Abstract
Food insecurity (FI) is a documented problem associated with adverse health outcomes among HIV-infected populations. Little is known about the relationship between alcohol use and FI. We assessed whether heavy alcohol use was associated with FI among HIV-infected, antiretroviral therapy (ART)-naïve cohorts in Uganda and Russia. Inverse probability of treatment weighted logistic regression models were used to evaluate the association using cross-sectional baseline data. FI was experienced by half of the Russia cohort (52%) and by a large majority of the Uganda cohort (84%). We did not detect an association between heavy alcohol use and FI in either cohort (Russia: AOR = 0.80, 95% CI = 0.46, 1.40; Uganda: AOR = 1.00, 95% CI = 0.57, 1.74) or based on the overall combined estimate (AOR = 0.89, 95% CI = 0.60, 1.33). Future studies should explore the determinants of FI in HIV-infected populations to inform strategies for its mitigation.
Keywords: Food Insecurity, HIV, Alcohol
Introduction
Overview
Globally, 795 million people are undernourished, including 780 million people in developing countries [1]. Food insecurity (FI), the “limited or uncertain availability of nutritionally adequate and safe foods or limited or uncertain ability to acquire acceptable foods in socially acceptable ways” and HIV infection often co-occur [2, 3]. Improving food security among HIV-infected people is a global health priority, as this population is highly vulnerable to the effects of inadequate nourishment [4–7].
Food insecurity among individuals with HIV infection
Numerous studies have found an association between FI and ART non-adherence in such diverse populations as people with illicit drug use or alcohol use in the United States [8, 9], patients receiving ART at health facilities in the Democratic Republic of Congo, Namibia, and Senegal [10–12], and patients initiating ART in Peru [13]. In a study in Mbarara, Uganda in which participants were followed for a median of 2.1 years, 26% of those severely food insecure gave up ART for food during follow-up and 80% gave up “adequate food for themselves or their family” to access outpatient care [14].
Food insecurity has also been associated with adverse health outcomes in HIV-infected populations. These adverse outcomes include reduced health-related quality of life as measured by the Medical Outcomes Study – HIV (MOS-HIV) (i.e., mental health and/or physical health status scores) [14, 15]; low CD4 count [16, 17]; increased depressive symptom severity [18]; new opportunistic infections [14]; an increase in the number of hospitalizations [14]; incomplete viral suppression [19–23]; and mortality [24]. How FI affects these important HIV outcomes is not clear, as is evident in a study from San Francisco in which associations between FI and low CD4 count and unsuppressed viral load were not fully explained by ART non-adherence, supporting the proposition that FI affects clinical outcomes via multiple pathways [17].
Alcohol use and food insecurity
In a conceptual framework proposed by Weiser et al. (Figure 1) (2011, 2015) [25, 26], FI increases the risk of HIV acquisition and transmission and worsens HIV/AIDS morbidity and mortality via nutritional, mental health, and behavioral pathways. In this framework, along the mental health pathway, FI is hypothesized to be positively associated with drug and alcohol use which are thought to be 1) directly associated with worse clinical outcomes and 2) indirectly associated with worse clinical outcomes via positive associations with ART non-adherence, missed clinic visits, and treatment interruptions. The resulting morbidity leads to increased FI (e.g. by reducing one’s ability to work), thus forming a self-reinforcing downward cycle [25, 26]. In addition to the associations between FI and health outcomes, the association between FI and alcohol use may also be bidirectional [26], as FI is associated with poor mental health [27], anxiety symptoms [28], and depressive symptoms [18, 28–30].
Figure 1. Food insecurity and HIV/AIDS morbidity and mortality [25].
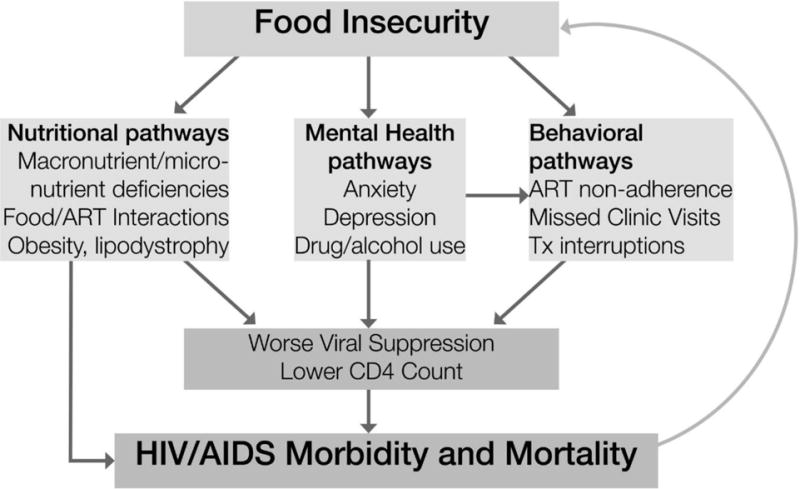
Weiser SD, Young SL, Cohen CR, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. Dec 2011;94(6):1729S–1739S.
Although several studies have explored the association between illicit drug use and FI in HIV-infected populations [31], the association between alcohol use and FI in HIV-infected populations has received less critical scrutiny. Normén et al. (2005) found a positive unadjusted association between ever having been in a drug or alcohol treatment program and FI among patients enrolled in a public ART program in British Columbia, Canada [32]. Among homeless and marginally housed HIV-infected persons in San Francisco, Weiser et al. (2009) found an unadjusted association between recent drug use and severe FI, but no such association with recent problem alcohol use [21]. A positive unadjusted association between recent heavy episodic drinking and FI was found among veterans receiving ART in the Veterans Aging Cohort Study, but no association was detected between past year hazardous alcohol consumption and FI [19]. Among HIV-HCV co-infected individuals in 6 Canadian provinces, Cox et al. (2016) did not find an independent association between current heavy alcohol use and FI [33].
