Abstract
Inorganic arsenic (iAs) is a well-characterized carcinogen, and recent epidemiologic studies have linked chronic exposures to non-cancer health outcomes, including cardiovascular disease, diabetes, skin lesions and respiratory disorders. Greater vulnerability has been demonstrated with early life exposure for health effects including lung and bladder cancer, immunotoxicity and neurodevelopment. Despite its well-known toxicity, there are important gaps in the regulatory oversight of iAs in food and in risk communication. This paper focuses on the US regulatory framework in relation to iAs in food and beverages. The state of existing regulatory agency toxicological assessments, monitoring efforts, standard setting, intervention policies and risk communication are explored. Regarding the approach for standard setting, risk-based evaluations of iAs in particular foods can be informative but are insufficient to create a numeric criterion, given current uncertainties in iAs toxicology and the degree to which traditional risk targets can be exceeded by dietary exposures. We describe a process for prioritizing dietary exposures for different lifestages and recommend a relative source contribution-based approach to setting criteria for arsenic in prioritized foods. Intervention strategies begin with an appropriately set criterion and a monitoring program that documents the degree to which this target is met for a particular food. This approach will promote improvements in food production to lower iAs contamination for those foods which initially do not meet the criterion. Risk communication improvements are recommended to ensure that the public has reliable information regarding sources and alternative dietary choices. A key recommendation is the consideration of meal frequency advice similar to what is currently done for contaminants in fish. Recent action level determinations by FDA for apple juice and infant rice cereal are evaluated and used as illustrations of how our recommended approach can further the goal of exposure mitigation from key sources of dietary iAs in the US.
Keywords: Arsenic, Food, Rice, Juice, EPA, FDA, Policy, Relative source contribution, Communication, Diet
GRAPHICAL ABSTRACT
1. Introduction
Inorganic arsenic (iAs) is a toxic metal that has been associated with numerous adverse outcomes in humans, including various cancers, cardiovascular disease, diabetes, respiratory disorders, skin lesions, immunotoxicity and neurodevelopmental effects in early life (National Research Council, 2014). While regulatory focus has historically been on controlling iAs in drinking water and soil, dietary sources predominate for many individuals (Kurzius-Spencer et al., 2014; Carlin et al., 2015). Given the variability in iAs content of foods and intra-population rates of consumption of iAs-containing foods, it can be challenging to single out individual food items for regulatory action and public education. Such efforts have also been hampered by the lack of consensus regarding the carcinogenic potency of iAs, and the risk of certain non-cancer outcomes at lower doses. Despite these difficulties, existing assessments converge on the possibility of elevated health risk and the need to consider mitigation strategies to limit iAs dietary exposure (Naujokas et al., 2013; Carlin et al., 2015).
In the US, few foods have been subjected to regulatory interventions aimed at reducing the public’s iAs exposure, and consequently, the appropriate incentives are not in place to promote exposure mitigation at the level of food production. Consumers have been confronted with media reports about the presence of arsenic in food, most notably with respect to apple juice and rice (Consumer Reports, 2012a; Consumer Reports, 2012b). Currently available information does not give consumers enough information to make informed choices about arsenic in the diet (Lai et al., 2015). Taken together, the emerging evidence regarding iAs’s association with myriad adverse outcomes combined with the importance of diet as a key exposure source underscore the need for regulatory oversight, mitigation strategies, and enhanced risk communication.
In this paper, we focus on the challenges and opportunities for improving the manner in which iAs in food is evaluated, monitored and controlled within the US regulatory framework, using recent action level determinations by the Food and Drug Administration (FDA) as case study examples. Our goals are to examine the adequacy of existing regulatory approaches and communication activities, to examine the utility of risk-based and alternative strategies for setting criteria for specific food items, and to recommend practical mitigation strategies that target the greatest sources of dietary iAs exposure.
This paper and the four others that accompany it (Cubadda et al., 2017; Davis et al., 2017; Punshon et al., 2017; Taylor et al., 2017) are products of the Collaborative on Food with Arsenic and Associated Risk and Regulation (C-FARR), a two-year effort led by the Dartmouth Superfund Research Program and Children’s Environmental Health and Disease Prevention Research Center. The goal of C-FARR is to synthesize the current information pertaining to arsenic from soil to plate, based on key questions and knowledge gaps identified by policy stakeholders and scientists from interdisciplinary backgrounds, to inform future regulatory and policy decisions affecting dietary arsenic exposure.
2. Defining the scope of the problem
2.1. Arsenic exposures and health effects
Unlike many other chemicals, the majority of evidence of adverse health outcomes for arsenic comes from studies of human populations instead of laboratory animal studies. A wealth of epidemiologic evidence supports the notion that chronic ingestion of iAs in water can elicit adverse health outcomes in exposed populations (National Research Council, 2014; Carlin et al., 2015). The National Academy of Sciences (NAS) Committee on Inorganic Arsenic provided a state of the evidence review of iAs to assist the EPA’s Integrated Risk Information System (IRIS) in developing a toxicological assessment for iAs (National Research Council, 2014). The Committee developed a three-tier hierarchy of health endpoints based upon whether evidence of a causal relationship with iAs has been demonstrated. The first tier (causality well documented) included lung, skin and bladder cancers, ischemic heart disease, and skin lesions. The second tier, termed priority endpoints (well defined, but evidence still emerging), included prostate and renal cancers, diabetes, non-malignant respiratory disease, infant morbidity, neurodevelopmental toxicity, and immune effects. The final tier included health effects worthy of further consideration, but for which data are less well developed, included liver and pancreatic cancers, renal disease, hypertension, stroke and other pregnancy outcomes, such as fetal loss, stillbirth and neonatal mortality. The NAS Committee stated that for a number of these endpoints, the doses required to elicit adverse effects may be close to or even overlap with levels of current human exposure (NRC, 2014).
2.2. Vulnerable populations
Particular sub-populations may be at increased risk for health effects resulting from arsenic exposure. This may result from altered metabolism or underlying genetic risk factors, lifestages that represent developmental windows of unique sensitivity to iAs toxicity, and factors that may increase dietary exposure such as individual preferences, age group, cultural factors, and dietary restrictions (e.g., gluten-free or allergy). A European study found that dietary exposure was about 3 times as great for children under 3 years of age compared with adults (European Food Safety Authority, 2014). Certain ethnic groups may receive greater exposure as average rice consumption and urinary iAs were both higher in Asian/other, Mexican, and Black children than in white children age 6–17 according to NHANES 2003–2008 data (Lai et al., 2015). The fetus and young children may be at particular risk as a result of developing organ systems and expected years of life in which to develop cancer and other chronic outcomes (Miller et al., 2002).
The vulnerability from early life exposure is perhaps best exemplified by the dramatic increases in rates of death from bronchiectasis in Chile for those exposed to iAs in utero (standardized mortality ratio [SMR] of 12.4, CI 3.3–31.7) and/or postnatally (SMR 46.2, CI 21.1–87.7) (Smith et al., 2006). In a Chilean case control study, those exposed to moderately elevated levels of arsenic in utero or as children (<100 μg L−1 in water) had an increased bladder and lung cancer as adults despite exposures ending as much as 40 years earlier (Steinmaus et al., 2014).
The distribution of arsenic forms (MMA, iAs, DMA) excreted in urine have been used as a marker of an individual’s ability to metabolize iAs via methylation (Marafante and Vahter, 1984). Inorganic arsenic is initially metabolized to MMA, an intermediate of substantial toxicity. In several studies, those who excreted a higher proportion of MMA in their urine had increased risk of lung, bladder and skin cancer suggesting that inadequate methylation capacity to DMA is a risk factor that may vary across the population (Smith and Steinmaus, 2009). Complicating matters, however, is recent research suggesting that higher percentage excretion of DMA is associated with higher body mass index (BMI) and an increased risk of type 2 diabetes and metabolic syndrome (Chen et al., 2012; Gribble et al., 2013; Kuo et al., 2015).
Multiple studies have suggested a synergistic relationship between inorganic arsenic and other carcinogens. Consequently, workers and others with co-exposures to carcinogens may represent an especially susceptible group. Several studies have found evidence of a synergistic relationship between exposure to arsenic and smoking in lung cancer (Ferreccio et al., 2000; Chen et al., 2004; Ferreccio et al., 2013). A case control study has described synergistic effects on lung and bladder cancer after co-exposure to arsenic and other known or suspected carcinogens including secondhand smoke, asbestos, silica, and wood dust (Ferreccio et al., 2013). In addition, in vitro studies have investigated potential co-mutagenic mechanisms through which arsenic may act with other carcinogens (Maier et al., 2002).
2.3. Sources of arsenic in food
Arsenic is ubiquitous in the environment, and can enter the food supply through myriad natural and anthropogenic processes. Arsenic is the 20th most abundant element in the Earth’s crust, and occurs naturally in geologic formations (Valberg et al., 1997). While contaminated drinking water is a widely recognized source of human exposure to iAs (Wu et al., 1989), impacted water may also be used as an irrigation source for the production of food crops (Heikens, 2006; Huq et al., 2006; Roberts et al., 2007). Contaminated soils may also play a role in the bioaccumulation of arsenic in food crops. A study of soils by the US Geological Survey found measurable levels of arsenic in nearly all collected samples, reporting a geometric mean total arsenic concentration of 5.2 μg g−1 with an observed range of <0.1 to 97 μg g−1 (Shacklette and Boerngen, 1984). Natural processes such as volcanic activity and the weathering of minerals can also release arsenic into soil and water (Aiuppa et al., 2006), and ultimately food.
Human activity is an important contributor to arsenic in the food system; Han et al. estimated that global industrial activities contributed 4.53 million metric tons of arsenic to the environment in the year 2000 alone, and identified the leading sources as mining, coal-fired power plants, and petroleum refining (Han et al., 2003). Other important anthropogenic legacy and ongoing sources of environmental arsenic include smelters (Pershagen, 1985), pesticide use (Peryea, 1991), pressure-treated lumber (Xue et al., 2006; Zartarian et al., 2006), arsenical drugs in animal agriculture (Nachman et al., 2005; Nachman et al., 2013; Nachman et al., 2016), and chemical weapons (Pitten et al., 1999; Fox et al., 2010). Arsenic can also be present in animal wastes and processed human biosolids, both of which can be used as soil amendments in crop agriculture (Nachman et al., 2005; Nachman et al., 2008).
2.4. The importance of dietary exposure
Evidence to support the notion that diet is an important contributor to population iAs burdens comes from a number of sources. A probabilistic exposure modeling study combined food intake data from NHANES with a smaller study detailing arsenic species distribution in a wide array of foods (Schoof et al., 1999). This study found that, on average, the US diet accounted for a daily iAs intake of 1.96 μg day−1, or twice the iAs contribution of drinking water (Xue et al., 2010). A more recent analysis used three population studies (NHEXAS, BAsES and 2003–04 NHANES) to examine the contribution of dietary iAs to overall iAs among non-seafood eaters with drinking water arsenic levels above and below the 10 μg L−1 maximum contaminant level (MCL). It found that among persons with drinking water above the MCL, 30% of iAs came from food, whereas among persons with drinking water below the MCL, the contribution of the diet ranged from 54 to 85% (Kurzius-Spencer et al., 2014). Dietary exposure estimates have been shown to be sensitive to the methods employed in their derivation. A study examining modeled exposures based on food-specific arsenic concentrations from market-basket surveys in combination with dietary recalls were poorer predictors of urinary arsenic levels than arsenic intakes constructed from duplicate diet sampling of those individuals in which the consumed foods were analyzed for iAs (Kurzius-Spencer et al., 2013).
