Abstract
It is unknown whether the addition of temozolomide (TMZ) to radiotherapy (RT) is associated with improved overall survival (OS) among older glioblastoma patients. We performed a retrospective cohort SEER-Medicare analysis of 1652 patients aged ≥65 years with glioblastoma who received ≥10 fractions of RT from 2005 to 2009, or from 1995 to 1999 before TMZ was available. Three cohorts were assembled based on diagnosis year and treatment initiated within 60 days of diagnosis: (1) 2005–2009 and TMZ/RT, (2) 2005–2009 and RT only, or (3) 1995–1999 and RT only. Associations with OS were estimated using Cox proportional hazards models and propensity score analyses; OS was calculated starting 60 days after diagnosis. Pre-specified sensitivity analyses were performed among patients who received long-course RT (≥27 fractions). Median survival estimates were 7.4 (IQR, 3.3–14.7) months for TMZ/RT, 5.9 (IQR, 2.6–12.1) months for RT alone in 2005–2009, and 5.6 (IQR, 2.7–9.6) months for RT alone in 1995–1999. OS at 2 years was 10.1 % for TMZ/RT, 7.1 % for RT in 2005–2009, and 4.7 % for RT in 1995–1999. Adjusted models suggested decreased mortality risk for TMZ/RT compared to RT in 2005–2009 (AHR, 0.86; 95 % CI, 0.76–0.98) and RT in 1995–1999 (AHR, 0.71; 95 % CI, 0.57–0.90). Among patients from 2005 to 2009 who received long-course RT, however, the addition of TMZ did not significantly improve survival (AHR, 0.91; 95 % CI, 0.80–1.04). In summary, among a large cohort of older glioblastoma patients treated in a real-world setting, the addition of TMZ to RT was associated with a small survival gain.
Keywords: Glioblastoma, Radiotherapy, Temozolomide, Elderly, Survival
Introduction
Glioblastoma is the most common primary brain malignancy, with a median age at diagnosis of 65 years [1]. Historically, surgical resection followed by adjuvant radiotherapy was the mainstay of treatment, with limited data to support the addition of chemotherapy [2, 3]. In 2005, publication of the EORTC 26981/NCIC CE.3 randomized trial established a new standard of care in glioblastoma, demonstrating that the addition of concurrent and adjuvant temozolomide to 6 weeks of radiotherapy was superior to radiotherapy alone, extending median survival by 2.5 months and leading to a cohort of longer-term survivors [4]. However the median age of that trial population was 56 years, and patients over age 70 were excluded, leaving open the question of whether temozolomide-based chemoradiation benefits the older glioblastoma population.
While several prospective trials have compared de-escalated treatment approaches for glioblastoma patients age ≥60, 65, or 70 years, including short-course radiotherapy or temozolomide monotherapy, none of these trials included standard concurrent chemoradiation over the course of 6 weeks as a comparison arm [5–9]. While recently presented data from the EORTC/NCIC/TROG randomized trial of short-course hypofractionated radiotherapy with or without concurrent temozolomide suggest a modest improvement in median OS with the addition of temozolomide [10], that trial did not address how short-course chemoradiation (2–3 weeks) compares to standard long-course chemoradiation (6 weeks), and specifically excludes patients who are considered to be candidates for standard long-course chemoradiation. At least half of all glioblastoma patients are elderly and the incidence is rising rapidly in this age group [11], yet it remains unknown whether the addition of temozolomide to radiotherapy is effective among older patients in a non-clinical trial population. Accordingly, we investigated survival outcomes of glioblastoma patients treated with radiotherapy with or without temozolomide in the Medicare population.
Methods
Data sources
Our study sample was drawn from linked Surveillance, Epidemiology and End Results (SEER)-Medicare data. SEER is a consortium of 17 population-based US cancer registries sponsored by the National Cancer Institute which collects incident cancer cases including glioblastoma, covering approximately 28 % of the US population [12]. SEER collects data regarding patient demographics, tumor characteristics, and primary surgical and radiation treatment. Medicare is the primary health insurer for approximately 97 % of Americans age ≥65 years. Medicare files document use of inpatient and outpatient healthcare services by patients enrolled in Medicare fee-for-service. This study was exempted from review by the institutional review board at the Harvard School of Public Health.
Patients
All subjects were Medicare beneficiaries age ≥65 years diagnosed with glioblastoma in SEER regions from January 1995-December 2009, who had continuous enrollment in Medicare Parts A and B from diagnosis to death. We used Medicare data from January 1, 1995 to December 31, 2010, to ensure minimum follow-up of 1 year after diagnosis. Glioblastoma was defined according to International Classification of Disease (ICD) for Oncology, 3rd edition (SEER codes 9440-9444). We excluded patients diagnosed at autopsy, without tissue, or without a known date, as well as patients with prior cancers except non-melanoma skin cancers. Date of diagnosis was based on biopsy/resection date in Medicare Part A claims (defined below), and cross-referenced against the diagnosis month in SEER. Patients enrolled in a health maintenance organization at diagnosis or not enrolled in Medicare fee-for-service for 12 months before diagnosis were excluded, in order to ascertain comorbidities [13, 14], and patients who did not survive at least 60 days following diagnosis were excluded to ensure equal treatment ascertainment between groups, as outlined below.