In studies outside the realm of HIV infection, the association between alcohol use and FI has been examined numerous ways with inconsistent results. In Ethiopia, any alcohol use by the head of the household was independently associated with an increase in the level of FI [34]. Among women with newborns in South Africa, FI was independently associated with hazardous drinking [35]. In South Africa, Eaton et al. (2014) found unadjusted associations between 2 measures of alcohol use and FI among women but not among men [36]. No significant independent association was found between heavy episodic drinking and FI among US military veterans in the Veterans Health Administration [37]. It is notable that most of the data about FI and health outcomes, both HIV and non-HIV-related, come from work in North America and Africa. Understanding alcohol’s relationship to FI in countries with particularly high per capita alcohol consumption (e.g., Sub-Saharan Africa and Eastern Europe) is of interest as the possibility of individuals buying alcohol rather than food is a real concern.
This study aims to contribute to the understanding of the relationship between alcohol use and FI in HIV-infected persons. Specifically, the primary objective is to evaluate the association between heavy alcohol use and FI among HIV-infected persons who have not initiated ART in Mbarara, Uganda and St. Petersburg, Russia. The pre-ART status allows an examination of the association between alcohol use and FI without the association being mediated by worse ART adherence. A secondary objective is to explore whether gender is an effect modifier of the relationship between alcohol use and FI. This secondary objective is intended to be descriptive and hypothesis-generating. There is evidence that women are at greater risk for FI than men [32, 38] and the association between alcohol use and FI may differ by gender [36].
Methods
Study Design
We analyzed baseline data from 2 prospective cohort studies within the Uganda Russia Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) consortium: Uganda ARCH conducted in Mbarara, Uganda and Russia ARCH conducted in St. Petersburg, Russia.
Participants
Overview
The study population in both cohorts is HIV-infected, ART-naïve adults with a range of current alcohol consumption, including abstinence.
Russia ARCH cohort
From November 2012 to June 2015, we enrolled 364 participants from clinical HIV and addiction sites, non-clinical sites, and via snowball recruitment in St. Petersburg, Russia. We obtained informed consent and conducted in-person assessments at First Saint Petersburg Pavlov State Medical University. Eligibility criteria included the following: 18–70 years of age; HIV infection and ART-naïve status verified with documentation from a healthcare provider; having a home or mobile telephone; living within 100 km of St. Petersburg; and providing the contact information of ≥2 friends or family members to assist in follow-up. Exclusion criteria were lack of fluency in Russian or cognitive impairment. Thirteen participants were subsequently unenrolled because they were determined to be HIV negative or due to unsuccessful phlebotomy, a key aspect of the main study. The study sample includes 351 participants, 3 of whom were excluded from adjusted analyses due to missing covariates.
Uganda ARCH cohort
From September 2011 to August 2014, we enrolled 484 participants from the Immune Suppression Syndrome (ISS) Clinic of the Mbarara Regional Referral Hospital in Uganda. Informed consent and in-person interviews were conducted at the ISS Clinic. Eligibility criteria included the following: ≥18 years of age; fluency in Runyankole or English; ability to give informed consent; residence within 60 km or less than a 2-hour drive from the clinic; no current or previous ART; not scheduled for initiation of ART within the next 3 months; diagnosis of WHO Stage I or II disease (asymptomatic or mild disease); and CD4 count >350 cells/mm3 (>500 as of February 19, 2014, due to a Ugandan guideline change for ART eligibility). Exclusion criteria were pregnancy or incarceration. Participants with low (<1000 copies/ml) or non-detected HIV viral load at baseline were tested for the presence of HIV antibodies or ART. Thirty-two of these participants were found to be either uninfected by HIV or had indeterminate results and 5 participants were positive for ART. These 37 participants were subsequently unenrolled from the study. Due to missing data, 445 participants were included in unadjusted analyses and 444 in adjusted analyses.
Ethics statement
The Russia ARCH study was approved by the institutional review boards of Boston University Medical Campus and First St. Petersburg Pavlov State Medical University. The Uganda ARCH study was approved by the institutional review boards of Boston University Medical Campus, University of California, San Francisco, Mbarara University of Science and Technology, and the Uganda National Council of Science and Technology.
Measurements
At both study sites, all baseline assessments were conducted in person by trained research assessors. In Mbarara, assessments were administered in Runyankole or English, based on each participant’s language preference. In St. Petersburg, all assessments were administered in Russian.
Outcomes
The primary outcome was any FI in the past 4 weeks as measured by 9 items of the Household Food Insecurity Access Scale (HFIAS) as revised in 2007 [39, 40]. The 9 items and 3 domains of the HFIAS were created to capture the experience of lack of food access “across countries and cultures” [39]. The HFIAS is used to categorize households as food secure, mildly food insecure, moderately food insecure, and severely food insecure [40]. In the main analysis, we dichotomized level of FI as any level of FI (mild, moderate, or severe) vs. food secure. In a secondary analysis, we dichotomized level of FI as severely food insecure vs. not severely food insecure.
Main independent variable
The main independent variable in this study was heavy alcohol use. For Uganda ARCH, we used the Alcohol Use Disorders Identification Test – Consumption (AUDIT-C) to assess drinking in the previous 3 months [41, 42]. Participants with AUDIT-C scores in the hazardous range (AUDIT-C score ≥4 for men; ≥3 for women) or a blood phosphatidylethanol (PEth) level of ≥50 ng/ml were considered heavy drinkers. We included PEth, a metabolite of ethanol, as part of our assessment of heavy drinking because previous studies in Uganda have found common underreporting of alcohol use in an HIV care setting [43–45].