Considering that chronic iAs exposure has been convincingly linked to adverse health outcomes across multiple organ systems, that certain population subgroups (due to genetic differences, lifestage, dietary patterns, or some combination of these and other factors) may be more vulnerable, and that thresholds for iAs health effects have not been demonstrated, efforts to limit iAs exposure merit consideration. Given existing evidence that diet can play a critical role in population iAs exposure, and that marked variation in arsenic content exists across foods, opportunities exist for both research and regulatory interventions aimed at addressing dietary arsenic exposure.
3. Existing assessment, management and communication efforts and associated shortcomings
3.1. Status of toxicological assessments of iAs
At present, two federal regulatory agencies have synthesized existing evidence to develop quantitative dose-response metrics for use in satisfying their regulatory mandates. EPA’s assessment is currently being conducted to update its IRIS database as well as to inform its Office of Groundwater and Drinking Water’s update to the arsenic drinking water MCL. FDA’s assessments of arsenic toxicology have been in support of the development of action levels for apple juice in 2013 and infant rice cereal in 2016. Of note, the FDA assessments address risks associated with early life exposure to arsenic at high consumption rates relative to adults, but for less than lifetime exposure. These federal assessments are briefly summarized and contrasted below.
The EPA IRIS database lists iAs as a known human carcinogen with a cancer slope factor (CSF) of 1.5 (mg kg−1 day−1)−1, derived in 1995 based upon skin cancer incidence in a large Taiwanese cohort (Tseng et al., 1968; Tseng, 1977). IRIS also contains a non-cancer reference dose (RfD) for iAs of 3 × 10−4 mg kg−1 day−1 based upon skin and vascular effects associated with Blackfoot disease that occurred in Taiwan from chronic arsenic ingestion in drinking water (Tseng, 1977). A threefold uncertainty factor was used to lower the NOAEL for these effects in creating the RfD. Subsequent analyses by EPA have focused on cancer effects; the 2001 assessment in support of the current MCL included a number of updates and improvements such as a focus on internal cancers (bladder, lung) from exposed Taiwanese villagers (United States Environmental Protection Agency, 2001). That cancer unit risk was very similar to the older value on IRIS (skin cancer basis). A review by the National Research Council (NRC) made suggestions for improving EPA’s cancer analysis and derived a potency that was approximately 10 times higher than the EPA derivations up to that time (National Research Council, 2001). A major difference was with respect to how the baseline cancer incidence for the reference Taiwanese population was constructed. EPA drafted an updated iAs cancer assessment in 2005 and another in 2010 with the goal of incorporating NRC’s (2001) recommendations (United States Environmental Protection Agency, 2010). That draft derived an arsenic cancer potency (25.7 [mg kg−1-day−1]−1) that was based upon the sum of bladder and lung cancer risks that was 17-fold higher than the value currently in the IRIS database. The draft 2010 CSF leads to a risk at the MCL of 70 per 10,000 or 7000 times greater than what is conventionally the target for carcinogens in drinking water, 1 per million (“de minimis” risk).
Residual, but still important uncertainties in EPA’s cancer assessment have prevented it from becoming a final document. Rather, a new NRC panel has reviewed EPA’s efforts and has made a series of recommendations for improving the assessment (National Research Council, 2014). This NRC panel recommended that EPA use the available epidemiology including studies in the US to develop a variety of RfDs for endpoints that have the strongest evidence of association with iAs (Tier 1 endpoints, described above). The committee recommended that EPA model these and the cancer endpoints (prostate and renal added as priorities to lung, bladder and skin) via empirical, low-dose modeling (e.g., spline fits) rather than assuming a particular extrapolation model or mode of action (e.g., linear, threshold or otherwise) (National Research Council, 2014). At the time of writing, EPA is still responding to these suggestions as they prepare new draft cancer and non-cancer assessments for iAs. An apparent goal is that the public health implications of arsenic in drinking water will be understood through updated assessments leading to a comprehensive benefit-cost analysis when EPA is ready to consider adjustments to the MCL.
FDA’s goal with its 2013 risk assessment was to evaluate a range of options for setting an apple juice action level for iAs to serve as guidance to juice producers (United States Food and Drug Administration, 2013). The risk assessment was based upon estimates of lifetime cancer risk to children and older lifestages from the consumption of iAs in apple juice. The toxicology portion of the assessment was based upon FDA’s modeling of the urinary tract and lung cancer incidence from the Taiwanese cohort (Chen et al., 2010a; Chen et al., 2010b), with extrapolation from high to lower doses explored via 8 different dose response models. This yielded potency estimates (linear low dose slope) nearly identical to that derived by EPA 2001 for MCL derivation. When this potency estimate was applied to the consumption of apple juice by a combination of child and adult windows of exposure (over a 50 year period), a population cancer risk of 8 per million (range 0–21.3) was predicted for an apple juice limit of 10 ppb. FDA chose the 10 ppb limit as the apple juice action level because it was readily achievable using good manufacturing practices, as the FDA market survey of 94 juice samples in 2011 found that the iAs content of all samples was below 10 ppb. Added rationale may have been an interest in matching EPA’s MCL and thus offer at least as much health protection as that exerted by EPA for drinking water. The MCL, however, is a regulatory value, while the apple juice action level serves as guidance and thus does not guarantee monitoring, detection of exceedances and enforcement that would accompany a regulatory standard.
A newer FDA risk assessment was developed in support of an action level issued for infant rice cereal and also evaluated rice and rice products consumed by children and the general public (United States Food and Drug Administration, 2016b). This assessment used a similar dose-response approach to cancer risk estimation as above for apple juice in terms of epidemiological data and extrapolation models. Modeling differences between this update and the 2013 assessment led to a 30% decrease in iAs potency (corresponding to combined bladder and lung cancers). This estimate generally agrees with the EPA cancer slope factors on IRIS and used by EPA in the MCL derivation (2001). To estimate cancer risk for young children, FDA considered the rice cereal ingestion rate of infants for the first year of life based upon NHANES dietary recall data in which the average infant ingested 5 g (2 tablespoons) of dry cereal per day. This is one-third of the recommended portion size (United States Department of Agriculture, 2012b) and infants may have several servings per day. These assumptions led to an estimated lifetime cancer risk stemming from brown rice cereal ingestion during the first year of life of 3.2 cases per million. In an alternative analysis, FDA provided a cancer estimate of 9.5 cases per million for 3 servings a day of brown rice cereal for the first year of life. Cancer risks as high as 162 per million were calculated for the combination of childhood and adult (0–50 years) exposures to rice products. FDA set the infant rice cereal action level at 100 ppb as a feasible way to decrease cancer risks by 2 to 47%. This action level is at the 36th to 47th percentile of the distribution of sampling results for rice cereal as sampled by FDA in two rounds (2011–2013, N = 81; 2014, N = 76).
Taken together, the current state of the toxicological science on iAs risk from food has a number of key uncertainties as follows: 1) uncertainty regarding the estimate of cancer potency, with EPA and FDA estimates spanning more than an order of magnitude; 2) uncertainty regarding non-cancer endpoints with an outdated RfD on IRIS and a planned reassessment expected to derive RfDs for a number of high priority non-cancer targets; 3) uncertainty with how to assess other As species in food, particularly methylated species and certain arsenosugars which may confer added toxicity beyond the iAs content of the food (Feldmann and Krupp, 2011; Molin et al., 2015); 4) uncertainty with respect to the extent of vulnerability differences across the population due to lifestage, genetics, pre-existing conditions and other factors.
These uncertainties make it evident that risk-based approaches, while useful to inform policy, are likely too uncertain to drive policy. In a later section, we further evaluate approaches to setting concentration targets for iAs in food, that while not risk-based, acknowledge the need to mitigate exposure to the extent possible. This goal-setting exercise begins with prioritizing foods and beverages for action based upon exposure, and then explores a variety of regulatory and communication steps that can help reduce the public’s exposure to iAs in food.
3.2. Regulatory policies concerning arsenic in the context of food production and consumption
Multiple agencies and policy mechanisms play direct and/or indirect roles in addressing dietary arsenic exposure in the US (Tables 1 and 2), a task complicated by the aforementioned historic and ongoing sources of arsenic to the food system. Rather than addressing the arsenic content in foods, however, the majority of existing regulation addresses the inputs to and environmental conditions of agriculture.
Table 1.
Regulatory actions concerning arsenic in food.
| Agency | Description | CFR | Relevant language | Reference | Regulation type/status |
|---|---|---|---|---|---|
| EPA | National primary drinking water standard | 40 CFR 9; 40 CFR 141; 40 CFR 142 | MCLG: 0 MCL: 0.01 mg/L |
https://federalregister.gov/a/01-1668 | Final rule |
| EPA | Standards for the use or disposal of sewage sludge | 40 CFR 503 | Bulk sewage sludge applied to agricultural land: Ceiling concentration: 75 mg per kg Cumulative pollutant loading rate: (41 kg per hectare) Pollutant concentration: monthly average concentration (41 mg per kg) |
https://federalregister.gov/a/99-18604 | Final rule |
| EPA | Effluent limitations guidelines and standards for the steam electric power generating point source category | 40 CFR 423 | Steam electric power generating effluent guidelines limit the levels of toxic metals, including arsenic, in wastewater that can be discharged from power plants | https://federalregister.gov/a/2015-25663 | Final rule |
| EPA | Mercury and air toxics standards | 40 CFR 60; 40 CFR 63 | Mercury and air toxics standards regulate metals emissions, including arsenic, from new and existing coal and oil-fired power plants. | https://federalregister.gov/a/2016-06563 | Final rule |
| FDA | Requirements for specific standardized beverages: bottled water | 21 CFR 165 | Allowable level of inorganic substances: 0.01 mg arsenic per liter. Bottled water producers would be subject to CGMP monitoring requirements in 21 CFR 129.35 and 129.80. | https://federalregister.gov/a/05-11406 | Final rule |
| FDA | Withdrawal of approval of new animal drug applications; carbarsone; roxarsone | 21 CFR 558 | The Food and Drug Administration (FDA) is amending the animal drug regulations to reflect the withdrawal approval of three new animal drug applications (NADAs) for roxarsone or carbarsone Type A medicated articles at the sponsor’s request because the products are no longer manufactured or marketed. | https://federalregister.gov/a/2013-27917 | Final rule |
| FDA | Withdrawal of approval of new animal drug applications; arsanilic acid | 21 CFR 558 | The FDA is amending the animal drug regulations to reflect the withdrawal of approval of a new animal drug application (NADA) for an arsanilic acid Type A medicated article at the sponsor’s request because the product is no longer manufactured or marketed. | https://federalregister.gov/a/2013-28256 | Final rule |
| FDA | Zoetis Inc., et al.; Withdrawal of approval of new animal drug applications for combination drug medicated feeds containing an arsenical drug | 21 CFR 556; 21 CFR 558 | The FDA is amending the animal drug regulations to reflect the withdrawal of approval of 69 new animal drug applications (NADAs) and 22 abbreviated new animal drug applications (ANADAs) for use of arsanilic acid, carbarsone, or roxarsone Type A medicated articles to manufacture combination drug Type B and Type C medicated feeds. | https://federalregister.gov/a/2014-02617 | Final rule |
| FDA | New animal drugs for use in animal feed; withdrawal of approval of new animal drug applications; nitarsone | 21 CFR 556; 21 CFR 558 | The FDA is amending the animal drug regulations to reflect the withdrawal of approval of three new animal drug applications (NADAs) providing for the use of nitarsone in medicated feed for chickens and turkeys. This action is being taken at the sponsor’s request because these products are no longer manufactured or marketed. | https://federalregister.gov/a/2015-31827 | Final rule |
| FDA | Food additives: sucrose fatty acid esters | 21 CFR 172.859 | Sucrose fatty acid esters: arsenic is not >3 ppm | https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.859 | Final rule |
Table 2.