Our primary aim was to compare overall survival (OS) for glioblastoma patients who received radiotherapy with or without concurrent temozolomide, which received US Food and Drug Administration (FDA) approval for recurrent anaplastic astrocytomas in late 1999 and glioblastoma in early 2005 [15]; it was assigned a ‘J’ code in January 2001, after which it was ascertainable through administrative claims. The primary comparison groups were patients diagnosed with glioblastoma from June 1, 2005 through December 31, 2009 receiving radiotherapy with or without concurrent temozolomide. We constructed a secondary control group of patients diagnosed from January 1, 1995 through December 31, 1999 receiving radiotherapy alone, a time period before temozolomide was FDA-approved for glioblastoma or widely available in the US. Since OS remained constant among older glioblastoma patients from 1995 to 2005 in population-based data [16], the 1995–1999 radiotherapy-alone cohort is less susceptible to any potential selection bias in the 2005–2009 time period when temozolomide was available. By the end of the followup period, 96.4 % of cohort members were known to be deceased.
Identification of treatment
Treatment-related Medicare claims were identified with the following codes, based on ICD-9, Current Procedural Terminology (CPT), and National Drug Code (NDC) classifications: surgical biopsy, 0113–0114 (ICD-9) and 61304–61305 (CPT); surgical resection, 0153–0159 (ICD-9); radiotherapy treatment delivery, 77413, 77418, 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77414, 77416, 0073T, G0174 (CPT); temozolomide, J8700 (CPT) and 000851XXXXX, 000853XXXXX, 545695XXXXX, 548685XXXXX (NDC). Extent of resection was dichotomized as either a subtotal resection (SEER coding)/biopsy (SEER or Medicare coding), or gross total resection (SEER coding).
Patients who initiated daily radiation treatments ≤60 days from the date of diagnosis, and who received ≥10 daily radiation treatments, were considered to have received adjuvant radiotherapy. In the landmark EORTC/NCIC trial, adjuvant radiotherapy was started at a median of 5 weeks after surgery [4], and it is standard to begin radiotherapy within 2 months from surgery. Patients were considered to have received temozolomide concurrently with radiotherapy if the first temozolomide claim also occurred ≤60 days from the date of diagnosis; temozolomide is an oral tablet taken daily on an outpatient basis, with practice variation in the number of tablets prescribed in a single claim. There were no inclusion/exclusion criteria regarding receipt of adjuvant temozolomide after completion of concurrent chemoradiation.
Outcomes
The primary outcome was OS, defined as the number of survival days starting 60 days after diagnosis until the date of death or the end of the observation period, to allow ascertainment of treatment initiation of radiotherapy with/without temozolomide, and reduce the chance of immortal time bias [17]. Date of death was reported in Medicare files and ascertained through December 31, 2010. Patients alive at the end of the followup period were censored.
Baseline characteristics
Baseline characteristics including age at diagnosis, sex, marital status, race/ethnicity, area median income, comorbidity, tumor location, tumor multifocality, tumor size, SEER region, extent of surgery, and hospital discharge location following index diagnosis hospitalization, are shown in Table 1. The Deyo adaptation [13] of the Charlson comorbidity index [14] was used to measure severity of comorbid diseases, modified to exclude cancer diagnoses. This method was applied to Medicare claims during the 12-month period prior to diagnosis. Index hospital discharge location was categorized as home vs. other location, including rehabilitation centers, skilled nursing and intermediate-care facilities, and long-term care centers, and were included to examine a dimension of patient performance status near the start of adjuvant therapies.
Table 1. Baseline characteristics of elderly glioblastoma patients in the 3 treatment cohorts.
| Characteristic | TMZ/RT (n = 705) | RT 2005–2009 (n = 714) | Pb | RT 1995–1999a (n = 233) | Pb |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |||
| Age at diagnosis | |||||
| 65–69 | 220 (31.2) | 207 (29) | .135 | 68 (29.2) | .803 |
| 70–74 | 235 (33.3) | 215 (30.1) | 76 (32.6) | ||
| 75–79 | 154 (21.8) | 167 (23.4) | 58 (24.9) | ||
| 80+ | 96 (13.6) | 125 (17.5) | 31 (13.3) | ||
| Sex | |||||
| Female | 338 (47.9) | 333 (46.6) | .623 | 123 (52.8) | .200 |
| Male | 367 (52.1) | 381 (53.4) | 110 (47.2) | ||
| Marital status | |||||
| Married | 502 (71.2) | 497 (69.6) | .510 | 154 (66.1) | .140 |
| Not married | 203 (28.8) | 217 (30.4) | 79 (33.9) | ||
| Race | |||||
| Non-Hispanic white | 636 (90.2) | 609 (85.3) | .005 | 206 (88.4) | .432 |
| Other | 69 (9.8) | 105 (14.7) | 27 (11.6) | ||
| Income tractc | |||||
| High | 482 (68.4) | 493 (69) | .783 | 141 (60.5) | .028 |
| Low | 223 (31.6) | 221 (31) | 92 (39.5) | ||
| SEER region | |||||
| Northeast | 147 (20.9) | 163 (22.8) | <.001 | 27 (11.6) | <.001 |