For Russia ARCH, we assessed alcohol use via the 30-day Timeline Followback method [46, 47]. With the aid of a calendar, the assessor and participant discussed alcohol consumption on each of the past 30 days and recorded the volumes of 4 alcoholic drink types (beer or low alcohol content cocktails, wine or high alcohol content cocktails, spirits, fortified wine) consumed on each day. The volumes were later converted to standard drinks with conversions for the alcohol content of each drink type [48]. Heavy alcohol use was defined in men as those who drank >4 drinks in a day or 14 drinks per week in the past 30 days and in women as those who drank >3 drinks in a day or 7 per week [49].
Covariates
Covariates were selected based on the literature and clinical knowledge about the 2 populations. Factors included as potential confounders for both Russia ARCH and Uganda ARCH included: age, gender, educational attainment beyond basic education in each setting (greater than 9 grades in Russia and greater than primary education in Uganda), marital status (defined in Uganda as having a spouse, defined in Russia as married or living with a partner), and income (self-reported monthly income tertiles for the Russia ARCH cohort; an asset index score categorized into low, medium, or high household wealth in Uganda).
In addition to the above, for Russia ARCH, we included the covariates of drug dependence and alcohol dependence in the past 12 months (DSM-IV criteria) with the Mini International Neuropsychiatric Interview 6.0 (MINI) drug instrument and alcohol instrument [50]; and MOS Social Support Survey score [51], dichotomized at the median. Baseline CD4 count was available for a subset of Russian participants and was included in a sensitivity analysis.
For the Uganda ARCH cohort, additional covariates included: religious affiliation, categorized as Catholic, Protestant/Anglican, and Muslim/Saved/other; literacy, evaluated by the research assistant and dichotomized as being able to read a full sentence vs. not being able to read a full sentence; CD4 count (continuous); and HIV Symptom Index (dichotomized at the sample’s median) [52]. Drug dependence was not assessed in Uganda due to the rarity of drug use.
Statistical Analysis
We stratified preliminary analyses by cohort to characterize the 2 study samples and assess whether the association between heavy alcohol use and FI was similar in the 2 cohorts. To characterize the study samples, we first computed descriptive statistics for baseline characteristics overall and stratified by heavy alcohol use. Propensity scores were used to minimize confounding due to measured covariates [53]. We applied the propensity scores using inverse probability of treatment weighted (IPTW) logistic regression models to evaluate the association between heavy alcohol use and FI. Predicted probabilities of heavy alcohol use (i.e., the propensity score for each participant) were calculated from a multiple logistic regression model, which included the potential confounders of the association between heavy alcohol use and FI. Subjects were then weighted by the inverse probability of being in their observed alcohol group, with probabilities determined based on estimated propensity scores. We evaluated covariate balance by assessing standardized differences by heavy drinking status in the weighted sample. An absolute difference of <0.20 was considered acceptable [54].
The Russia and Uganda ARCH cohorts come from 2 diverse populations with unique demographics. In addition, covariates were expected to differ for the 2 cohorts as described above. Thus although we have individual level data from the 2 cohorts, because covariates differed by cohort, the data were not pooled into a single analysis as this may produce biased results. A random effects meta-analysis was used to account for within and between study variability [55].
There was not a significant difference between the cohort-specific odds ratios (p=0.59). Therefore we conducted a subsequent analysis to provide a single overall estimate of the association between heavy alcohol use and FI. Secondary analyses of the outcome of severe FI were analyzed using the same approach. To explore and describe whether gender is an effect modifier of the relationship between heavy alcohol use and FI, we stratified analyses by gender within each cohort, developing propensity score models separately for men and women. We also conducted post hoc analyses to explore whether income (Russia cohort)/household wealth (Uganda cohort) were effect modifiers of the relationship between heavy alcohol use and FI and whether drug dependence was a mediator of the association between heavy alcohol use and FI in the Russia cohort. An alpha level of 0.05 was used for all tests. Analyses were conducted using SAS version 9.3 (SAS Institute Inc., 2011).
Results
The Russia ARCH cohort study sample included 351 participants with a mean age of 34 years and 71% male; 250 (71%) reported current heavy alcohol use. Educational attainment greater than basic education was common (79%). Nearly half (43%) were married or were living with a partner and over a third (37%) injected drugs in the past 30 days. A majority (62%) met criteria for alcohol dependence in the past 12 months and a similar proportion (56%) for drug dependence. Food insecurity in the past 4 weeks was common, with 52% food insecure, including 23% severely food insecure. In the propensity score weighted sample (Table I), participants without heavy drinking were similar to participants with heavy drinking on all characteristics which were considered potential covariates in the Russia ARCH cohort.
Table I.