Legislative actions and non-binding policy activity concerning arsenic in food.
| Agency | Description | CFR | Relevant language | Reference (link) | Regulation type/status |
|---|---|---|---|---|---|
| EPA | Organic arsenicals; product cancellation order and amendments to terminate uses | 74 FR 50187 | Cancellation and amendments to terminate uses of end-use and manufacturing-use organic arsenical products registered under section 3 of FIFRA. | https://federalregister.gov/a/2013-07074 | Notice: unclear why this is not listed as a final rule; delay in peer review resulted in 2013 reversal of prohibition of application of organic arsenicals on certain types of land |
| Reregistration eligibility decision | Organic arsenical use is cancelled unconditionally for food-producing land (still permitted for use in certain non-crop producing land) | ||||
| USDA | USDA national list of allowed and prohibited substances | 7 CFR 205.602 | Nonsynthetic substances prohibited for use in organic crop production: Arsenic prohibited in organic crop production |
http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&SID=9874504b6f1025eb0e6b67cadf9d3b40&rgn=div6&view=text&node=7:3.1.1.9.32.7&idno=7#se7.3.205_1602 | Only applies to organic food producers |
| NOP | FR title 7 part 205 national organic program structural pest management | NOP §205.206(f) | A producer must not use lumber treated with arsenate or other prohibited materials for new installations or replacement purposes in contact with soil and livestock. | https://www.ams.usda.gov/sites/default/files/media/GuideForOrganicCropProducers.pdf | Only applies to organic food producers |
| Congress | APPLE Juice Act of 2012 | H.R. 3984 – 112th Congress | Directs the Commissioner of Food and Drugs (FDA) to promulgate final regulations establishing tolerances under the Federal Food, Drug, and Cosmetic Act (FFDCA) to limit the quantity of total arsenic and lead in beverages containing fruit juice. | https://www.congress.gov/bill/112th-congress/house-bill/3984 | Latest action: 2/10/2012: referred to subcommittee on health |
| Congress | RICE Act of 2012 | H.R. 6509- 112th Congress | Directs the Secretary of Health and Human Services (HHS) to promulgate a final regulation under the Federal Food, Drug, and Cosmetic Act establishing the minimum quantity of total arsenic contained in rice or a rice product that will cause the rice or rice product to be deemed to be adulterated under the act. | https://www.congress.gov/bill/112th-congress/house-bill/6509 | Latest action: 09/26/2012: referred to the subcommittee on health |
| Congress | RICE Act of 2015 | H.R. 2529 – 114th Congress | This bill directs the FDA to promulgate a final regulation establishing the minimum quantity of inorganic arsenic contained in rice or a rice product that will cause sale of the rice or rice product to be prohibited. | https://www.congress.gov/bill/114th-congress/house-bill/2529 | Latest action: 05/22/2015: referred to the subcommittee on health |
| FDA | Specific tolerances for residues of approved and conditionally approved new animal drugs | 21 CFR 556.60 |
Tolerances. The tolerances for total residue of combined arsenic (calculated as As) are: (1) Turkeys—(i) muscle and eggs: 0.5 ppm (ppm). (ii) Other edible tissues: 2 ppm. |
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=556.60 Removal of 556.60: https://federalregister.gov/a/2015-31827 |
21 CFR 556.60 was removed along with the withdrawal of approval for nitarsone on 12/18/2015 Dec |
| FDA | Draft guidance for industry on arsenic in apple juice: action level | 78 FR 42086 | The FDA is announcing the availability of a draft guidance for industry entitled “Arsenic in Apple Juice: Action Level” and two supporting documents entitled “Supporting Document for Action Level for Arsenic in Apple Juice” (the draft supporting document) and “A Quantitative Assessment of Inorganic Arsenic in Apple Juice” (the risk assessment document). The supporting documents are referenced in the draft guidance. The draft guidance identifies for the industry an action level for inorganic arsenic in apple juice that FDA considers protective of human health and achievable with the use of good manufacturing practices. It also describes FDA’s intended sampling and enforcement approach. | https://federalregister.gov/a/2013-16719 | Notice |
| FDA | Inorganic arsenic in rice cereals for infants: action level | 81 FR 19976 | The FDA is announcing the availability of a draft guidance for industry entitled “Inorganic Arsenic in Rice Cereals for Infants: Action Level,” a supporting document entitled “Supporting Document for Action Level for Inorganic Arsenic in Rice Cereals for Infants” (the supporting document), and a risk assessment report entitled “Arsenic in Rice and Rice Products Risk Assessment: Report” (the risk assessment report). The draft guidance, when finalized, will identify for industry an action level for inorganic arsenic in rice cereals for infants that will help protect public health and is achievable with the use of current good manufacturing practice. | https://federalregister.gov/a/2016-07840 | Notice of availability |
| FDA | Detention without physical examination and surveillance of fruit juices and fruit juice concentrates due to heavy metal contamination | Import alert #20-05 | Districts may detain without physical examination fruit juices and fruit juice concentrates from specified fruits from the firm(s) listed on the red list for this alert. | http://www.accessdata.fda.gov/cms_ia/importalert_56.html | DWPE with surveillance |
The EPA regulates arsenic levels in public drinking water supplies, though no US agency directly regulates the arsenic content of irrigation water, which can contribute to the accumulation of arsenic in food crops (Heikens, 2006). EPA also regulates arsenic emissions into air and water from power plants through the Mercury and Air Toxics Standards (United States Environmental Protection Agency, 2016) and the Steam Electric Power Generating Effluent Guidelines (United States Environmental Protection Agency, 2015). While these regulatory mechanisms may influence the arsenic content of soil and water involved in agricultural production, they do not specifically address the arsenic content of food commodities. The EPA also regulates arsenic levels in processed human biosolids, but not animal wastes, applied to agricultural land as fertilizer. This situation was improved in 2015 when FDA withdrew approval for all arsenic-based animal drugs in the U.S., therefore eliminating a major source of arsenic in animal wastes (Nachman et al., 2016). Lead arsenate, an early insecticide, saw extensive agricultural use in the early 20th century, but was largely replaced in practice with DDT and ultimately banned in 1988 (Peryea, 1988). Organic arsenical pesticides that were once used on agricultural lands are now permitted for application only on certain non-food producing lands (United States Environmental Protection Agency, 2009). USDA Organic-certified food producers are also prohibited from using arsenical pesticides or lumber treated with chromated copper arsenate to avoid increased arsenic levels in crops and livestock (United States Department of Agriculture, 2012a; United States Department of Agriculture, 2013).
The mandate of regulating arsenic in food lies with the FDA, which can develop both nonbinding guidance documents and enforceable policy; the agency retains the authority to mitigate the presence of heavy metals such as arsenic in food under the interstate commerce clause of the Federal Food, Drug and Cosmetic Act (United States Congress, 2005). Despite this, at present, enforceable maximum levels of iAs in foods are not currently established in the US; instead, the FDA currently deals with arsenic in foods and beverages on a case-by-case basis and as noted above has set draft action levels for two commodities which only serve as guidance to producers. In 2005, the FDA amended its bottled water quality standard regulations to adopt EPA’s arsenic MCL. Requirements for monitoring and enforcement are included in the rule (United States Food and Drug Administration, 2005).
FDA activity regarding arsenic in foods and beverages has increased in recent years, possibly in response to interest from legislators and public attention sparked by non-governmental organizations (Consumer Reports, 2012b). In recent years Congress proposed but did not pass bills that would have required FDA to promulgate tolerances for arsenic in beverages containing fruit juice (United States Congress, 2012a) and rice products (United States Congress, 2012b). Non-profit organizations have also called for FDA to move forward with the development and implementation of enforceable standards for arsenic in rice and juice (Consumers Union, 2013).
Based on previously discussed risk assessments, FDA has developed and released proposed action levels for arsenic in apple juice (10 ppb) and infant rice cereal (100 ppb), accompanied by draft guidance documents consistent with the FDA’s good guidance practices regulation (United States Food and Drug Administration, 2015). To date, both action levels and accompanying guidance documents remain in draft form. FDA has not developed monitoring or intervention plans for the implementation of these action levels, though available online is a non-binding warning letter to an apple juice producer whose arsenic content was above the action level (United States Food and Drug Administration, 2016e). It is unclear whether such letters are a common occurrence. FDA has not proposed action levels or regulatory interventions for any other food items, with potential areas of intervention being beer and wine, grains, grape and other fruit juices, and non-infant rice products as these have shown elevated levels of iAs (United States Food and Drug Administration, 2016c).
On the global level, policy addressing arsenic in food is an area of interest for many countries and international organizations. Enforceable maximum limits for arsenic in food currently exist in several countries, though the regulated food products, allowable concentrations, and the monitoring and enforcement programs accompanying the established limits differ dramatically between countries (Table 3). Most regulations focus on iAs content, and the most commonly regulated food categories are grains (particularly rice), and marine products (fish, shellfish and seaweed) (Petursdottir et al., 2015).
Table 3.
Selected food-related arsenic regulation in other countries.
| Country | Regulation type | Regulation content | Reference |
|---|---|---|---|
| EU | Maximum limit for iAs in rice and rice-based products | Polished rice: 0.2 mg kg−1, Parboiled and husked rice: 0.25 mg kg−1 Rice waffies, rice wafers, rice crackers and rice cakes: 0.3 mg kg−1 Rice destined for the production of food for infants and young children: 0.1 mg kg−1 |
http://faolex.fao.org/docs/pdf/eur146756.pdf |
| France | Limits on iAs in algae | Algae: 3 mg kg−1 | CEVA (2010) Régelementation algues alimentaires Synthése CEVA au 1/04/2010. France |
| China | Maximum limit for iAs and total As | e.g. Grain: 0.5 mg kg−1 Rice 0.2 mg kg−1, Veg: 0.5 mg kg−1 Fish: 0.1 mg kg−1 |
Ministry of Health of the People’s Republic of China (2012) National Food Safety Standard. Maximum Levels of Contaminants in Food. GB 2762–2012 |
| Canada | Maximum limit for iAs | Fish protein: 3.5 mg kg−1 Edible bone meal: 1 mg kg−1 Fruit juices and beverages: 0.1 mg kg−1 |
CFIA (2014) Food and Drugs Act Regulations (FDAR). Section B.15.001 Tables I and III |
| Australia and New Zealand | Maximum limit on iAs in food | Cereals: 1 mg kg−1 total As Fish, seaweed, shellfish: 1–2 mg kg−1 iAs |
Australia New Zealand Food Standards Code: Standard 1.4.1: Contaminants and Natural Toxicants http://www.comlaw.gov.au/Details/F2011C00121 |
3.3. Monitoring for arsenic in food
It is well established that measurable arsenic is ubiquitous in the food system, though the limited available data would suggest that among many commodities, the amounts and species composition can vary substantially (EFSA CONTAM Panel, 2009). Decision-making would benefit greatly from analyses of arsenic species in an extensive array of food items, especially targeting items of cultural significance or that are consumed during a particularly vulnerable lifestage.
