Abstract
3-acetylpyridine (3-AP) is a metabolic antagonist used in research to decrease levels of nicotinamide (niacinamide) in laboratory animals. The administration of 3-AP followed by nicotinamide to rats leads to the selective destruction of neurons in the medial inferior olive, resulting in a loss of climbing fibers innervating cerebellar Purkinje cells and a consequent ataxia manifest by alterations in both balance and gait. Although 3-AP has also been administered to mice to destroy neurons in the inferior olive, there are limited studies quantifying the consequent effects on balance, and no studies on gait. Further, the relationship between 3-AP-induced lesions of the inferior olive and behavior has not been elucidated. Because 3-AP continues to be used for experiments involving mice, this study characterized the effects of this toxin on both balance and gait, and on the neuronal integrity of several brain regions involved in motor coordination. Results indicate that C57BL/6 mice are less sensitive to the neurotoxic effects of 3-AP than rats, and a dose more than 6.5 times that used for rats produces deficits in both balance and gait comparable to those in rats. This dose led to a significant (p< 0.05) loss of NeuN(+) neurons in several subregions of the inferior olive including the rostral medial nucleus, dorsomedial cell column, ventrolateral protrusion, and cap of Kooy. Further, the number of NeuN(+) neurons in these subregions, with the exception of the dorsomedial cell column, was significantly (p<0.05) related to rotorod performance, implicating their involvement in this behavior.
Keywords: 3-acetylpyridine, mouse, gait, ataxia, balance, inferior olive
1. Introduction
3-acetylpyridine (3-AP), also known as methyl β-pyridyl ketone, is an analogue of nicotinic acid (niacin) containing a methyl group in place of the hydroxyl. When administered to rats, 3-AP functions as a metabolic antagonist, leading to decreased levels of nicotinamide (niacinamide) and consequent inhibition of NAD+-dependent reactions. Early studies investigating the effects of 3-AP in rats indicated that a single dose of 65 mg/kg, administered intraperitoneally (i.p.), led to a near total destruction of both the inferior olive and nucleus ambiguus, as well as lesions of the substantia nigra and dorsal raphe nucleus, and degenerating fibers in the solitary tracts (Desclin and Escubi, 1974; Sotelo et al., 1975). However, when nicotinamide (300 mg/kg) was administered 3.5 hours after administration of the toxin (70-75 mg/kg, i.p.), a greater than 90% loss of inferior olivary neurons (Rondi-Reig et al., 1997), predominantly in the rostral medial accessory olive (Seoane et al., 2005; Wecker et al., 2013), was apparent leaving the hippocampus intact (Rondi-Reig et al., 1997) and sparing other nuclei (Seoane et al., 2005). Further, experiments demonstrated that nicotinamide could also protect rats from the lethality of 3-AP with an optimal survival rate when administered 3.5 hours after the toxin (Jones et al., 1994). Characterization of motor behavior following the administration of 70-75 mg/kg 3-AP (i.p.) followed at 3.5 hours by 300 mg/kg nicotinamide (i.p.) demonstrated that rats exhibited impaired balance on both a stationary and rotating rod (Gasbarri et al., 2003; Rondi-Reig et al., 1997; Wecker et al., 2013), and decreased velocity and distance moved in an open field (Wecker et al., 2013). Further, animals exhibited an altered gait characterized by excess flexion and extension of the hindlimbs (Watanabe et al., 1997), decreased stride length and increased stride width of both forelimbs and hindlimbs (Seoane et al., 2005; Wecker et al., 2013), and increased stride frequency, braking duration, step and paw angle, with decreased stride, swing and propulsion durations and paw area (Lambert et al., 2014). Thus, findings strongly support the idea that the toxin-induced degeneration of inferior olivary neurons, resulting in the loss of climbing fibers innervating cerebellar Purkinje cells in the rat (Hodgson et al., 2015), underlies the motor incoordination observed in rats following 3-AP administration.
