989 May 30;28(11):4914–4922. doi: 10.1021/bi00437a058. [DOI] [PubMed] [Google Scholar]
- Kellis J. T., Jr, Nyberg K., Sali D., Fersht A. R. Contribution of hydrophobic interactions to protein stability. Nature. 1988 Jun 23;333(6175):784–786. doi: 10.1038/333784a0. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Becktel W. J., Matthews B. W. Hydrophobic stabilization in T4 lysozyme determined directly by multiple substitutions of Ile 3. Nature. 1988 Aug 4;334(6181):406–410. doi: 10.1038/334406a0. [DOI] [PubMed] [Google Scholar]
- Mauguen Y., Hartley R. W., Dodson E. J., Dodson G. G., Bricogne G., Chothia C., Jack A. Molecular structure of a new family of ribonucleases. Nature. 1982 May 13;297(5862):162–164. doi: 10.1038/297162a0. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Sandberg W. S., Terwilliger T. C. Influence of interior packing and hydrophobicity on the stability of a protein. Science. 1989 Jul 7;245(4913):54–57. doi: 10.1126/science.2787053. [DOI] [PubMed] [Google Scholar]
- Tidor B., Karplus M. Simulation analysis of the stability mutant R96H of T4 lysozyme. Biochemistry. 1991 Apr 2;30(13):3217–3228. doi: 10.1021/bi00227a009. [DOI] [PubMed] [Google Scholar]
- Wolfenden R., Andersson L., Cullis P. M., Southgate C. C. Affinities of amino acid side chains for solvent water. Biochemistry. 1981 Feb 17;20(4):849–855. doi: 10.1021/bi00507a030. [DOI] [PubMed] [Google Scholar]
- Wood R. H., Thompson P. T. Differences between pair and bulk hydrophobic interactions. Proc Natl Acad Sci U S A. 1990 Feb 1;87(3):946–949. doi: 10.1073/pnas.87.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]