The monitoring of arsenic in feed for food-producing animals and in food is divided between several programs across two agencies. With regard to arsenic in animal feed, the United States Department of Agriculture (USDA) Food Safety and Inspection Program operates the National Residue Program (NRP), which conducts surveillance and targeted monitoring of domestic and imported meat, poultry, and egg products (United States Department of Agriculture, 2015), the results of which are published in the NRP’s Red Book (United States Department of Agriculture, 2014). It is not clear, however, whether arsenic-based drugs served as the primary rationale for NRP arsenic sampling, and whether its inclusion in the NRP protocols will continue now that these drugs are no longer permitted for use in the US (and arsenic tolerance levels in animal products have been formally withdrawn from the CFR as of April 2016) (United States Department of Agriculture, 2015; United States Food and Drug Administration, 2016a). The FDA receives a list of and is responsible for investigating producers whose samples exceed regulatory arsenic tolerance limits; the agency may take legal action if conditions at production operations with repeat violations (United States Department of Agriculture, 2015). FDA also operates a Feed Compliance Program for contaminants of Veterinary Medicine (CVM), although the program only provides FDA staff with non-binding guidance regarding the monitoring and control of contaminants, including arsenic (outside of the context of arsenic-based drugs), in animal feed (United States Food and Drug Administration, 2010).
The FDA operates several programs that monitor arsenic levels in the food supply. The Total Diet Study (TDS), operating continuously since 1961, annually evaluates total arsenic in approximately 4 samples each of 280 U.S. food items prepared as consumed from across the country (Tao and Bolger, 1999). Food items are updated approximately every ten years to reflect changes in the typical U.S. diet, and include juices, wines, vegetables, meat products, and rice products, among others. Results are made public (albeit slowly in the case of arsenic) and are used in FDA risk assessments to determine priority foods and contaminants (United States Food and Drug Administration, 2016d). While the scope of the TDS is inclusive of a wide array of commonly-consumed food items, the number of samples taken per year for each commodity may be of limited value with regard to market representativeness for a specific food.
The Food Safety Modernization Act (FSMA) of 2011 mandates that FDA identify high-risk foods and contaminants and determine, where necessary, guidance and action levels for decreasing population exposure (United States 111th Congress, 2011); the Most Significant Food Contaminants and High Risk Foods programs were adopted to meet FSMA requirements, and both rely on TDS sampling data (United States Food and Drug Administration, 2014). Both of these programs specifically note that toxic elements would be either ranked among contaminant prioritization lists, or would be an important factor in decision-making regarding high-risk foods.
Beyond TDS and the implementation of FSMA, FDA monitors arsenic levels in specific food items that garner public concern under the Toxic Elements Program, including apple and pear juices beginning in 2005 and rice and rice products beginning in 2012 (United States Food and Drug Administration, 2016c). In some cases, regulatory monitoring of specific food items appears to occur in tandem with, or possibly in response to, concern generated by sampling of iAs in food items by nongovernmental organizations such as the 2012 reports on arsenic levels in rice and apple juice products by Consumer Reports (Consumer Reports, 2012b; Consumer Reports, 2012a).
In summary, arsenic is monitored to varying extents at selected points in the food system, including animal feed, meat, and some foods as consumed. The majority of existing monitoring programs are limited to evaluation of total arsenic which does not provide the information needed to assess the relative contribution of different foods to iAs exposure. When considering data needs in support of decision-making, current monitoring programs in the U.S. fall short. Existing regulatory mandates, and by extension Agency activities, concerning arsenic were not designed or implemented in a coordinated fashion, but instead evolved over time, often in a reactive manner. Consequently, the lack of a coordinated strategy for addressing dietary iAs exposures results in specific monitoring or intervention activities of limited scope that fail to account for the holistic nature of exposure and typically deal with foods on an individual basis.
3.4. Existing risk communication efforts surrounding arsenic in food
Communicating health risks resulting from arsenic exposure poses significant challenges for a variety of reasons. Even the word “arsenic” evokes its historical reputation as a poison (Hughes et al., 2011). The complex chemistry of arsenic, including inorganic and organic forms with varying toxicity, contributes to public confusion regarding risks. Specific health outcomes associated with arsenic exposure such as cancer and cardiovascular disease, as well as particular vulnerabilities of children, are of high public concern. Intense media coverage stimulated by periodic reports of arsenic contamination in a range of foods, combined with the release of scientific publications related to arsenic’s propensity to elicit human health effects, and announcements of regulatory interventions to address arsenic exposure has generated a cascade of headlines and social media activity. These cyclical bursts of information related to dietary arsenic predictably raise public concern about exposure and risk, and are typically accompanied by disparate and often conflicting responses from scientists, food manufacturers, regulators and other key stakeholders.
An illustrative example occurred in September 2011 when Dr. Mehmet Oz, on his nationally-broadcasted television show, announced results of arsenic testing he had commissioned of over 30 commercial apple juices, in which total arsenic concentrations in popular brands exceeded the MCL for drinking water. His report focused public attention on the potential presence of arsenic in apple juice, information that had been known to the FDA and monitored through the TDS since 1991, but was newsworthy and alarming to consumers who were hearing this for the first time. This public concern was heightened by the association of apple juice with consumption by children and absence of an FDA regulatory standard. The initial FDA response was to acknowledge the known presence of trace amounts of arsenic in apple juice, but at levels below which they believed there was a health concern or need for a standard (United States Food and Drug Administration, 2011a). The FDA also challenged the testing protocols used by the Oz laboratory as well as evaluation of total arsenic rather than the more health relevant iAs species (United States Food and Drug Administration, 2011a; United States Food and Drug Administration, 2011b). Attention in the media included weighing in by a former acting director of the CDC, congressmen, and Consumers Reports representing varying levels of concern and suggested responses. In July 2013, the FDA announced their risk assessment results and decision to set a limit of 10 ppb for arsenic in apple juice which was widely reported in the media as a confirmation of the safety concerns raised in 2011 (Aubrey, 2013) (United States Food and Drug Administration, 2013). The lasting impact of this example on public risk perceptions is unclear, though it has been suggested that consumers tend to be more risk averse when a perception exists that experts disagree about risks (Groth, 2016).
Applying the research of Paul Slovic to this particular example would predict heightened public risk perception about arsenic contamination in apple juice with influencing factors including the involuntary nature of the exposure, particular risk to a vulnerable population, conflicting messages from experts, the serious nature of associated health risks, and an appearance of regulatory agency delay that might impact perceived credibility (Slovic, 1987).
A similar scenario ensued in September 2012 when Consumer Reports identified a widely-consumed food staple, rice, as containing a range of iAs concentrations varying by rice type and country of origin (Consumer Reports, 2012a). This fragmented, episodic pattern of public risk communication about arsenic in distinct food products has persisted, most recently with the April 2016 FDA announcement of a proposed action level for infant rice cereal but not other rice-based commodities (United States Food and Drug Administration, 2016b).
Additional factors unique to arsenic exposure contribute to the difficulty in providing science-based, actionable communication. These include the inadequacy of existing toxicological metrics for estimating risk, which make it difficult to compare iAs risks to other environmental or dietary constituent risks, and the challenge of prioritizing foods for intervention among many food sources. Food manufacturers have sought to reassure consumers either through dismissal of the presence of a significant health concern (USA Rice Federation, 2016) or by distinguishing their product in the marketplace through producer-initiated arsenic testing (Lundberg Farms, 2016). Healthcare providers face particular challenges in responding to their patients’ concerns about current and prior exposures to arsenic, particularly in the perinatal period and infancy, when research suggests heightened vulnerability (Lai et al., 2015).
It is clear that recent high profile media attention to arsenic in specific foods, in the form of television talk shows, magazine articles, and other lay venues succeeds in piquing the public’s interest. In both the juice and rice examples, public attention on the hazard has resulted from reports from non-governmental entities. In general, this attention did not provide consumers the information needed to decide whether personal action was needed and what that action might entail. The situation was made worse by conflicting information and inconsistent advice from a variety of governmental and independent sources. Each of these media cycles raised public concern but left important questions unanswered since there was no trusted regulatory or medical authority that could provide clear risk communication. These cycles of media attention, public interest, limited regulatory activity and poor risk communication highlight the need for a proactive regulatory approach to managing arsenic exposures through food.
4. Prioritizing opportunities for intervention
Intervention strategies for arsenic may best focus on means to minimize the public’s exposure from common dietary sources to the greatest extent possible rather than focus on a particular risk-based (e.g., de minimis cancer risk) goal. In some respects, the challenges presented by iAs are similar to those from lead; both are natural crustal elements which have no known physiological function, but for which exposure is widespread and toxicity is multi-faceted. Similarly to lead, evidence is accumulating for arsenic producing negative effects on cardiovascular function and neurodevelopment at exposure levels that are common in the general population (National Research Council, 2014). EPA has not been able to derive an RfD or CSF for lead, and while such values are available for iAs, they are outdated. It is uncertain as to where new levels may be set. Thus, for both lead and arsenic, levels that can be found in food or other environmental media present risk assessment and risk management challenges. Similar to the ongoing effort with lead to reduce exposures from a multitude of sources, the presence of arsenic in a variety of foods offers multiple intervention opportunities including adoption of best production practices aimed at delivering low arsenic foods to the market, and consumer education aimed at limiting the ingestion of high arsenic foods. The latter can include meal consumption advisories akin to what is provided for fish due to methylmercury and polychlorinated biphenyl (PCB) contamination. While it is important to consider the nutritional benefits of food items (e.g., fish), it is also critical to recognize the contaminant exposure and look to alternative foods and meal frequency limits until such time as the contaminant content of the food is more compatible with public health goals (e.g., de minimis risk, or non-cancer risks below thresholds of concern). This approach has generally worked for fish consumption for high risk groups (e.g., pregnant women) but care is needed in crafting advisories that don’t completely discourage consumption of the food item (Cohen et al., 2005). It is also important to maintain sensitivity to the cultural values and practices and the degree to which a food product is a key nutritional source for some populations in considering interventions. This is particularly true in the case of rice.