Characteristics of HIV-infected ART-naïve cohorts from Uganda and Russia stratified by heavy alcohol use in the propensity score weighted samples
| Characteristic | Russia ARCH cohort n=348 |
Uganda ARCH cohort n=444 |
||||
|---|---|---|---|---|---|---|
| Current abstinence or non-heavy alcohol use | Current heavy alcohol use | Standardized Difference | Current abstinence or non-heavy alcohol use | Current heavy alcohol use | Standardized Difference | |
| Male | 71.0% | 70.6% | −0.01 | 32.1% | 31.1% | −0.02 |
| Age mean (SD) | 34.1 (9.7) | 33.7 (7.1) | −0.04 | 34.1 (13.6) | 34.1 (15.0) | 0.00 |
| Greater than basic education | 76.2% | 77.9% | 0.04 | 30.0% | 32.8% | 0.06 |
| Married1 | 42.8% | 43.1% | 0.01 | 49.7% | 43.0% | −0.13 |
| Income tertile or asset category | ||||||
| Lowest | 37.2% | 37.4% | 0.00 | 38.5% | 37.1% | −0.03 |
| Middle | 31.3% | 31.3% | 0.00 | 41.1% | 42.7% | 0.03 |
| Highest | 31.5% | 31.3% | −0.01 | 20.4% | 20.2% | 0.00 |
| Current injection drug use (30 days) | 33.4% | 40.6% | 0.15 | 0% | 0% | – |
| Alcohol dependence (12 months) | 59.0% | 61.4% | 0.05 | 3.1% | 37.0% | 0.93 |
| Drug dependence (12 months) | 56.2% | 55.7% | −0.01 | – | – | – |
| Low social support | 49.1% | 49.7% | 0.01 | 51.4% | 55.8% | 0.09 |
| CD4 count2 mean (SD) | 558 (587) | 520 (342) | −0.08 | 571 (271) | 571 (296) | 0.00 |
| HIV symptom count high (4 weeks) | 73.2% | 43.4% | −0.63 | 62.6% | 65.1% | 0.05 |
| Religion | ||||||
| Catholic | – | – | – | 36.5% | 35.7% | −0.02 |
| Protestant/Anglican | – | – | – | 47.6% | 45.9% | −0.03 |
| Muslim/Saved/other | – | – | – | 15.9% | 18.4% | 0.07 |
| Literate | – | – | – | 68.3% | 67.6% | −0.01 |
| Food insecurity (insecure vs. secure) | 53.8% | 48.2% | −0.11 | 83.5% | 83.4% | 0.00 |
|
Severe food insecurity (severe vs. moderate, mild, secure) |
24.8% | 21.9% | −0.07 | 28.2% | 36.6% | 0.18 |
In Russia ARCH cohort, married or living with partner
In Russia ARCH cohort, n=246; in Uganda ARCH cohort, n=444
The Uganda ARCH cohort study sample included 445 participants with a mean age of 34 years and 32% male; 192 (43%) had current heavy alcohol use and half (49%) were married. Two-thirds (68%) were literate and 30% had education beyond basic education. No participants had injected drugs in the past 30 days. A small minority (18%) met criteria for alcohol dependence in the past 12 months. Half (49%) were Protestant or Anglican, 35% were Catholic, and 16% were Muslim, Saved, or other. FI in the past 4 weeks was very common, with 84% food insecure, including 32% severely food insecure. In the propensity score weighted sample (Table I), participants without heavy drinking were similar to participants with heavy drinking on all characteristics which were considered potential covariates in the Uganda ARCH cohort.
Heavy Alcohol Use and Food Insecurity
In unadjusted analyses (Table II), we did not find a significant association between heavy alcohol use and FI in the Russia ARCH cohort (OR=0.90, 95% CI = 0.57 to 1.43) or in the Uganda ARCH cohort (OR=0.85, 95% CI: 0.51 to 1.42).
Table II.
Unadjusted associations between heavy alcohol use and food insecurity among HIV-infected ART-naïve cohorts from Uganda and Russia
| Russia ARCH cohort | Uganda ARCH cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Current abstinence or non-heavy alcohol use n=101 |
Current heavy alcohol use n=250 |
Crude OR (95% CI) |
p-value | Current abstinence or non-heavy alcohol use n=253 |
Current heavy alcohol use n=192 |
Crude OR (95% CI) |
p-value |
| Food insecurity (insecure vs. secure) | 54 (53.5%) | 127 (50.8%) | 0.90 (0.57, 1.43) |
0.65 | 215 (85.0%) | 159 (82.8%) | 0.85 (0.51, 1.42) |
0.54 |
| Severe food insecurity (severe vs. moderate, mild, secure) | 23 (22.8%) | 56 (22.4%) | 0.98 (0.56, 1.70) |
0.94 | 73 (28.9%) | 68 (35.4%) | 1.35 (0.91, 2.02) |
0.14 |
In the weighted analyses, adjusted for potential confounders (Table III), we did not detect an association between heavy alcohol use and FI in either cohort (Russia: AOR = 0.80, 95% CI = 0.46 to 1.40; Uganda: AOR = 1.00, 95% CI = 0.57 to 1.74) or based on the overall combined estimate from the 2 cohorts (AOR = 0.89, 95% CI = 0.60 to 1.33). A confirmatory analysis adjusting for baseline CD4 count in the subset of the Russian cohort with CD4 count results available (n=246) yielded similar results to the primary results above (AOR = 0.59, 95% CI = 0.14 to 2.46).
Table III.
Adjusteda associations between heavy alcohol use and food insecurity among HIV-infected ART-naïve cohorts from Uganda and Russia
| N | Adjusted* OR (95% CI) | p-value | |
|---|---|---|---|
| Primary Outcome: Food Insecurity | |||
| Russia ARCH | 348 | 0.80 (0.46, 1.40) | 0.43 |
| Uganda ARCH | 444 | 1.00 (0.57, 1.74) | 0.99 |
| Overall Combined Estimate | 792 | 0.89 (0.60, 1.33) | 0.59 |
| Secondary Outcome: Severe Food Insecurity | |||
| Russia ARCH | 348 | 0.85 (0.45, 1.61) | 0.62 |
| Uganda ARCH | 444 | 1.47 (0.93, 2.31) | 0.10 |
| Overall Combined Estimate | 792 | 1.17 (0.69, 1.99) | 0.17 |
Based on inverse probability treatment weighted logistic regression models
In exploratory gender-stratified analysis (Table IV) we did not find an association between heavy alcohol use and FI among women (AOR = 0.44, 95% CI = 0.16 to 1.23) or men (AOR = 1.02, 95% CI = 0.53 to 1.99) in the Russia ARCH cohort. Similarly, in the Uganda ARCH cohort, the analysis did not find an association between heavy alcohol use and FI among women (AOR = 1.14, 95% CI = 0.55 to 2.39) or men (AOR = 0.65, 95% CI = 0.25 to 1.70).