| Midwest | 93 (13.2) | 98 (13.7) | 64 (27.5) | ||
| South | 148 (21) | 117 (16.4) | 20 (8.6) | ||
| West | 317 (45) | 336 (47.1) | 122 (52.4) | ||
| Deyo comorbidity score [13] | |||||
| 0 | 434 (61.6) | 438 (61.3) | .365 | 165 (70.8) | .033 |
| 1 | 193 (27.4) | 181 (25.4) | 51 (21.9) | ||
| ≥2 | 78 (11.1) | 95 (13.3) | 17 (7.3) | ||
| Tumor location | |||||
| Supratentorial | 556 (78.9) | 564 (79) | .953 | 159 (68.2) | .001 |
| Other | 149 (21.1) | 150 (21) | 74 (31.8) | ||
| Tumor multifocality | |||||
| No | 691 (98) | 700 (98) | .973 | 216 (92.7) | <.001 |
| Yes | 14 (2) | 14 (2) | 17 (7.3) | ||
| Tumor size (pre-operative) | |||||
| >3 cm | 498 (70.6) | 511 (71.6) | .699 | 132 (56.7) | <.001 |
| ≤3 cm | 207 (29.4) | 203 (28.4) | 101 (43.3) | ||
| Extent of resection | |||||
| GTR | 221 (31.3) | 230 (32.2) | .726 | 27 (11.6) | <.001 |
| Biopsy/STR | 484 (68.7) | 484 (67.8) | 206 (88.4) | ||
| Discharge locationd | |||||
| Home | 390 (55.3) | 357 (50) | .045 | 137 (58.8) | .353 |
| Other facility | 315 (44.7) | 357 (50) | 96 (41.2) | ||
Percentages for some categories do not total 100 % due to rounding
TMZ temozolomide, RT radiotherapy, SEER surveillance, epidemiology, and end results, GTR gross total resection, STR sub-total resection
Radiotherapy alone in 1995–1999 when temozolomide was not available (1995 to mid-1999) or not approved (late 1999) for glioblastoma
P value for comparison with TMZ/RT treatment group
Dichotomized median income based on median household or per capita income by US Census tract or zip code
Following index hospitalization for glioblastoma diagnosis, location the patient was discharged to including home (with or without care services), skilled nursing facility, rehabilitation facility, intermediate care facility, or long-term care facility
Statistical analysis
Distribution of baseline characteristics between the temozolomide/radiotherapy group diagnosed 2005–2009 (TMZ/RT), radiotherapy alone group diagnosed 2005–2009 (RT 2005–2009), and radiotherapy alone group diagnosed 1995–1999 (RT 1995–1999) were evaluated with the χ2 test. Median survival was estimated using the Kaplan–Meier method. Univariable and multivariable Cox proportional hazards models were constructed using the Table 1 characteristics, to examine whether the addition of temozolomide to radiotherapy improved OS, compared to both radiotherapy-alone cohorts; all Table 1 characteristics were included in the multivariable model to best estimate treatment effects.
Propensity score analyses were performed to balance measurable confounders between the TMZ/RT, RT 2005–2009, and RT 1995–1999 treatment groups, using multivariable logistic regression to predict adjuvant treatment received, based on covariates from Table 1 [18]. Propensity scores indicating likelihood of receiving temozolomide were estimated, and the cohort was divided into quintiles of the estimated propensity scores [19, 20]. Cox proportional hazards models were conducted separately within each quintile to compare OS among patients who did vs. did not receive temozolomide, and a hazard ratio (HR) was estimated for the entire cohort [21]. We also performed Cox models using propensity scores in 3 different ways to adjust for differences in covariates between treatment groups, including regression adjustment, propensity score matching, and inverse probability of treatment weighting [22, 23].
Finally, we performed subgroup analyses for characteristics that were less balanced between groups, specifically SEER region and age. We also examined comorbidity as a potential confounder of the association between treatment and OS, given the potential for chemotherapy decisions to be made based on comorbid conditions, and the lack of patient performance status data within SEER-Medicare. Two sensitivity analyses were also performed: (1) receipt of standard long-course radiotherapy over the course of 6 weeks as per the EORTC/NCIC trial, in which nearly all patients (94 %) received ≥90 % of the planned dose (≥27 fractions), and (2) hospital discharge location to home vs. other location. All analyses were performed using R Studio (version 0.98.1062) running R (version 3.1.1). Statistical significance was set at P < .05, and all tests were two-tailed.
Results
Patients
We identified 12,393 patients age ≥65 years with glioblastoma diagnosed from 1995 to 2009 (Fig. 1). After exclusion criteria were applied, the final cohort consisted of 1652 patients. Within the study cohort, 1419 patients met inclusion criteria in the 2005–2009 time period, including 705 patients (50 %) who received concurrent temozolomide with radiotherapy, and 999 patients (70 %) who received long-course radiotherapy. Among the 705 patients receiving concurrent temozolomide, 53.6 % had at least 1 claim for temozolomide within the 90 days following receipt of the final fraction of radiotherapy, consistent with adjuvant temozolomide delivered following concurrent chemoradiation. Our secondary control group consisted of 233 patients from the 1995–1999 pre-temozolomide time period.
Fig. 1.