4.1. A coordinated approach to minimizing exposure to iAs through food
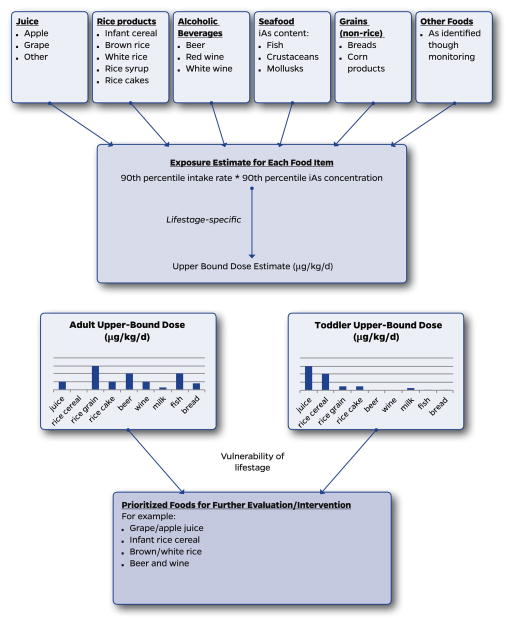
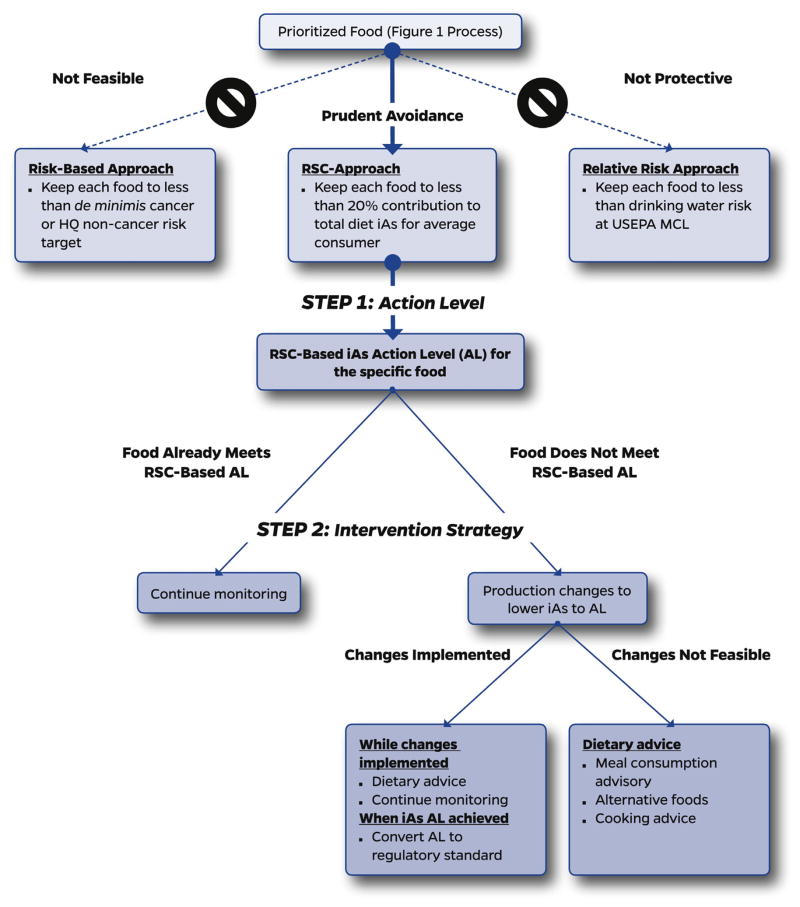
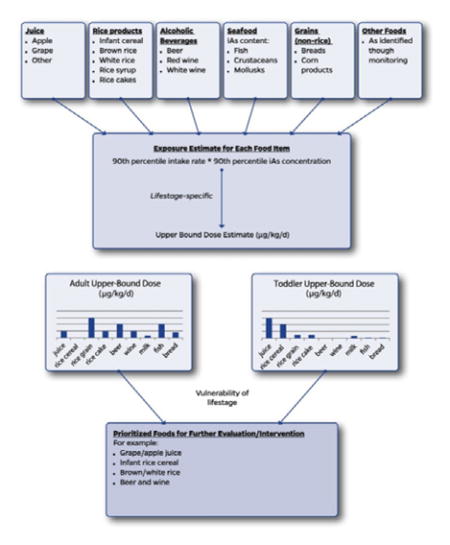
We hereby propose a coordinated approach for reducing arsenic exposure via the diet as a combination of three key elements: a) prioritization of foods for further evaluation and intervention (Fig. 1); b) development of an action level in the food based upon a relative source contribution (RSC) approach which ensures that no particular food contributes a disproportionate amount to aggregate dose (Fig. 2, Step 1); and c) intervention strategies that bring the iAs concentration of the food down to the action level, or if this is not immediately feasible, provides dietary advice that limits consumption of that food to meet RSC-based exposure goals (Fig. 2, Step 2).
Fig. 1.
Exposure-based approach to prioritize foods for further evaluation/intervention.
Fig. 2.
Relative source contribution (RSC) approach to targeted interventions for reducing dietary exposure to iAs.
The centerpiece of this strategy is the RSC-based approach for iAs in food. As described above and shown in Fig. 2, the traditional risk-based approach to developing an action level is not practical for iAs, given that approaches for developing iAs dose-response metrics are still under development and with emerging information suggesting that typical dietary exposures may be associated with elevated risk for both cancer and non-cancer endpoints. In light of difficulties using a risk-based approach, the arsenic drinking water MCL is often used as a touchpoint to imply a safe level of exposure, despite the fact that this value was not risk-based and was a compromise on the basis of economic feasibility (United States Environmental Protection Agency, 2000). The 10 ppb MCL is associated with an estimated risk that is far higher than is typically tolerated for other MCLs (400 to 7000 times greater than de minimis). A possible justification is that since 10 ppb is allowed in public water supplies, many consumers are at that level of exposure, making it inconsistent to hold dietary sources to a more stringent target. However, only 3% of the US population are estimated to be drinking public water served by a supply at or above the MCL (Mushak et al., 2000). In these populations, it is likely that food sources dominate aggregate arsenic exposures. Taken together, interventions aimed at achieving iAs intakes comparable to water consumption at the MCL are inadequately protective and thus inconsistent with public health goals.
The RSC approach starts from the assumption that the aggregate iAs dietary exposure is at elevated health risk and thus does not require specification of risk. The strategy recognizes that it is important to bring iAs exposures under control, driven by an action level for a food item that limits the exposure possible from that item to less than a pre-set percentage of the aggregate dose. Here, we take advantage of the RSC concept, which is currently employed in EPA’s MCL derivation process to limit the fraction of the reference dose that drinking water can contribute with the knowledge that, for many agents, diet or various environmental media may also contribute to the aggregate dose. The RSC is a means to ensure that the aggregate dose does not increase above a risk-based target. But one does not need an RfD or risk-based targets to employ the RSC approach, as it can be based off of the current baseline level of exposure in the diet minus the food item in question. In this manner, it endeavors to prevent the baseline diet from undue influence by any single iAs source and to ensure that the overall aggregate exposure, even for high end consumers of a high iAs food, is not driven to an extreme of exposure.
The manner in which the percentage of the RSC is set for drinking water has been by default and convention as a 20% contribution to overall exposure. We adopt this convention for the current purpose of illustrating the concept (Fig. 2, Step 1), although we recognize that setting this percentage can also take into account the number of foods which make a substantive contribution to dietary iAs so that there is “room” for these various sources when considering the variability inherent in those sources.
The following two case examples use high-profile foods previously addressed by the FDA to illustrate the RSC approach to setting an action level and intervention strategies to achieve this level of iAs mitigation (Fig. 2 concepts). The prioritization of foods to receive this level of attention is shown in Fig. 1 based upon a systematic evaluation of the exposures possible from the array of foods most relevant to a given lifestage. This approach is in part exemplified in the following examples because FDA’s prioritization of apple juice and rice cereal was based upon their relatively high content of iAs and they are disproportionately ingested in early life at what may be a vulnerable lifestage (both elements of Fig. 1). However, the selection of these foods by FDA did not involve the type of systematic evaluation outlined in Fig. 1.
Two intervention strategies are outlined in Fig. 2: 1) the adoption of best production practices to minimize the iAs content of specific foods; and 2) risk communication strategies (including meal consumption frequencies) aimed at limiting the public’s iAs exposure through food. Ideally, best production practices would be the basis for meeting the action level. This is most feasible if market sampling indicates a broad range of arsenic concentrations in a particular food and if follow-up research suggests that certain sources of raw commodities or ingredients, or even certain brands, are consistently lower in iAs than others. If this can be documented, then controllable factors, such as growing conditions, soil concentrations, soil amendments, water concentrations, packaging, processing, etc. can be addressed (Davis et al., 2017; Punshon et al., 2017). This research could then lead to best production practices that guide the marketplace to produce food items with lower iAs content. FDA’s role in setting action levels and regulatory standards for iAs in foods is a crucial element to such an approach. This is exemplified below with respect to the FDA action level for apple juice, which could potentially be set lower and thus help to drive the development of best production practices.
Unfortunately, best production practices may have limited success in cases where iAs is high in a food commodity with little variance in the sampling data. While research may still be warranted to further explore sources and feasibility for production practices to reduce iAs content of that food, the more immediate approach to protect public health moves in the direction of risk communication strategies to limit consumption of the high iAs food (and perhaps promote consumption of alternatives). Meal frequency advice akin to what is commonly done for fish may be a central part of this outreach and communication. We focus upon this option in the area of rice and rice products where the best production practice approach is first evaluated followed by a consideration of meal consumption advice. Other intervention strategies dependent upon risk communication such as alternative food preparation practices can also be considered.
4.2. Apple juice
One example where there are diet-related iAs exposures of concern is with regards to children’s consumption of fruit juices which consistently contain amounts of iAs of potential concern. While apple, pear and grape juices have had frequent iAs detections, we use apple juice as an illustration given FDA’s 2013 focus on this commodity. FDA’s approach to setting an action level for apple juice was based upon feasibility. Market basket surveys were used to establish the range of arsenic levels in juice and then FDA set an action level that was above all the sampled juice results. This approach prevents higher levels of iAs but does not lower the level in juice. FDA did not provide a feasibility assessment for considering lower action levels. It would appear that a lower target may be feasible as many samples were well below the FDA action level; the median of the distribution was an iAs level of approximately 5 ppb (United States Food and Drug Administration, 2013). While some of this variability may be random, a systematic difference in the manner in which the samples/brands with less iAs content source their raw materials or process their juice may explain some of the variation. If FDA had set an apple juice goal of 5 ppb, perhaps phased in over a few years, it may have encouraged juice manufacturers to research the sources of iAs in apple juice and move towards practices that meet this lower action level. By setting the value at 10 ppb, a level that is already being met in the marketplace, there is no incentive for manufacturers to try to limit iAs in their products.
There would be little public health issue with setting the value at 10 ppb if the health risks associated with juice ingestion were below a level of concern. FDA’s risk assessment, however, found that an action level of 10 ppb is associated with a cancer risk of 8 per million for average consumers, and because of uncertainties in the current non-cancer assessment, FDA’s action level-setting process did not take into account non-cancer endpoints. Further, to the extent that the juice action level is predicated on consistency with USEPA’s drinking water MCL, this is not a health protective basis, as described above.
Our proposed approach sets an iAs action level for juice that is not risk-based or MCL-based, but rather relies on the food’s RSC to total iAs intake (Fig. 1). This approach requires knowledge of the baseline level of iAs in a young child’s diet and then to set the amount that can come from apple juice to a percentage (20% for purposes of illustration) of that total background. As summarized in the ATSDR Toxicological Profile for Arsenic (Agency for Toxic Substances and Disease Registry, 2007), market basket surveys for iAs in foods combined with central tendency estimates of dietary ingestion of these foods yield iAs exposures of 4.5–9 μg/day for children aged 2–6 years of age (Tao and Bolger, 1999; Yost et al. 2004). This comports with estimates from a variety of European studies in which the central estimate of aggregate iAs exposure for toddlers (1–3 years old) is 8.1 μg/day (European Food Safety Authority, 2014). An RSC goal of 20% would mean that any particular food item would not contribute >0.9 to 1.8 μg/day of iAs for the average 2–6 year old consumer in the US. The exposure side of this RSC calculation is to develop a central estimate of children’s apple juice ingestion rate, multiplied by the FDA action level (10 ppb) to determine whether the action level is sufficient to keep children’s iAs exposure from apple juice within the 20% RSC. We note that the apple juice exposure assessment provided in FDA (2013) does not focus upon the ingestion rate of young children, and modeled the risks for the average consumer of apple juice for a 0–50 year age window, since this exposure scenario provided the greatest lifetime cancer risk. The FDA exposure estimate was based upon 2-day dietary recall data from NHANES averaged across consumers and non-consumers, with FDA also providing a high end consumer estimate of triple this intake rate. The long-term and consumer and non-consumer averaging of juice consumption, along with the 3 times bounding assumption yields a daily intake of 76 to 229 mL of apple juice for 3–6 year old children (based upon their body weight). The upper end of this range is approximately what is contained in one mid-sized juice box (6.75 oz or 200 mL). This (200 mL) is a reasonable first assumption for child consumers of apple juice. This ingestion rate at the FDA action level would deliver 2 μg/day of iAs, which exceeds the upper end of the target RSC range and would represent 22–44% of the aggregate background dietary exposure.