Table IV.
Adjusteda associations between heavy alcohol use and food insecurity by gender
| Cohort | Stratum | N | Current abstinence or non-heavy alcohol use | Current heavy alcohol use | Adjusted* OR (95% CI) |
p-value |
|---|---|---|---|---|---|---|
| Primary Outcome: Food Insecurity | ||||||
| Russia ARCH |
Women | 102 | 18 (66.7%) | 38 (50.7%) | 0.44 (0.16, 1.23) | 0.12 |
| Men | 246 | 35 (48.0%) | 89 (51.4%) | 1.02 (0.53, 1.99) | 0.95 | |
| Uganda ARCH |
Women | 300 | 166 (86.0%) | 94 (87.9%) | 1.14 (0.55, 2.39) | 0.72 |
| Men | 144 | 48 (81.4%) | 65 (76.5%) | 0.65 (0.25, 1.70) | 0.38 | |
| Secondary Outcome: Severe Food Insecurity | ||||||
| Russia ARCH |
Women | 102 | 11 (40.7%) | 16 (21.3%) | 0.45 (0.16, 1.30) | 0.14 |
| Men | 246 | 12 (16.4%) | 40 (23.1%) | 1.30 (0.55, 3.03) | 0.55 | |
| Uganda ARCH |
Women | 300 | 57 (29.5%) | 50 (46.7%) | 1.74 (1.02, 2.97) | 0.04 |
| Men | 144 | 16 (27.1%) | 18 (21.2%) | 0.95 (0.38, 2.38) | 0.92 | |
Based on inverse probability treatment weighted logistic regression models
There were no significant relationships between current heavy alcohol use and the secondary outcome of severe FI in unadjusted analyses (Table II, Russia: OR=0.98, 95% CI: 0.56 to 1.70; Uganda: OR=1.35, 95% CI: 0.91 to 2.02) or adjusted analyses (Table III, Russia: AOR = 0.85, 95% CI = 0.45 to 1.61; Uganda: AOR = 1.47, 95% CI = 0.93 to 2.31; overall combined estimate: AOR = 1.17, 95% CI = 0.69 to 1.99).
In gender-stratified analyses (Table IV), we did not find an association between heavy alcohol use and severe FI among women (AOR = 0.45, 95% CI = 0.16 to 1.30) or men (AOR = 1.30, 95% CI = 0.55 to 3.03) in the Russia ARCH cohort. In the Uganda ARCH cohort there was a significant positive association between heavy alcohol use and severe FI among women (AOR = 1.74, 95% CI = 1.02 to 2.97) but not among men (AOR = 0.95, 95% CI = 0.38 to 2.38).
In exploratory post hoc analyses, we did not detect interactions between heavy alcohol use and income for either the Russia cohort (p=0.71) or the Uganda cohort (p=0.27). Subsequent stratified analyses showed greater variability across income levels for the Uganda cohort compared with the Russia cohort (Russia: lowest income tertile (AOR = 0.67, 95% CI = 0.25 to 1.75), middle income tertile (AOR = 0.69, 95% CI = 0.22 to 2.11), highest income tertile (AOR = 1.11, 95% CI = 0.40 to 3.08); Uganda: lowest asset level (AOR = 0.96, 95% CI = 0.36 to 2.57), middle asset level (AOR = 1.56, 95% CI = 0.56 to 4.35), highest asset level (AOR = 0.62, 95% CI = 0.23 to 1.68)).
An additional post hoc analysis was performed to assess potential mediation by drug dependence in the Russia cohort. We found that the adjusted odds ratio for heavy alcohol was similar in models with (AOR = 0.80, 95% CI = 0.46 to 1.40) and without drug dependence as a covariate (AOR = 0.73, 95% CI = 0.42 to 1.28), suggesting drug dependence is not a mediator of the relationship between heavy alcohol use and FI.
Although propensity score analyses do not generate measures of association between potential confounders and the outcome of interest, we ran multiple logistic regression models in preliminary analyses. Among the common covariates (age, gender, education, marital status, and income/wealth), female gender was associated with increased odds of FI in the Uganda cohort (AOR = 2.13, 95% CI = 1.19 to 3.78) but not in the Russia cohort (AOR = 1.15, 95% CI = 0.66 to 1.99). In both cohorts, low income or wealth vs. high was associated with increased odds of FI (Uganda: AOR = 3.33, 95% CI = 1.55 to 7.14; Russia: AOR = 4.63, 95% CI = 2.50 to 8.57) and middle income or wealth vs. high was associated with increased odds of FI (Uganda: AOR = 2.68, 95% CI = 1.36 to 5.26; Russia: AOR = 3.68, 95% CI = 2.02 to 6.70).
Discussion
In this study of ART-naïve, HIV-infected individuals in Mbarara, Uganda and St. Petersburg, Russia, we were unable to detect an association between current heavy alcohol use and the primary outcome of food insecurity (FI) or the secondary outcome severe FI.