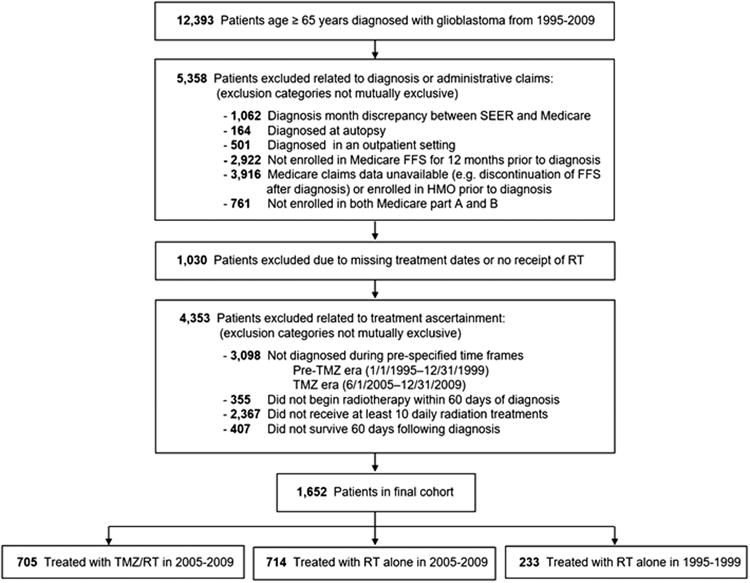
Assembly of the study cohort. SEER surveillance, epidemiology, and end results, FFS fee-for-service, HMO health maintenance organization, RT radiotherapy, TMZ temozolomide
Baseline characteristics among the TMZ/RT, RT 2005–2009, and RT 1995–1999 groups are shown in Table 1. Patients who received temozolomide were more likely than those who received radiotherapy alone in 2005–2009 to be white (90.2 vs. 85.3 %, P = .005), from the South (21.0 vs. 16.4 %, P < .001), and to be discharged to home after initial diagnosis (55.3 vs. 50.0 %, P = .045). Patients in the RT 1995–1999 group were more likely than patients treated with TMZ/RT to live in regions with lower median income (P = .028); have lower comorbidity burden (P = .033); have tumors that were infratentorial (P = .001), multifocal (P < .001), or larger (P < .001); and have undergone subtotal resection/biopsy (P < .001).
Survival
Survival curves for the TMZ/RT, RT 2005–2009, and RT 1995–1999 treatment groups are shown in Fig. 2. Median OS was 7.4 (IQR, 3.3–14.7) months for TMZ/RT, 5.9 (IQR, 2.6–12.1) months for RT alone in 2005–2009, and 5.6 (IQR, 2.7–9.6) months for RT in 1995–1999. Unadjusted 1-year and 2- year survival probabilities were, respectively, 31.2 % (95 % CI, 27.8–34.8 %) and 10.1 % (95 % CI, 8.0–12.6 %) for TMZ/RT, 25.5 % (95 % CI, 22.4–28.9 %) and 7.1 % (95 % CI, 5.4–9.3 %) for RT 2005–2009, and 14.2 % (95 % CI, 10.1–19.5 %) and 4.7 % (95 % CI, 2.5–8.5 %) for RT 1995–1999.
Fig. 2.
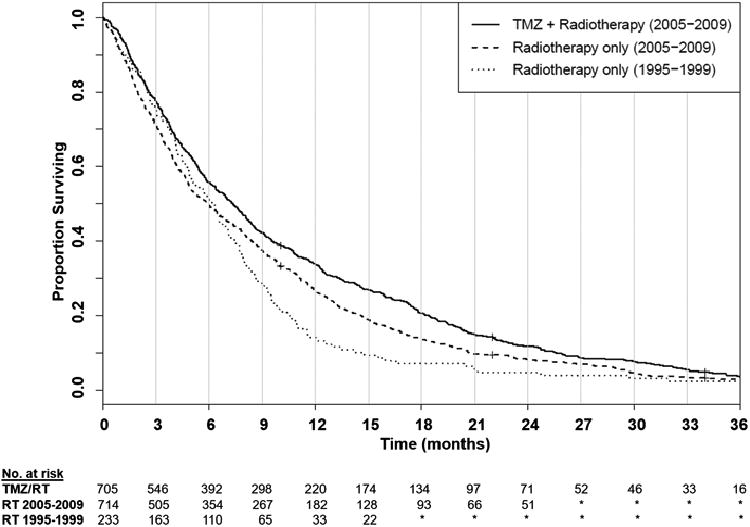
Overall survival according to treatment group, starting 60 days following diagnosis. *Numbers less than 11, or changes in number of less than 11 cases between timepoints, are not reported consistent with SEER-medicare confidentiality policies. TMZ temozolomide, RT radiotherapy, SEER surveillance, epidemiology and end results
Multivariable Cox proportional hazards modeling, adjusting for baseline characteristics, showed longer OS among patients in the TMZ/RT group compared to RT 2005–2009 (HR, 0.82; 95 % CI, 0.73–0.91) and RT 1995–1999 (HR, 0.66; 95 % CI, 0.54–0.79; Table 2). Stratified models by propensity score quintiles revealed a persistent but attenuated OS advantage to TMZ/RT vs. RT 2005–2009 (HR, 0.86; 95 % CI, 0.76–0.98) and vs. RT 1995–1999 (HR, 0.71; 95 % CI, 0.57–0.90). These findings were maintained for models that used regression adjustment, propensity score matching, and inverse probability of treatment weighting.
Table 2. Effect of temozolomide added to radiotherapy on hazard ratios for overall survival.