While it is reasonable to use broad averages when extrapolating to a 50-year exposure (United States Food and Drug Administration, 2013), the alternative approach just described is to narrow the focus on the RSC for apple juice in a vulnerable lifestage (young children). In this manner, the regulatory action level can be evaluated with respect to whether it prevents this food commodity from being a main source of iAs ingestion for average consumers of a particular age, and whether it causes the aggregate exposure of high end consumers to become excessive relative to the rest of the population of that lifestage.
The upper end of children’s juice intake can be estimated from a study of 168 preschool children (2 or 5 years of age) which found that 11% of children ingested 12 oz (355 mL) of fruit juice per day (Dennison et al. 1997). If we assume this to be a high end (e.g., 90th percentile) rate of consumption, this represents an iAs dose of 3.5 μg/day which increases the aggregate dietary dose of arsenic by 39 to 78%. Thus, the FDA juice action level of 10 ppb can be seen to allow an increase in the background rate of exposure during a potentially vulnerable lifestage by 22–44% and 39–78% for average and high-end consumers of this lifestage, respectively. To meet an RSC goal of 20% would involve a lower juice action level, with 5 ppb coming closer to achieving that goal; this would shift the distribution of sampling results to below 5 ppb, which would likely have a larger benefit than what is assumed where all samples are at the action level. As improvements are made in apple juice production practices to meet this RSC-based AL, further research and advances can hopefully make even lower action levels feasible and thus, as in the case of childhood lead exposures, result in a decline in iAs exposures over time.
Exceedances of the RSC goal for those children at the high end of ingestion could be addressed via frequency consumption advice. Pediatricians have for several decades recognized that early life juice intake can be excessive in their patient population leading to a higher risk of obesity and dental decay (American Academy of Pediatrics, 2001). Official American Academy of Pediatrics (AAP) recommendations are for young children to ingest no >4–6 oz (118–177 mL)/day of fruit juice. An iAs-motivated message to limit excessive juice consumption could provide additional public health incentive to encourage families to meet the AAP recommendation.
4.3. Infant rice cereal: BMP analysis combined with meal frequency advice
As described above, FDA set an action level for infant rice cereal of 100 ppb, a concentration that was the 36th to 47th percentile of the distribution of rice cereal sampling results (United States Food and Drug Administration, 2016b). Thus, the majority of samples did not meet the action level. It may be difficult to achieve a much lower action level in the short term given that only 3 to 20% of samples were able to meet a target of 75 ppb, but fortunately this is an active research area involving both agricultural practices and cooking methods (Zhao et al., 2010; Sohn, 2015; Signes-Pastor et al., 2016).
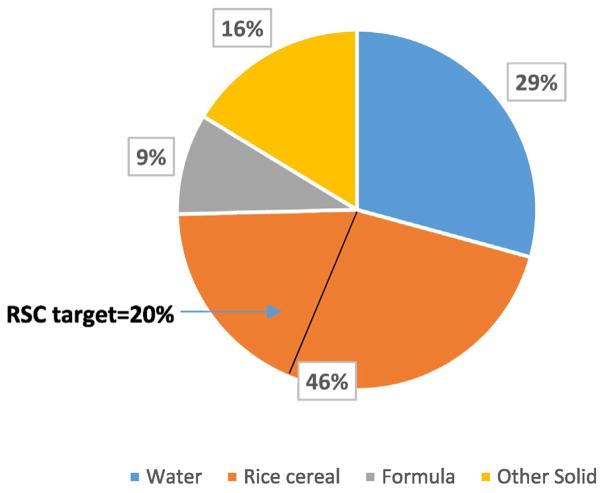
FDA’s cancer assessment suggested greater than de minimis risk for infants ingesting rice cereal for one year, 3.2 to 9.5 per million. The infant exposure associated with this action level can be put into the context of the RSC approach, with sources of exposure during this lifestage (approximately 6 months of age) being infant formula or breast milk, and baby foods containing fruits, vegetables or meats, as well as drinking water. This array of iAs exposures was compiled in a Monte Carlo analysis based upon rice cereal containing 91 ppb as a central estimate (range 23 to 283 ppb) and assuming one serving of rice cereal (4.5 tablespoons dry cereal) per day (Shibata et al., 2016). The analysis showed rice cereal to contribute 45% of a 6–8 month old’s iAs exposure for the median consumer (49% if using the FDA action level of 100 ppb), well above a target RSC of 20% as discussed above for juice (Fig. 3). Consistent with Fig. 2, two intervention choices to reduce the rice cereal exposure to an RSC of 20% are: a) setting the action level to 41 (rounded to 40) ppb and through research and best production practices achieving this goal over time (Zhao et al., 2010); b) in the meantime, risk communication and meal frequency advice which educates parents to utilize a variety of cold and hot infant foods while limiting the number of rice cereal meals to 2 meals/week. This is based upon rice cereal at the FDA action level and a 20.4 g meal size (Shibata et al., 2016). At this meal frequency, rice cereal at 100 ppb would contribute <20% of the aggregate iAs exposure in a 6 month old infant.
Fig. 3.
Inorganic arsenic pathways analysis in 6–8 month old infants. (Adapted from Shibata et al., 2016.)
These intervention recommendations for apple juice (lower the action level to 5 ppb) and infant rice cereal (2 meals/wk advisory) are provided as examples of the approaches needed when considering iAs across a wide array of foods. Another priority area for children is iAs in brown rice syrup which can be a driver of exposure when present as a sweetener in infant formula or snack bars (Jackson et al., 2012).
5. Conclusions
While many uncertainties exist with regard to iAs dose response for the multiple endpoints for which it is active as well as the timing of critical windows of vulnerability, there is sufficient evidence that dietary exposures can be a public health concern (EFSA CONTAM Panel, 2009; Gundert-Remy et al., 2015). We have identified key gaps and limitations of current food monitoring programs, along with a dearth of regulatory standards or guidance for many commonly-consumed foods known to accumulate arsenic. Without clear signals from regulatory agencies, the food industry lacks iAs concentration benchmarks for their products, and without a carefully conceived and transparently reported monitoring system, there is even less incentive for producers to pursue iAs mitigation. The general public has not received consistent messages about iAs in the diet from FDA and other stakeholder groups; in fact, some of the sources the public may rely upon are ill-equipped to provide advice or are conflicted by other interests (USA Rice Federation, 2016). While the issue of arsenic in food has been examined by intergovernmental bodies and investigators outside of the US (EFSA CONTAM Panel, 2009), no clear template emerges that describes how the various domestic stakeholders can collaboratively address this complex issue.
As described above, numerous uncertainties in assessing dose-response relationships for arsenic for cancer and other outcomes have, to date, precluded the derivation of quantitative toxicity metrics that are needed to replace outdated values. Similar to lead, a risk management approach reliant on estimates of cancer risks or reference doses is not possible or recommended at this time. Consequently, for dietary arsenic exposures, we advocate for a prudent avoidance approach, recognizing that exposure reductions that would reduce aggregate dietary iAs intake to levels equal to or below traditional risk targets may not be achievable.
This prudent avoidance approach we recommend is based upon the RSC concept such that any particular food’s contribution to aggregate exposure is limited to a set percentage. Implementation of this approach may begin with currently available resources (e.g. the Total Diet Study arsenic data and dietary intake information from NHANES/What We Eat In America), but full realization of its potential is dependent on the development of a more rigorous and comprehensive monitoring strategy that includes arsenic speciation. These types of market analyses are beginning to be implemented in other countries, such as Italy (F. Cubbada, personal communication). While public pressures may prompt action on a narrow set of commodities (including some that may well be justified by an RSC examination, Fig. 2), it is likely that specific foods that have not entered the public awareness (and thus have not been addressed from a regulatory perspective) may be contributing significantly to aggregate dietary exposures. A more holistic and tematic approach to monitoring foods that enter the marketplace will foster confidence that potential exposures are understood, that opportunities for intervention are prioritized and that goals are being met. As a complement to better arsenic monitoring, tracking dietary patterns to identify commonly- and highly-consumed foods, especially among population subgroups or lifestages with increased vulnerability to arsenic exposure, is needed.
Beyond prioritizing foods for intervention and working towards reductions in the arsenic content of foods, there exist other opportunities for mitigating exposure. Improved communication among stakeholders is a key need; specifically, research is needed to identify means of communication about food-based arsenic risks in a manner that provides actionable information to consumers without creating unnecessary alarm. Further, additional work is needed to better understand how the targeting of messages both to the general population and to uniquely vulnerable subpopulations can be managed to maximize the impact on exposure and risk reductions. Thinking upstream from the consumer, research and communication opportunities may exist between food producers, regulators and academics that would facilitate the engineering of a safer product lower in arsenic and meeting regulatory goals. Also noteworthy is the role of the marketplace in fostering action; consumer demands for a safer product can provide food manufacturers with the oft-requisite economic incentive needed to take steps towards reductions in arsenic content.
There are certain foods for which controlling arsenic levels may be particularly challenging. While eventual interventions to lower arsenic concentrations are warranted, alternative approaches involving risk communication may be necessary in the immediate term in order to limit those foods’ contributions to aggregate exposures. While not ideal, these situations provide an opportunity for FDA to issue food-specific meal frequency advice. Provision of such advice can achieve multiple purposes, by both giving consumers clear and actionable plans for making dietary modifications, and signaling to the marketplace that source reduction interventions are needed for that particular food commodity. Careful attention to the communication strategy and its reception are important, however, especially when the food item of interest confers nutritional or cultural benefits.
Recent FDA interventions for arsenic in food have been limited to the setting of action levels for two specific foods, though the activities involved in their implementation are not well described in publicly-accessible regulatory documents. These action plans have not included monitoring and enforcement plans that would be likely to provide appropriate signals to producers and other market forces, and do not provide those in public health and risk communication with the data needed to properly advise the public. While promulgation of standards may prove to be more politically challenging and time consuming than action levels, future regulatory interventions could initiate with an action level with the intention of giving the industry time to transition into a firm regulatory standard.
The proposed approach can be a useful tool for making risk management decisions about targeting arsenic concentration reductions in specific foods, although we acknowledge that other considerations play a critical role in decision-making. Among these is the nutritional benefit of the foods under consideration, as well as the importance of specific foods to those with health-related dietary restrictions. The case of methylmercury (MeHg) in fish is an example where efforts have been made to consider the dietary benefits of n-3 polyunsaturated fatty acids against the cardiovascular and neurological risks related to MeHg exposure (Mahaffey, 2004; Cohen et al., 2005; Ginsberg and Toal, 2009; Mahaffey et al., 2011). Other important considerations in decision-making around item-specific interventions are the availability of alternative food items to meet nutritional needs, and the cultural significance of the item in question.
Moving forward, a broader consideration of other arsenic species is critical. The majority of epidemiologic focus on arsenic has been centered around drinking water exposures, and thus exclusively examines iAs; while the evidence of biological significance is strongest for these species, less is known about the toxicity of other organic forms, such as methylated species, arsenosugars and arsenolipids which are common in certain foods (Francesconi et al., 2002; Schmeisser et al., 2006; Sele et al., 2012). Given the complex mixture of arsenic species that are present in various foods, future toxicological research can be made more relevant to dietary exposure via studies that explore the effects of organic arsenicals and mixtures of iAs with such organic species. Exposure profiles for non-inorganic species in the diet should also be a focus of future research.