Exploratory gender-stratified analyses found a significant association between heavy alcohol use and severe FI among women but not among men in the Uganda ARCH cohort. However, given the multiple comparisons conducted and the exploratory nature of these analyses, we interpret these results as preliminary, hypothesis-generating analyses that merit further investigation.
In post hoc analyses, income/household wealth did not appear to be an effect modifier of the association between heavy alcohol use and FI by income in either cohort. We also did not find evidence that drug dependence is a mediator of the relationship between heavy alcohol use and FI in the Russia cohort. We did not explore mediation by drug use in the Uganda cohort due to the rarity of drug use in that cohort. The Uganda cohort affords us the opportunity to explore alcohol use in the almost complete absence of other substance use.
This study is unique in its exploration of FI in Eastern Europe and its examination of FI and alcohol use in 2 very different contexts. The majority of participants in Uganda ARCH work in agriculture while residents in the St. Petersburg region live in a densely populated city. Although previous studies have explored FI in Mbarara, Uganda, this is the first study in that setting to investigate the association between alcohol use and FI. Despite the different cultural contexts, the prevalence of FI and alcohol use in these HIV-infected populations is very high and the estimated associations between FI and alcohol were similar.
Given food insecurity’s association with adverse outcomes in HIV-infected persons, further research that may inform strategies for mitigating FI and the adverse consequences associated with FI in these populations is warranted. It is unlikely however, given the absence of a significant association between heavy alcohol use and FI in both cohorts, that addressing alcohol consumption per se will ameliorate FI or its possible undesirable associations.
Weiser et al. (2012) explores competing demands for resources in a resource-poor setting in the context of the tradeoff between food and healthcare services [14]. Anema et al. (2010) found that injection drug users in British Columbia who spend more than $50 per day on drugs had increased odds of FI in adjusted analyses compared with those spending less than $50 per day on drugs [56]. It may be that the relative expenditure for alcohol in Uganda and Russia was not as high, which may in part explain the absence of the association of alcohol use and FI. In a study in Mbarara, Uganda among HIV-infected participants with alcohol use in the past year, Asiimwe et at. (2015) calculated that the median total expenditure on alcohol was less than $20 over the past 3 months combined [57].
Limitations
The Household Food Insecurity Access Scale was modified in the assessment administered to the Russia ARCH cohort. The modified questions ask only about a participant’s experience with FI rather than the experience of all members of the participant’s household, as this was perceived by Russian collaborators as a response that could be better assessed. This may result in misclassifying a participant in a food insecure household as food secure if, for example, another household member is forgoing food to aid the participant. In addition, the modified questions do not distinguish between eating less often, for example, due to a lack of resources versus other reasons. This may result in misclassifying a food secure participant as food insecure if they forgo food because of illness or other reasons rather than a lack of resources. These changes may affect the generalizability of our results with other household FI research. Assessing individual-level FI in the Russia ARCH cohort and household FI in the Uganda ARCH cohort may account for some of the differences seen in the 2 cohorts.
We cannot assess causality or directionality of associations due to the observational and cross-sectional design of this study. The outcome and main independent variables were acquired primarily via self-report. However, we believe that including the PEth biomarker in the Uganda ARCH sample addressed the area of most concern for underreporting, alcohol use in Uganda.
Conclusions
Despite FI and heavy alcohol use being very common in 2 HIV-infected ART-naïve cohorts in Mbarara, Uganda and St. Petersburg, Russia, these 2 characteristics, each associated in the literature with adverse HIV outcomes, were not significantly associated with each other.
Acknowledgments
We thank all Uganda ARCH and Russia ARCH subjects for their participation and our colleagues at First St. Petersburg Pavlov State Medical University, Mbarara Regional Referral Hospital, Mbarara University of Science and Technology, Boston University, Boston Medical Center, and University of California – San Francisco for their support. We also thank Marlene Lira for her translation of the abstract.
This project was supported by the National Institute on Alcohol Abuse and Alcoholism U01AA020776, U01AA020780, U24AA020778, U24AA020779, U01AA021989. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.FAO, IFAD and WFP. Meeting the 2015 international hunger targets: taking stock of uneven progress. Rome: FAO; 2015. The State of Food Insecurity in the World 2015. http://www.fao.org/3/a-i4646e.pdf. Accessed October 22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Guidance Note: Food and Nutrition. Geneva: UNAIDS; 2014. http://www.unaids.org/sites/default/files/media_asset/food-nutrition_en.pdf. Accessed October 22, 2015. [Google Scholar]
- 3.Life Sciences Research Office. Core indicators of nutritional state for difficult-to-sample populations. J Nutr. 1990;120(Suppl 11):1559–600. doi: 10.1093/jn/120.suppl_11.1555. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Nutrient Requirements for People Living with HIV/AIDS. Geneva: WHO; 2003. http://apps.who.int/iris/bitstream/10665/42853/1/9241591196.pdf?ua=1. Accessed 2003. October 22 2015. [Google Scholar]
- 5.The World Bank. A synthesis of international guidance. Washington, DC: World Bank; HIV/AIDS, Nutrition, and Food Security: What we can do. http://siteresources.worldbank.org/NUTRITION/Resources/281846-1100008431337/HIVAIDSNutritionFoodSecuritylowres.pdf. Accessed October 22, 2015. [Google Scholar]
- 6.UNAIDS. Policy Brief: HIV, Food Security, and Nutrition. 2008 http://www.unaids.org/sites/default/files/media_asset/jc1515_policy_brief_nutrition_en_1.pdf. Accessed October 22, 2015.
- 7.USAID. The Essential Role of Nutrition in the HIV and AIDS Response. Updated April 2015. https://www.usaid.gov/what-we-do/global-health/hiv-and-aids/technical-areas/essential-role-nutrition-hiv-and-aids-response. Accessed October 22, 2015.