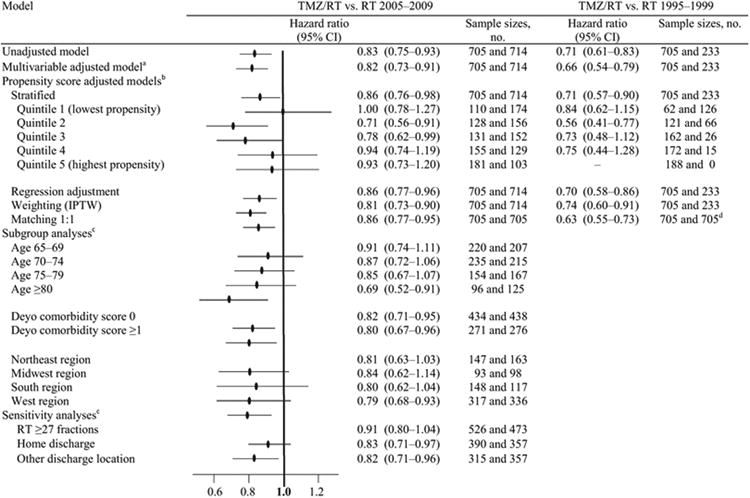
|
Overall survival defined as starting 60 days following diagnosis, for equal period of treatment ascertainment TMZ temozolomide, RT radiotherapy, SEER surveillance, epidemiology, and end results, PS propensity score, IPTW inverse probability of treatment weighting
Model was adjusted for age, sex, marital status, race, median income in the census tract of residence, Deyo comorbidity score [13], tumor location, tumor size, surveillance, epidemiology, and end results (SEER) region, hospital discharge location, and extent of resection
Propensity of receiving temozolomide with radiotherapy was estimated using a multivariable regression model that included age, sex, marital status, race, median income in the census tract of residence, Deyo comorbidity score, tumor location, tumor size, SEER region, hospital discharge location, and extent of resection
For subgroup and sensitivity analyses, IPTW modeling was used, adjusting for same baseline covariables listed above in the multivariable adjusted model, with removal of the specific covariable being analyzed
Matches were obtained to the RT 1995–2009 treatment group with replacement
Subgroup analyses showed no significant survival benefit among subgroups of patients age 65–69, 70–74, or 75–79 years receiving TMZ/RT vs. RT 2005–2009, but patients in the age ≥80 years subgroup had longer survival in the TMZ/RT group compared to RT 2005–2009 (HR, 0.69; 95 % CI, 0.52–0.91). Sensitivity analyses showed no significant survival advantage to TMZ/RT compared to RT in 2005–2009 among the subgroup of patients receiving long-course RT (≥27 fractions; HR, 0.91; 95 % CI, 0.80–1.04). We repeated survival analyses after excluding patients ≥80 years old, to observe if the overall findings were being driven disproportionately by this oldest subgroup. After excluding these patients, all HRs, including for the multivariable and propensity score models, were essentially unchanged (data not shown).
We also evaluated the association between baseline characteristics and overall survival, comparing the TMZ/RT group to the RT 2005–2009 and the RT 1995–1999 group. Inferior survival was associated with older age, residence in a lower median income census tract, hospital discharge location to non-home location, and subtotal resection/biopsy (Table 3).
Table 3. Median survival in the 3 treatment cohorts, and hazard ratios for overall survival adjusted for baseline characteristics.
| Characteristic | Crude median survival (IQR), monthsa | Hazard ratio (95 % CI) | |||
|---|---|---|---|---|---|
|
|
|
||||
| TMZ/RT | RT 2005–2009 | RT 1995–1999b | TMZ/RT vs. RT 2005–2009 | TMZ/RT vs. RT 1995–1999 | |
| Treatment | |||||
| TMZ/RT | 7.4 (3.3–14.7) | – | – | 0.82 (0.73–0.91) | 0.66 (0.54–0.79) |
| RT | – | 5.9 (2.6–12.1) | 5.6 (2.7–9.6) | 1 [ref.] | 1 [ref.] |
| Age at diagnosis | |||||
| 65–69 | 12.0 (6.1–19.6) | 10.9 (5.9–18.1) | 7.9 (4.7–13.6) | 1 [ref.] | 1 [ref.] |
| 70–74 | 7.4 (3.8–14.4) | 6.4 (2.8–12.9) | 5.4 (2.5–8.0) | 1.31 (1.14–1.51) | 1.40 (1.18–1.66) |
| 75–79 | 7.0 (3.2–14.3) | 5.5 (2.7–11.6) | 4.9 (2.4–8.4) | 1.27 (1.09–1.48) | 1.24 (1.02–1.50) |
| 80+ | 4.2 (1.7–8.0) | 3.2 (1.6–6.5) | 4.1 (2.7–7.7) | 2.02 (1.69–2.42) | 1.66 (1.31–2.09) |
| Sex | |||||
| Female | 7.3 (3.2–15.6) | 5.9 (2.7–13.2) | 5.8 (2.5–9.9) | 0.86 (0.77–0.97) | 0.90 (0.78–1.03) |
| Male | 7.4 (3.3–14.3) | 5.9 (2.3–11.6) | 5.5 (2.8–8.9) | 1 [ref.] | 1 [ref.] |
| Marital status | |||||
| Married | 8.2 (3.7–16.3) | 6.4 (2.6–12.6) | 6.3 (2.8–9.7) | 0.93 (0.82–1.05) | 0.83 (0.72–0.97) |
| Not married | 5.4 (2.5–11.1) | 5.0 (2.4–11.0) | 4.8 (2.0–9.3) | 1 [ref.] | 1 [ref.] |
| Race | |||||