As the epidemiologic evidence connecting arsenic to numerous health outcomes advances, dose-response relationships and windows of heightened vulnerability may become clarified. This progress will enable more refined targets for arsenic exposure reductions. In the interim, we have outlined an approach that is dependent on bolstering monitoring resources, and which encourages exposure mitigation via the setting of appropriate iAs concentration targets in particular foods, best production practices and improved communication strategies. These recommendations are likely to provide a rational, scientifically defensible and clearer path to dietary arsenic reductions than what has occurred up until the present time.
HIGHLIGHTS.
Our assessment of regulatory oversight of dietary arsenic found that foods are addressed on individual bases, and not comprehensively.
A process for prioritizing dietary inorganic arsenic exposures by different lifestages is proposed.
A relative source contribution-based approach to setting criteria for arsenic in prioritized foods is recommended.
Acknowledgments
Funding
This paper, a product of the Collaborative on Food with Arsenic and associated Risk and Regulation (C-FARR), is supported by the Dart-mouth College Toxic Metals Superfund Research Program through funds from the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number 1R13ES026493-01 to C. Chen and Award Number P42ES007373 to B. Stanton, and the Children’s Environmental Health and Disease Prevention Research Center at Dartmouth through funds from the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P01ES022832 to M. Karagas.
Footnotes
Competing interests
The authors declare that no competing interests exist.
References
- Agency for Toxic Substances and Disease Registry. [accessed 6 July 2016];Toxicological Profile for Arsenic. 2007 Available: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3. [PubMed]
- Aiuppa A, Avino R, Brusca L, Caliro S, Chiodini G, D’Alessandro W, et al. Mineral control of arsenic content in thermal waters from volcano-hosted hydrothermal systems: insights from island of Ischia and Phlegrean fields (Campanian Volcanic Province, Italy) Chem Geol. 2006;229:313–330. [Google Scholar]
- American Academy of Pediatrics. The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107:1210–1213. doi: 10.1542/peds.107.5.1210. [DOI] [PubMed] [Google Scholar]
- Aubrey A. How Much Arsenic Is Safe in Apple Juice? [accessed 30 June 2016];FDA Proposes New Rule. 2013 Available: http://www.npr.org/sections/thesalt/2013/07/12/201489247/how-much-arsenic-is-safe-in-apple-juice-fda-proposes-new-rule.
- Carlin DJ, Naujokas MF, Bradham KD, Cowden J, Heacock M, Henry HF, et al. Arsenic and environmental health: state of the science and future research opportunities. Environ Health Perspect. 2015 doi: 10.1289/ehp.1510209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Hsu LI, Chiou HY, Hsueh YM, Chen SY, M-M W, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. JAMA. 2004;292:2984–2990. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, M-M W, Chen CJ. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ Res. 2010a;110:455–462. doi: 10.1016/j.envres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Wang YH, et al. Arsenic in drinking water and risk of urinary tract cancer: a follow-up study from northeastern Taiwan. Cancer Epidemiol Biomark Prev. 2010b;19:101–110. doi: 10.1158/1055-9965.EPI-09-0333. [DOI] [PubMed] [Google Scholar]
- Chen JW, Wang SL, Wang YH, Sun CW, Huang YL, Chen CJ, et al. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere. 2012;88:432–438. doi: 10.1016/j.chemosphere.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Cohen JT, Bellinger DC, Connor WE, Kris-Etherton PM, Lawrence RS, Savitz DA, et al. A quantitative risk–benefit analysis of changes in population fish consumption. Am J Prev Med. 2005;29:325–334. e6. doi: 10.1016/j.amepre.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Consumer Reports. [accessed 14 June 2016];Arsenic in Your Food. 2012a Available: http://www.consumerreports.org/cro/magazine/2012/11/arsenic-in-your-food/index.htm?loginMethod=auto.
- Consumer Reports. Arsenic in Your Juice: How Much Is too Much? [accessed 4 June 2016];Federal Limits Don’t Exist. 2012b Available: http://www.consumerreports.org/cro/magazine/2012/01/arsenic-in-your-juice/index.htm?loginMethod=auto. [PubMed]
- Consumers Union. [accessed 14 June 2016];Letter to Dr. Margaret Hamburg. 2013 Available: http://consumersunion.org/wp-content/uploads/2013/07/Arsenic_FDA_HHS_0713.pdf.
- Cubadda F, Jackson B, Kuzurius-Spencer M, Cottingham K, Ornelas Van Horne Y. Human exposure to dietary inorganic arsenic and other arsenic species: state of knowledge, gaps and uncertainties. Sci Total Environ. 2017 doi: 10.1016/j.scitotenv.2016.11.108. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Argos J, Slaughter F, Gossai A, Ahsan H, Karagas M. Assessment of human dietary exposure to arsenic through rice. Sci Total Environ. 2017 doi: 10.1016/j.scitotenv.2017.02.119. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison BA, Rockwell HL, Baker SL. Excess fruit juice consumption by pre-school-aged children is associated with short stature and obesity. Pediatrics. 1997;99:15–22. [PubMed] [Google Scholar]
- EFSA CONTAM Panel. Scientific opinion on arsenic in food. EFSA J. 2009;7:1351. [Google Scholar]
- European Food Safety Authority. [accessed 30 October 2015];Dietary Exposure to Inorganic Arsenic in the Euro-pean Population. 2014 Available: http://www.efsa.europa.eu/en/efsajournal/pub/3597.
- Feldmann J, Krupp EM. Critical review or scientific opinion paper: arsenosugars—a class of benign arsenic species or justification for developing partly speciated arsenic fractionation in foodstuffs? Anal Bioanal Chem. 2011;399:1735–1741. doi: 10.1007/s00216-010-4303-6. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, González C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11:673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Yuan Y, Calle J, Benítez H, Parra RL, Acevedo J, et al. Arsenic, tobacco smoke, and occupation: associations of multiple agents with lung and bladder cancer. Epidemiology. 2013;24:898–905. doi: 10.1097/EDE.0b013e31829e3e03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Curriero F, Kulbicki K, Resnick B, Burke T. Evaluating the community health legacy of WWI chemical weapons testing. J Community Health. 2010;35:93–103. doi: 10.1007/s10900-009-9188-y. [DOI] [PubMed] [Google Scholar]
- Francesconi KA, Tanggaar R, McKenzie CJ, Goessler W. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem. 2002;48:92–101. [PubMed] [Google Scholar]
- Ginsberg GL, Toal BF. Quantitative approach for incorporating methylmercury risks and omega-3 fatty acid benefits in developing species-specific fish consumption advice. Environ Health Perspect. 2009;117:267. doi: 10.1289/ehp.11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble MO, Crainiceanu CM, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Body composition and arsenic metabolism: a cross-sectional analysis in the strong heart study. Environ Health. 2013;12:1. doi: 10.1186/1476-069X-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth E. Scientific foundations of fish-consumption advice for pregnant women: epidemiological evidence, benefit-risk modeling, and an integrated approach. Environ Res. 2016 doi: 10.1016/j.envres.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, et al. High exposure to inorganic arsenic by food: the need for risk reduction. Arch Toxicol. 2015;89:2219–2227. doi: 10.1007/s00204-015-1627-1. [DOI] [PubMed] [Google Scholar]
- Han FX, Su Y, Monts DL, Plodinec MJ, Banin A, Triplett GE. Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften. 2003;90:395–401. doi: 10.1007/s00114-003-0451-2. [DOI] [PubMed] [Google Scholar]
- Heikens A. Arsenic Contamination of Irrigation Water, Soil and Crops in Bangladesh: Risk Implications for Sustainable Agriculture and Food Safety in Asia. RAP Publication (FAO) 2006 [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq SI, Joardar J, Parvin S, Correll R, Naidu R. Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation. J Health Popul Nutr. 2006:305–316. [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL. Arsenic, organic foods, and brown rice syrup. Environ Health Perspect. 2012;120:623. doi: 10.1289/ehp.1104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care. 2015;38:620–627. doi: 10.2337/dc14-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, O’Rourke MK, Hsu CH, Hartz V, Harris RB, Burgess JL. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J Expo Sci Environ Epidemiol. 2013;23:442–449. doi: 10.1038/jes.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, et al. Contribution of diet to aggregate arsenic exposures—an analysis across populations. J Expo Sci Environ Epidemiol. 2014;24:156–162. doi: 10.1038/jes.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai PY, Cottingham KL, Steinmaus C, Karagas MR, Miller MD. Arsenic and rice: translating research to address health care providers’ needs. J Pediatr. 2015;167:797. doi: 10.1016/j.jpeds.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg Farms. [accessed 30 June 2016];A Letter From the CEO. 2016 Available: http://www.lundberg.com/info/arsenic-in-food.
- Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the -3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: risks and benefits. Environ Res. 2004;95:414–428. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Mariën K, et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev. 2011;69:493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Schumann BL, Chang X, Talaska G, Puga A. Arsenic co-exposure potentiates benzo[a]pyrene genotoxicity. Mutat Res Genet Toxicol Environ Mutagen. 2002;517:101–111. doi: 10.1016/s1383-5718(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Marafante E, Vahter M. The effect of methyltransferase inhibition on the metabolism of [74 As] arsenite in mice and rabbits. Chem Biol Interact. 1984;50:49–57. doi: 10.1016/0009-2797(84)90131-5. [DOI] [PubMed] [Google Scholar]
- Miller MD, Marty MA, Arcus A, Brown J, Morry D, Sandy M. Differences between children and adults: implications for risk assessment at California EPA. Int J Toxicol. 2002;21:403–418. doi: 10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- Molin M, Ulven SM, Meltzer HM, Alexander J. Arsenic in the human food chain, biotransformation and toxicology–review focusing on seafood arsenic. J Trace Elem Med Biol. 2015;31:249–259. doi: 10.1016/j.jtemb.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Mushak P, McKinzie M, Olson E. Arsenic and Old Laws. National Resources Defense Council; 2000. [Google Scholar]
- Nachman KE, Graham JP, Price LB, Silbergeld EK. Arsenic: a roadblock to potential animal waste management solutions. Environ Health Perspect. 2005;113:1123. doi: 10.1289/ehp.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman KE, Mihalic JN, Burke TA, Geyh AS. Comparison of arsenic content in pelletized poultry house waste and biosolids fertilizer. Chemosphere. 2008;71:500–506. doi: 10.1016/j.chemosphere.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a US-based market basket sample. Environ Health Perspect. 2013;121:818–824. doi: 10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman KE, Love DC, Baron PA, Nigra AE, Murko M, Raber G, et al. Nitarsone, inorganic arsenic, and other arsenic species in Turkey meat: exposure and risk assessment based on a 2014 U.S. market basket sample. Environ Health Perspect. 2016 doi: 10.1289/EHP225. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Arsenic in Drinking Water: 2001 Update. Washington, DC: 2001. [Google Scholar]
- National Research Council. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic: Interim Report. National Academies Press; 2014. [Google Scholar]
- Naujokas M, Anderson B, Ahsan H, Aposhian H, Graziano J, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershagen G. Lung cancer mortality among men living near an arsenic-emitting smelter. Am J Epidemiol. 1985;122:684–694. doi: 10.1093/oxfordjournals.aje.a114147. [DOI] [PubMed] [Google Scholar]
- Peryea FJ. [accessed 10 August 2016];Historical Use of Lead Arsenate Insecticides, Resulting Soil Contamination and Implications for Soil Remediation. 1988 Available: http://soils.tfrec.wsu.edu/leadhistory.htm.