- 8.Chen Y, Kalichman SC. Synergistic effects of food insecurity and drug use on medication adherence among people living with HIV infection. J Behav Med. 2015 Jun;38(3):397–406. doi: 10.1007/s10865-014-9612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalichman SC, Grebler T, Amaral CM, et al. Food insecurity and antiretroviral adherence among HIV positive adults who drink alcohol. J Behav Med. 2014 Oct;37(5):1009–1018. doi: 10.1007/s10865-013-9536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musumari PM, Wouters E, Kayembe PK, et al. Food insecurity is associated with increased risk of non-adherence to antiretroviral therapy among HIV-infected adults in the Democratic Republic of Congo: a cross-sectional study. PLoS One. 2014;9(1):e85327. doi: 10.1371/journal.pone.0085327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SY, Fanelli TJ, Jonas A, et al. Household food insecurity associated with antiretroviral therapy adherence among HIV-infected patients in Windhoek, Namibia. J Acquir Immune Defic Syndr. 2014;67(4):e115–22. doi: 10.1097/QAI.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benzekri NA, Sambou J, Diaw B, et al. High prevalence of severe food insecurity and malnutrition among HIV-infected adults in Senegal, West Africa. Plos One. 2015 Nov;10(11):e0141819. doi: 10.1371/journal.pone.0141819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke MF, Murray MB, Muñoz M, et al. Food insufficiency is a risk factor for suboptimal antiretroviral therapy adherence among HIV-infected adults in urban Peru. AIDS and behavior. 2011 Oct;15(7):1483–1489. doi: 10.1007/s10461-010-9789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiser SD, Tsai AC, Gupta R, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS. 2012 Jan;26(1):67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palermo T, Rawat R, Weiser SD, Kadiyala S. Food access and diet quality are associated with quality of life outcomes among HIV-infected individuals in Uganda. PLoS One. 2013;8(4):e62353. doi: 10.1371/journal.pone.0062353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiser SD, Bangsberg DR, Kegeles S, et al. Food insecurity among homeless and marginally housed individuals living with HIV/AIDS in San Francisco. AIDS Behav. 2009 Oct;13(5):841–8. doi: 10.1007/s10461-009-9597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiser SD, Yuan C, Guzman D, et al. Food insecurity and HIV clinical outcomes in a longitudinal study of urban homeless and marginally housed HIV-infected individuals. AIDS. 2013 Nov;27(18):2953–2958. doi: 10.1097/01.aids.0000432538.70088.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palar K, Kushel M, Frongillo EA, et al. Food insecurity is longitudinally associated with depressive symptoms among homeless and marginally-housed individuals living with HIV. AIDS Behav. 2015 Aug;19(8):1527–34. doi: 10.1007/s10461-014-0922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang EA, McGinnis KA, Fiellin DA, et al. Food insecurity is associated with poor virologic response among HIV-infected patients receiving antiretroviral medications. J Gen Intern Med. 2011 Sep;26(9):1012–1018. doi: 10.1007/s11606-011-1723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalichman SC, Cherry C, Amaral C, et al. Health and treatment implications of food insufficiency among people living with HIV/AIDS, Atlanta, Georgia. J Urban Health. 2010 Jul;87(4):631–641. doi: 10.1007/s11524-010-9446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009 Jan;24(1):14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiser SD, Palar K, Frongillo K, et al. Longitudinal assessment of associations between food insecurity, antiretroviral adherence and HIV treatment outcomes in rural Uganda. AIDS. 2014 Jan;28(1):115–120. doi: 10.1097/01.aids.0000433238.93986.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koss CA, Natureeba P, Nyafwono D, et al. Brief report: Food insufficiency Is associated with lack of sustained viral suppression among HIV-infected pregnant and breastfeeding Ugandan women. J Acquir Immune Defic Syndr. 2016 Mar 1;71(3):310–5. doi: 10.1097/QAI.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anema A, Chan K, Chen Y, Weiser S, Montaner JS, Hogg RS. Relationship between food insecurity and mortality among HIV-positive injection drug users receiving antiretroviral therapy in British Columbia, Canada. PLoS One. 2013;8(5):e61277. doi: 10.1371/journal.pone.0061277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiser SD, Young SL, Cohen CR, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. 2011 Dec;94(6):1729S–1739S. doi: 10.3945/ajcn.111.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiser SD, Palar K, Hatcher AM, et al. Food insecurity and health: A conceptual framework. In: Ivers L, editor. Food Insecurity and Public Health. 1st. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 27.Cole SM, Tembo G. The effect of food insecurity on mental health: panel evidence from rural Zambia. Soc Sci Med. 2011 Oct;73(7):1071–9. doi: 10.1016/j.socscimed.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Hadley C, Tegegn A, Tessema F, et al. Food insecurity, stressful life events and symptoms of anxiety and depression in east Africa: evidence from the Gilgel Gibe growth and development study. J Epidemiol Community Health. 2008 Nov;62(11):980–6. doi: 10.1136/jech.2007.068460. [DOI] [PubMed] [Google Scholar]
- 29.Heflin CM, Siefert K, Williams DR. Food insufficiency and women’s mental health: findings from a 3-year panel of welfare recipients. Soc Sci Med. 2005 Nov;61(9):1971–82. doi: 10.1016/j.socscimed.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Alaimo K, Olson CM, Frongillo EA. Family food insufficiency, but not low family income, is positively associated with dysthymia and suicide symptoms in adolescents. J Nutr. 2002 Apr;132(4):719–25. doi: 10.1093/jn/132.4.719. [DOI] [PubMed] [Google Scholar]