| White | 7.4 (3.3–14.5) | 5.8 (2.4–12.1) | 5.6 (2.7–9.8) | 1.14 (0.96–1.36) | 1.01 (0.80–1.27) |
| Other | 6.0 (3.2–17.2) | 6.7 (3.1–12.8) | 6.2 (2.4–8.9) | 1 [ref.] | 1 [ref.] |
| Income tractc | |||||
| High | 8.2 (3.8–16.3) | 6.4 (2.8–13.1) | 5.0 (2.2–9.3) | 0.78 (0.69–0.88) | 0.89 (0.76–1.03) |
| Low | 5.5 (2.5–10.8) | 4.7 (2.3–10.0) | 5.9 (0.3–10.0) | 1 [ref.] | 1 [ref.] |
| SEER region | |||||
| Northeast | 10.6 (6.8–19.2) | 9.4 (4.6–16.6) | – | 1 [ref.] | 1 [ref.] |
| Midwest | 5.5 (2.6–12.3) | 6.2 (2.5–12.4) | 5.9 (3.1–9.8) | 1.18 (0.97–1.43) | 1.15 (0.90–1.47) |
| South | 5.8 (2.8–11.1) | 4.8 (2.3–10.7) | 4.2 (2.7–7.1) | 1.22 (1.02–1.45) | 1.32 (1.05–1.66) |
| West | 7.7 (3.5–15.1) | 5.9 (2.8–11.9) | 5.7 (2.6–9.5) | 1.22 (1.06–1.41) | 1.28 (1.05–1.55) |
| Deyo comorbidity score [13] | |||||
| 0 | 10.2 (5.9–18.2) | 9.5 (5.0–15.9) | 8.4 (6.0–11.8) | 1 [ref.] | 1 [ref.] |
| 1 | 5.7 (2.4–12.2) | 5.1 (2.7–10.9) | 4.9 (2.1–8.4) | 1.08 (0.94–1.23) | 1.13 (0.96–1.33) |
| ≥2 | 7.7 (3.3–12.4) | 2.9 (1.7–6.9) | 6.3 (2.8–7.3) | 1.05 (0.87–1.27) | 0.88 (0.69–1.13) |
| Tumor location | |||||
| Supratentorial | 5.9 (2.7–13.4) | 4.4 (2.0–10.5) | 5.3 (2.6–9.6) | 1 [ref.] | 1 [ref.] |
| Other | 7.6 (3.5–15.3) | 6.4 (2.8–12.8) | 5.7 (2.7–9.6) | 1.07 (0.93–1.23) | 1.00 (0.84–1.17) |
| Tumor multifocality | |||||
| No | 9.5 (4.1–24.7) | 4.3 (1.7–13.6) | 11.7 (1.4–16.3) | 1.02 (0.70–1.50) | 3.18 (2.08–4.86) |
| Yes | 7.3 (3.3–14.6) | 6.0 (2.6–12.1) | 5.3 (2.6–9.0) | 1 [ref.] | 1 [ref.] |
| Tumor size | |||||
| >3 cm | 7.1 (3.1–13.5) | 5.9 (2.6–12.1) | 5.7 (2.7–9.5) | 1.09 (0.96–1.22) | 1.17 (1.01–1.35) |
| ≤3 cm | 8.1 (3.9–17.5) | 5.9 (2.5–12.2) | 5.4 (2.7–9.6) | 1 [ref.] | 1 [ref.] |
| Extent of resection | |||||
| GTR | 10.7 (5.0–19.5) | 8.3 (3.9–14.6) | 6.7 (3.4–10.2) | 0.74 (0.65–0.84) | 0.73 (0.62–0.86) |
| Biopsy/STR | 6.1 (2.8–11.6) | 4.7 (2.0–11.0) | 5.3 (2.7–9.3) | 1 [ref.] | 1 [ref.] |
| Discharge locationd | |||||
| Home | 8.7 (3.5–16.4) | 7.7 (3.3–13.1) | 7.0 (3.6–10.9) | 0.78 (0.69–0.87) | 0.70 (0.60–0.81) |
| Other | 5.8 (3.1–12.1) | 4.7 (2.0–10.9) | 4.2 (2.1–6.9) | 1 [ref.] | 1 [ref.] |
IQR interquartile range, TMZ temozolomide, RT radiotherapy, SEER surveillance, epidemiology, and end results, GTR gross total resection, STR sub-total resection
Overall survival defined as starting 60 days following diagnosis, for equal period of treatment ascertainment
Radiotherapy alone treatment for diagnoses in 1995–1999 when temozolomide was not available (1995 to mid-1999) or not approved (late 1999) for glioblastoma
Dichotomized median income based on median household or per capita income by US Census tract or zip code
Following index hospitalization for glioblastoma diagnosis, location the patient was discharged to including home (with or without care services), skilled nursing facility, rehabilitation facility, intermediate care facility, or long-term care facility
Discussion
In this SEER-Medicare cohort of glioblastoma patients who received radiotherapy with or without temozolomide, we observed that following the 2005 FDA approval decision [15], adoption of temozolomide concurrently with radiotherapy was high, with 50 % (705 of 1419) of patients ≥65 years receiving temozolomide-based chemoradiation from 2005 to 2009. Given the lack of other effective systemic agents for this lethal disease, it is unsurprising that concurrent temozolomide was administered frequently among elderly patients. The survival advantage we observed with temozolomide-based chemoradiation in this older cohort persisted on propensity score adjustment models intended to limit confounding, yet was small, at approximately 1 month, and there was no apparent increase in longer-term survivors.