- Peryea FJ. Phosphate-induced release of arsenic from soils contaminated with lead arsenate. Soil Sci Soc Am J. 1991;55:1301–1306. [Google Scholar]
- Petursdottir AH, Sloth JJ, Feldmann J. Introduction of regulations for arsenic in feed and food with emphasis on inorganic arsenic, and implications for analytical chemistry. Anal Bioanal Chem. 2015;407:8385–8396. doi: 10.1007/s00216-015-9019-1. [DOI] [PubMed] [Google Scholar]
- Pitten FA, Müller G, König P, Schmidt D, Thurow K, Kramer A. Risk assessment of a former military base contaminated with organoarsenic-based warfare agents: uptake of arsenic by terrestrial plants. Sci Total Environ. 1999;226:237–245. doi: 10.1016/s0048-9697(98)00400-8. [DOI] [PubMed] [Google Scholar]
- Punshon T, Guerinot M, Jackson B, Schekel K, Warczak T, Meharg A. Understanding arsenic dynamics in arable systems to decrease human dietary arsenic exposure. Sci Total Environ. 2017 doi: 10.1016/j.scitotenv.2016.12.111. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LC, Hug SJ, Dittmar J, Voegelin A, Saha GC, Ali MA, et al. Spatial distribution and temporal variability of arsenic in irrigated rice fields in Bangladesh. 1 Irrigation water. Environ Sci Technol. 2007;41:5960–5966. doi: 10.1021/es070298u. [DOI] [PubMed] [Google Scholar]
- Schmeisser E, Goessler W, Francesconi KA. Human metabolism of arsenolipids present in cod liver. Anal Bioanal Chem. 2006;385:367–376. doi: 10.1007/s00216-006-0401-x. [DOI] [PubMed] [Google Scholar]
- Schoof R, Yost L, Eickhoff J, Crecelius E, Cragin D, Meacher D, et al. A market basket survey of inorganic arsenic in food. Food Chem Toxicol. 1999;37:839–846. doi: 10.1016/s0278-6915(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Sele V, Sloth JJ, Lundebye AK, Larsen EH, Berntssen MH, Amlund H. Arsenolipids in marine oils and fats: a review of occurrence, chemistry and future research needs. Food Chem. 2012;133:618–630. [Google Scholar]
- Shacklette HT, Boerngen JG. Element Concentrations in Soils and Other Surficial Materials of the Conterminous United States. USGPO 1984 [Google Scholar]
- Shibata T, Meng C, Umoren J, West H. Risk assessment of arsenic in rice cereal and other dietary sources for infants and toddlers in the US. Int J Environ Res Public Health. 2016;13:361. doi: 10.3390/ijerph13040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Carey M, Meharg AA. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016;191:128–134. doi: 10.1016/j.foodchem.2014.11.078. [DOI] [PubMed] [Google Scholar]
- Slovic P. Perception of risk. Science. 1987:280–285. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn E. [accessed 28 November 2016];Simple Cooking Methods Flush Arsenic out of Rice. 2015 Available: http://www.nature.com/news/simple-cooking-methods-flush-arsenic-out-of-rice-1.18034.
- Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J, Durán V, et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomark Prev. 2014;23:1529–1538. doi: 10.1158/1055-9965.EPI-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao SSH, Bolger MP. Dietary arsenic intakes in the United States: FDA total diet study, September 1991–December 1996. Food Addit Contam. 1999;16:465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwedtle T, Reimer K, Conklin S, et al. Human exposure to organic arsenic species from seafood. Sci Total Environ. 2017 doi: 10.1016/j.scitotenv.2016.12.113. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WP. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977;19:109. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng W, Chu HM, How S, Fong J, Lin C, Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968;40:453–463. [PubMed] [Google Scholar]
- FDA Food Safety Modernization Act §104; United States 111th Congress; 2011. [accessed 14 June 2016]. Available: https://www.gpo.gov/fdsys/pkg/PLAW-111publ353/pdf/PLAW-111publ353.pdf. [Google Scholar]
- United States Congress. Federal Food, Drug and Cosmetic Act § 342. [accessed 7 July 2016];Adulterated Food. 2005 Available: https://www.gpo.gov/fdsys/pkg/USCODE-2011-title21/pdf/USCODE-2011-title21-chap9-subchapIV-sec342.pdf.
- United States Congress. [accessed 7 July 2016];H.R.3984 - APPLE Juice Act of 2012. 2012a Available: https://www.congress.gov/bill/112th-congress/house-bill/3984.
- United States Congress. [accessed 7 July 2016];H.R.6509 - RICE Act. 2012b Available: https://www.congress.gov/bill/112th-congress/house-bill/6509.
- United States Department of Agriculture. [accessed 14 June 2016];Guide for Organic Crop Producers. 2012a Available: https://www.ams.usda.gov/sites/default/files/media/GuideForOrganicCropProducers.pdf.
- United States Department of Agriculture. [accessed 2 July 2016];Household USDA Foods Fact Sheet: Infant Rice Cereal. 2012b Available: http://www.fns.usda.gov/sites/default/files/HHFS_INFANT-RICE-CEREAL_110241Nov2012.pdf.
- United States Department of Agriculture. [accessed 14 June 2016];§205.602 Nonsynthetic Substances Prohibited for Use in Organic Crop Production. 2013 Available: http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&SID=9874504b6f1025eb0e6b67cadf9d3b40&rgn=div6&view=text&node=7:3.1.1.9.32.7&idno=7#se7.3.205_1602.
- United States Department of Agriculture. [accessed 14 June 2016];US National Residue Program for Meat, Poultry, and Eggs: 2014 Residue Sample Results. 2014 Available: http://www.fsis.usda.gov/wps/wcm/connect/2428086b-f8ec-46ed-8531-a45d10bfef6f/2014-Red-Book.pdf?MOD=AJPERES.
- United States Department of Agriculture. [accessed 14 June 2016];US National Residue Program for Meat, Poultry, and Eggs: 2015 Residue Sampling Plans. 2015 Available: http://www.fsis.usda.gov/wps/wcm/connect/04c818ed-9bb1-44b2-9e3f-896461f1ffb9/2015-Blue-Book.pdf?MOD=AJPERES.
- United States Environmental Protection Agency. [accessed 4 July 2016];Arsenic in Drinking Water Rule Economic Analysis. 2000 Available: http://nepis.epa.gov/Exe/ZyNET.exe/20001YQT.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C00thru05%5CTxt%5C00000001%5C20001YQT.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=p%7Cf&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL.
- United States Environmental Protection Agency. 40 CFR parts 9, 141, and 142, national primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Final rule Fed Regist. 2001;66(14):6975–7066. (Available: [accessed]) [Google Scholar]
- United States Environmental Protection Agency. [accessed 15 June 2016];Organic Arsenicals; Product Cancellation Order and Amendments to Terminate Uses. 2009 Available: https://www.federalregister.gov/articles/2009/09/30/E9-23319/organic-arsenicals-product-cancellation-order-and-amendments-to-terminate-uses.
- United States Environmental Protection Agency. IRIS Toxicological Review of Inorganic Arsenic (Cancer) (2010 External Review Draft) Washington, DC: 2010. [Google Scholar]
- United States Environmental Protection Agency. [accessed 15 June 2016];Effluent Limitations Guidelines and Standards for the Steam Electric Power Generating Point Source Category. 2015 Available: https://www.federalregister.gov/articles/2015/11/03/2015-25663/effluent-limitations-guidelines-and-standards-for-the-steam-electric-power-generating-point-source.
- United States Environmental Protection Agency. National Emission Standards for Hazardous Air Pollutants From Coal- and Oil-fired Electric Utility Steam Generating Units and Standards of Performance for Fossil-fuel-fired Electric Utility, Industrial-Commercial-Institutional, and Small Industrial-Commercial-Institutional Steam Generating Units; Technical Correction. [accessed 15 June 2016];2016 Available: https://www.federalregister.gov/articles/2016/04/06/2016-06563/national-emission-standards-for-hazardous-air-pollutants-from-coal-and-oil-fired-electric-utility.
- United States Food and Drug Administration. [accessed 7 July 2016];Beverages: Bottled Water. 2005 Available: https://www.federalregister.gov/articles/2005/06/09/05-11406/beverages-bottled-water.
- United States Food and Drug Administration. [accessed 14 June 2016];Compliance Program Guidance Manual: Feed Contaminants Program. 2010 Available: http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/ComplianceEnforcement/UCM113409.pdf.
- United States Food and Drug Administration. [accessed 30 June 2016];Letter From FDA to Dr. Oz Show Regarding Apple Juice and Arsenic (9/11/2011) 2011a Available: http://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm271630.htm.
- United States Food and Drug Administration. [accessed 30 June 2016];Second Letter From FDA to Dr. Oz Show Regarding Apple Juice and Arsenic (9/13/2011) 2011b Available: http://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm271632.htm.
- United States Food and Drug Administration. [accessed 14 June 2016];FDA Proposes “Action Level” for Arsenic in Apple Juice. 2013 Available: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm360466.htm.
- United States Food and Drug Administration. [accessed 14 June 2016];Food Advisory Committee Meeting Charge and Questions 29–30 September 2014. 2014 Available: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/UCM414448.pdf.
- United States Food and Drug Administration. [accessed 3 July 2016];21 CFR 10.115: Good Guidance Practices. 2015 Available: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=10.115.
- United States Food and Drug Administration. [accessed 14 June 2016];C.F.R. § 556.00. 2016a Available: http://www.ecfr.gov/cgi-bin/text-idx?SID=4d2d115917a6a0206318ab21d1961f0e&mc=true&tpl=/ecfrbrowse/Title21/21cfr556_main_02.tpl.
- United States Food and Drug Administration. [accessed 14 June 2016];FDA Proposes Limit for Inorganic Arsenic in Infant Rice Cereal. 2016b Available: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm493740.htm.
- United States Food and Drug Administration. [accessed 14 June 2016];Metals: Arsenic. 2016c Available: http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals.
- United States Food and Drug Administration. [accessed 14 June 2016];Total Diet Study. 2016d Available: http://www.fda.gov/Food/FoodScienceResearch/TotalDietStudy/default.htm.
- United States Food and Drug Administration. [accessed 4 August 2016];Valley Processing, Inc. 6/2/16. 2016e Available: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2016/ucm506526.htm.
- USA Rice Federation. [accessed 30 June 2016];An Online Resource for Information on Arsenic in Rice. 2016 Available: http://arsenicfacts.com/
- Valberg P, Beck B, Bowers T, Keating J, Bergstrom P, Boardman P. Issues in setting health-based cleanup levels for arsenic in soil. Regul Toxicol Pharmacol. 1997;26:219–229. doi: 10.1006/rtph.1997.1148. [DOI] [PubMed] [Google Scholar]
- Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian VG, Özkaynak H, Dang W, Glen G, Smith L, et al. A probabilistic arsenic exposure assessment for children who contact chromated copper arsenate (CCA)-treated playsets and decks, part 2: sensitivity and uncertainty analyses. Risk Anal. 2006;26:533–541. doi: 10.1111/j.1539-6924.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118:345. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost LJ, Tao SH, Egan SK, Barraj LM, Smith KM, Tsuji JS, et al. Estimation of Dietary Intake of Inorganic Arsenic in U.S. Children. Hum Ecol Risk Assess Int J. 2004;10:473–483. [Google Scholar]
- Zartarian VG, Xue J, Özkaynak H, Dang W, Glen G, Smith L, et al. A probabilistic arsenic exposure assessment for children who contact CCA-treated playsets and decks, part 1: model methodology, variability results, and model evaluation. Risk Anal. 2006;26:515–531. doi: 10.1111/j.1539-6924.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP, Meharg AA. Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol. 2010;61:535–559. doi: 10.1146/annurev-arplant-042809-112152. [DOI] [PubMed] [Google Scholar]