- 31.Anema A, Mehra D, Weiser S, et al. Drivers and consequences of food insecurity among illicit drug users. In: Watson RS, editor. Health of HIV Infected People: Food, Nutrition and Lifestyle with Antiretroviral Drugs [Volume 1] Elsevier Publishing Inc; 2015. [Google Scholar]
- 32.Normén L, Chan K, Braitstein P, et al. Food insecurity and hunger are prevalent among HIV-positive individuals in British Columbia, Canada. J Nutr. 2005 Apr;135(4):820–825. doi: 10.1093/jn/135.4.820. [DOI] [PubMed] [Google Scholar]
- 33.Cox J, Hamelin AM, McLinden T, et al. Food insecurity in HIV-hepatitis C virus co-infected individuals in Canada: The importance of co-morbidities. AIDS Behav. 2016 Feb; doi: 10.1007/s10461-016-1326-9. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regassa N, Stoecker BJ. Household food insecurity and hunger among households in Sidama district, southern Ethiopia. Public Health Nutr. 2012 Jul;15(7):1276–1283. doi: 10.1017/S1368980011003119. [DOI] [PubMed] [Google Scholar]
- 35.Dewing S, Tomlinson M, le Roux IM, Chopra M, Tsai AC. Food insecurity and its association with co-occurring postnatal depression, hazardous drinking, and suicidality among women in peri-urban South Africa. J Affect Disord. 2013 Sep;150(2):460–465. doi: 10.1016/j.jad.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eaton LA, Cain DN, Pitpitan EV, et al. Exploring the relationships among food insecurity, alcohol use, and sexual risk taking among men and women living in South African townships. J Prim Prev. 2014 Aug;35(4):255–265. doi: 10.1007/s10935-014-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang EA, McGinnis KA, Goulet J, et al. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep 2015. 2015 May-Jun;130(3):261–268. doi: 10.1177/003335491513000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiser SD, Leiter K, Bangsberg DR, et al. Food insufficiency is associated with high-risk sexual behavior among women in Botswana and Swaziland. PLos Med. 2007 Oct;4(10):1589–97. doi: 10.1371/journal.pmed.0040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swindale A, Bilinsky P. Development of a universally applicable household food insecurity measurement tool: process, current status, and outstanding issues. J Nutr. 2006 May;136(5):1449S–1452S. doi: 10.1093/jn/136.5.1449S. [DOI] [PubMed] [Google Scholar]
- 40.Coates J, Swindale A, Bilinsky P. Household food insecurity access scale (HFIAS) for measurement of household food access: Indicator guide (v. 3) Washington, D.C: 2007. http://www.fao.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hfias.pdf. Accessed October 23, 2015. [Google Scholar]
- 41.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998 Sep;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 42.Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003 Apr;163(7):821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 43.Bajunirwe F, Haberer JE, Boum Y, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLoS One. 2014;9(12):e113152. doi: 10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn JA, Fatch R, Kabami J, Mayanja B, Emenyonu NI, Martin J, Bangsberg DR. Self-report of alcohol use increases when specimens for alcohol biomarkers are collected in persons with HIV in Uganda. J Acquir Immune Defic Syndr. 2012 Dec;61(4):e63–64. doi: 10.1097/QAI.0b013e318267c0f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahn JA, Emenyonu NI, Fatch R, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction. 2016 Feb;111(2):272–9. doi: 10.1111/add.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: HumanaPress; 1992. [Google Scholar]
- 47.Sobell LC, Sobell MB. Alcohol Timeline Followback (TLFB) Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 48.National Institute of Alcohol Abuse and Alcoholism. Rethinking Drinking: Alcohol and Your Health. Rockville, MD: National Institutes of Health; 2015. http://pubs.niaaa.nih.gov/publications/RethinkingDrinking/Rethinking_Drinking.pdf. Accessed October 23, 2015. [Google Scholar]
- 49.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician’s Guide. Bethesda, MD: National Institutes of Health; 2007. Updated 2005 edition. [Google Scholar]
- 50.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 51.Fleishman JA, Sherbourne CD, Crystal S, et al. Coping, conflictual social interactions, social support, and mood among HIV-infected persons. HCSUS consortium. Am J Community Psychol. 2000;28(4):421–453. doi: 10.1023/a:1005132430171. [DOI] [PubMed] [Google Scholar]
- 52.Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001 Dec;54(Suppl 1):S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 53.Stuart EA, Marcus SM, Horvitz-Lennon MV, Gibbons RD, Normand SL. Using non-experimental data to estimate treatment effects. Psychiatr Ann. 2009 Jul;39(7):41451. doi: 10.3928/00485713-20090625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanza ST, Moore JE, Butera NM. Drawing causal inference using propensity scores: A practical guide for community psychologists. Am J Community Psychol. 2013;54(3–4):380–92. doi: 10.1007/s10464-013-9604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin DY, Zeng D. On the relative efficiency of using summary statistics versus individual-level data in meta-analysis. Biometrika. 2010 Jun;97(2):321–32. doi: 10.1093/biomet/asq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anema A, Wood E, Weiser SD, Qi J, Montaner JS, Kerr T. Hunger and associated harms among injection drug users in an urban Canadian setting. Subst Abuse Treat Prev Policy. 2010;5:20. doi: 10.1186/1747-597X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asiimwe SB, Fatch R, Emenyonu NI. Comparison of traditional and novel self-report measures to an alcohol biomarker for quantifying alcohol consumption among HIV-infected adults in Sub-Saharan Africa. Alcohol Clin Exp Res. 2015;39(8):1518–27. doi: 10.1111/acer.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]