The benefits of concurrent temozolomide among older glioblastoma patients have remained controversial [24]. Prior reports have observed temporal increases in survival among older patients since 2005 but lacked information about temozolomide use [16], and a recent study did not describe outcomes for the elderly [25]. Subgroup analyses from the EORTC/NCIC trial [26, 27] have suggested reduced benefit for temozolomide among older patients, but with limited power to detect differences. In contrast to the trial's overall hazard ratio of 0.6 for combined chemoradiation [4, 26], there was no survival advantage to concurrent temozolomide (HR, 0.8; P = .34) among the 83 patients age 65–70 years [27]. However with a much larger sample of over 1600 patients age ≥65 in the current study, we were able to detect a small survival increase associated with temozolomide, though based on retrospective data. Hazard ratios ranged generally from 0.8 to 0.9 for individual age subgroups in our data, though often losing statistical significance, reflective of the small effect and underpowering within subgroups even in this large sample. The larger effect estimate we observed among the oldest subgroup (age ≥80 years) may reflect residual confounding at the extremes of age that persisted despite adjustments, and while speculative, could indicate that patients aged ≥80 years with the highest performance status were more likely to receive temozolomide but also independently more likely to live longer. Removal of the oldest patients from analysis did not affect our overall results, potentially because these patients represented only 15 % of the total sample and our analyses already adjusted for age. Of note, the cohort of patients treated with radiotherapy alone in 1995–1999, before temozolomide was available, was included as an additional control for potential patient selection in the 2005–2009 era, since no secular survival trends have been observed in prior SEER investigations among the older glioblastoma population from 1995 to 2005 [16].
Less intensive treatment regimens among older glioblastoma patients have been tested in prospective trials [5–9], including monotherapy with either radiotherapy or temozolomide, yet no published studies to date have included chemoradiation as a comparator arm. Roa et al. [7] reported that among glioblastoma patients ≥60 years old and KPS ≥50, 40 Gy delivered in 15 daily fractions was comparable with 60 Gy in 30 daily fractions, with median survival of 5.6 months in the hypofractionated arm and 5.1 months in the standard arm (P = .57). The Nordic trial [5] randomized glioblastoma patients ≥60 years old to hypofractionated radiotherapy (34 Gy/10 fractions), standard radiotherapy (60 Gy/30 fractions), or temozolomide monotherapy for 6 cycles, with median survival of 7.5 months, 6.0 months, and 8.3 months, respectively (P = .01 for the temozolomide arm vs. standard radiotherapy arm). These trials suggest that shorter courses of radiotherapy are associated with similar survival to longer radiotherapy courses. In general, treatment de-escalation approaches have been pursued due to short survival [28, 29] and toxicity concerns [29–31] among older patients. A single-arm prospective study among glioblastoma patients ≥70 years old [31] demonstrated neurotoxicity among 40 % of patients which was largely grade 2 and reversible, and grade 3–4 hematologic toxicity in 28 % of patients. The landmark EORTC/NCIC/TROG randomized trial (ClinicalTrials.gov NCT00482677) of short-course hypofractionated radiotherapy with or without concurrent temozolomide will provide important prospective data on efficacy and toxicity of a combined modality approach in the elderly, and recently presented initial results suggest a modest improvement in median OS with the addition of temozolomide (9.3 vs. 7.6 months; HR, 0.67; 95 % CI, 0.56–0.80) [10]. Crucially, however, that trial did not address how short-course chemoradiation (2–3 weeks) compares to standard long-course chemoradiation (6 weeks), and specifically excludes patients who are considered to be candidates for standard long-course chemoradiation. Two recent retrospective reports [32, 33] suggest that short-course chemoradiation may be preferable to long-course chemoradiation, given the equivalent survival observed. Given that we detected no survival advantage to temozolomide among 999 patients who received long-course radiotherapy (≥27 fractions), yet we found a small advantage in the entire cohort, it is possible but speculative that concurrent temozolomide may have a larger proportional benefit among shorter courses of radiation.
Limitations of our study relate primarily to its observational design. SEER-medicare does not include details on radiation dose or O6-methylguanine DNA methyltransferase (MGMT) methylation status. While MGMT promoter methylation can be prognostic and predictive of benefit from temozolomide [34], and is an important part of guideline-concordant care [35], unfortunately there has not yet been universal adoption of MGMT testing in routine clinical care [36, 37], partly due to technical challenges in existing MGMT assays and lack of effective alternatives to temozolomide. Thus our dataset reflects real-world practice wherein treatment decisions are often made without biomarker information. Second, since patient performance status information is not captured in registry data, we performed multiple propensity score analyses and added a second control group to reduce the risk of confounding bias, which appears to have been largely mitigated except for perhaps among the extreme elderly subgroup as outlined above. Third, under-ascertainment of temozolomide is possible in our cohort, if some Medicare beneficiaries had temozolomide covered instead by supplemental non-Medicare insurance plans [38, 39]. If present, this would tend to bias our results towards the null and underestimate the effect estimate for temozolomide. We attempted to mitigate the possibility of imbalance in treatment ascertainment between cohorts by calculating survival starting 60 days after diagnosis, as treatment initiation for both radiotherapy and temozolomide was defined as starting with 60 days of diagnosis, though there remains the possibility that either cohort still contained residual bias related to early toxic deaths that were not captured. Finally, we did not examine receipt of salvage therapies between treatment groups, yet this is not anticipated to have affected survival substantially, given the lack of effective salvage treatments in this disease; even bevacizumab, which received FDA approval as salvage therapy in 2009 [40], has not been shown to increase overall survival in glioblastoma.
In summary, our retrospective population-level analysis suggests that the addition of temozolomide to radiotherapy among older glioblastoma patients is associated with a small survival advantage, which was not observed among the large subgroup receiving standard long-course radiotherapy. The potential benefits of temozolomide for those receiving hypofractionated regimens are being addressed in the EORTC/NCIC/TROG trial. Given the limited evidence on concurrent temozolomide in the rapidly growing population of older glioblastoma patients, this study provides the first data on population-based effectiveness. Physicians making treatment decisions will need to weigh risks and benefits of concurrent temozolomide for individual patients, incorporating a multitude of patient parameters including age, comorbidities, performance status, molecular markers, and patient preferences.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. This study used the linked SEER-medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Funding This work was supported by grants from the National Cancer Institute of the National Institutes of Health (P01CA134294-06 to F.D.), and from the Agency for Healthcare Research and Quality (K18 HS21991 to F.D.). Contents of this publication are solely the responsibility of the authors and no official endorsement by the National Cancer Institute, National Institutes of Health, Agency for Healthcare Research and Quality, or the U.S. Department of Health and Human Services is intended or should be inferred.
Footnotes
This work was presented at the Society for Neuro-Oncology Annual Meeting, November 13–16, 2014, Miami, Florida.
Compliance with ethical standards: Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker MD, Green SB, Byar DP, et al. Randomized comparisons of raditherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 3.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Malmström A, Gronberg BG, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 6.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 7.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 8.Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29:2050–2055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]
- 9.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 10.Perry JR, Laperriere N, O'Callaghan CJ, et al. A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (CCTG CE.6, EORTC 26062-22061, TROG 08.02, NCT00482677) J Clin Oncol. 2016;34(18_suppl):LBA2. [Google Scholar]
- 11.Chakrabarti I, Cockburn M, Cozen W, et al. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104:2798–2806. doi: 10.1002/cncr.21539. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Surveillance, Epidemiology and End Results. [Accessed Mar 2016]; Available at: http://seer.cancer.gov/about/overview.html.
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MH, Johnson JR, Pazdur R. Food and drug administration drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11:6767–6771. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 16.Darefsky AS, King JT, Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of surveillance, epidemiology, and end results registries. Cancer. 2012;118:2163–2172. doi: 10.1002/cncr.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to the validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1365–1373. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 19.D'Agostino RB, Jr, D'Agostino RB., Sr Estimating treatment effects using observational data. JAMA. 2007;297:314–316. doi: 10.1001/jama.297.3.314. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 21.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Sharma DB, Gray SW, et al. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307:1593–1601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvold ND, Reardon DA. Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging. 2014;9:357–367. doi: 10.2147/CIA.S44259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubrow R, Darefsky AS, Jacobs DI, et al. Time trends in glioblastoma multiforme survival: the role of temozolomide. Neuro Oncol. 2013;15:1750–1761. doi: 10.1093/neuonc/not122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 27.Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39:350–357. doi: 10.1016/j.ctrv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto FM, Reiner AS, Panageas KS, et al. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64:628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 29.Arvold ND, Wang Y, Zigler C, et al. Hospitalization burden and survival among older glioblastoma patients. Neuro Oncol. 2014;16:1530–1540. doi: 10.1093/neuonc/nou060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 31.Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 32.Arvold ND, Tanguturi SK, Aizer AA, et al. Hypofractionated versus standard radiation therapy with or without temozolomide for older glioblastoma patients. Int J Radiat Oncol Biol Phys. 2015;92:382–389. doi: 10.1016/j.ijrobp.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Minniti G, Scaringi C, Lanzetta G, et al. Standard (60 Gy) or short-course (40 Gy) irradiation plus concomitant and adjuvant temozolomide for elderly patients with glioblastoma: a propensity-matched analysis. Int J Radiat Oncol Biol Phys. 2015;91:109–115. doi: 10.1016/j.ijrobp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 35.Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 36.Wick W, Weller M, van den Bent M, et al. MGMT testing–the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10:372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 37.Weller M, Stupp R, Hegi ME, et al. Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol. 2012;14:iv 100–108. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidoff AJ, Shaffer T, Erten MZ, et al. Use and spending on antineoplastic therapy for medicare beneficiaries with cancer. Med Care. 2013;51:351–360. doi: 10.1097/MLR.0b013e3182726ceb. [DOI] [PubMed] [Google Scholar]
- 39.Ray S, Bonafede MM, Mohile NA. Treatment patterns, survival, and healthcare costs of patients with malignant gliomas in a large US commercially insured population. Am Health Drug Benefits. 2014;7:140–149. [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]