Although the effects of 3-AP have been well investigated in the rat, it is unclear whether similar effects are present in mice, the vertebrate species most commonly used for research. The earliest studies investigating the effects of 3-AP in mice reported that doses ranging from 114-450 mg/kg (i.p.) led to lesions in the hypothalamus, hippocampus, amygdala and inferior olive (Coggeshall and Maclean, 1958; Hicks, 1955; Montgomery and Christan, 1976) indicating that high doses of the toxin can lead to damage in multiple brain regions. Further, surviving mice had a ‘high-stepping’ gait (Coggeshall and Maclean, 1958), mild weakness of the hindlimbs (Montgomery and Christan, 1976), decreased activity in the open field (Ozaki et al., 1983), and difficulty coordinating movements (Ikeda et al., 1993). Because the administration of 3-AP by itself led to non-selective changes in several brain regions, it was impossible to relate behaviors to neuronal integrity in a specific brain region until nicotinamide was administered after 3-AP to mice to restrict the lesion to the inferior olive (Caddy and Vozeh, 1997), as had been done for rats. These investigators reported that the administration of 3-AP (200 mg/kg, i.p.) followed by nicotinamide (300 mg/kg, i.p.) at 3 hours led to the selective degeneration of inferior olivary neurons in Lurcher mutant mice, but wild-type mice were unaffected; unfortunately, motor behavior was not assessed. Similarly, the administration of 3-AP (75 mg/kg, i.p.) followed by harmaline (15 mg/kg, i.p.) to increase neuronal firing, and nicotinamide (300 mg/kg, i.p.) to C57BL/6 mice led to neuronal depletion throughout the rostrocaudal inferior olive (Zhang et al., 2003), and the administration of the toxin (500 mg/kg, i.p.) followed by nicotinamide (500 mg/kg, i.p.) at 3 hours led to lesions of the inferior olive (Shutoh et al., 2006), but again, motor behavior was not assessed in either of these studies.
Sillitoe et al. (2003) reported that BALB/c mice exhibited both ataxia and the degeneration of ‘most’ (sic) neurons in the inferior olive at 7-11 days following the administration of 50 mg/kg 3-AP (i.p.), although quantitative data were not presented and it is unclear that lesions were limited to the inferior olive. Further, Kotajima et al. (2014) administered this dose of 3-AP (i.p.) followed at 3-3.5 hours with 500 mg/kg (i.p.) nicotinamide to C57BL/6 mice to restrict the lesion, and reported that mice could not maintain their balance on the rotorod and exhibited a 50% loss of cells in the inferior olive. In contrast, several studies have indicated that doses of 500 mg/kg 3-AP followed by 500 mg/kg nicotinamide are necessary to lead to degeneration of inferior olivary neurons in C57BL/6 mice (Katoh et al., 1998; Shutoh et al., 2006) suggesting that mice may be less sensitive than rats to the neurotoxic effects of 3-AP, an idea supported by findings more than 60 years ago indicating that mice can metabolize 3-AP to nicotinamide (Kaplan et al., 1954).
Thus, several unanswered questions remain regarding the behavioral and neurotoxic effects of 3-AP in mice. The goals of the present study were to determine: 1) the effects of 3-AP on rotorod balance and neuronal integrity of several brain regions involved in motor coordination; 2) whether impaired rotorod performance was related to the loss of neurons in a specific brain region; and 3) whether a dose of 3-AP that altered rotorod performance and neuronal integrity also affected spatial and temporal gait parameters.
2. Materials and Methods
2.1. Animals and chemicals
Mice (12 BALB/c and 76 C57BL/6) obtained from Jackson Laboratories (Bar Harbor, ME) were 7 weeks of age upon arrival and were housed 4 per cage on a 12 hour light:dark cycle (6 am:6 pm) in a pathogen-free vivarium maintained at 21-23°C with 40-50% humidity. Cages (polycarbonate rectangular box, 340 × 190 × 130 mm) were lined with Harlan Teklad pelleted paper bedding (Envigo, Madison, WI) and enriched with a polycarbonate domed igloo (100 mm diameter × 60 mm tall). Mice had ad libitum access to both food (Harlan Teklad Global 2018 18% Protein Rodent Diet) and water, and the care and use of animals were approved by the University of South Florida Institutional Animal Care and Use Committee in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were conducted in accordance with ARRIVE guidelines. Mice were acclimated to the vivarium for at least 1 week prior to experimentation, and all behavioral assessments were conducted between 8 am and 1 pm.
All chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise noted.
2.2. Behavioral assessments
A rotorod (Rotamex 5, Columbus Instruments, Columbus, OH) was used to assess balance and motor coordination. For comparison between strains of mice, a 3 cm diameter spindle rod was used. To reduce variability in baseline performance, mice were familiarized to the procedure by placing them on the rod rotating at 20 rpm for a maximal time of 180 sec with 3 trials/day for 3 consecutive days. Baseline performance was determined on days 4 and 7 (with 3 trials/day) using an accelerating paradigm in which mice were placed on the rod rotating at 4 rpm with an acceleration rate of 0.2 revolutions/sec, achieving a speed of 40 rpm during a 180 sec trial. Baseline latency for each animal was expressed as the average of the 3 longest times the animal could remain on the rod during both assessment days. The day following baseline determinations, mice received injections (i.p.) of 3-AP [50-75 mg/kg in phosphate-buffered saline (PBS; 7.7 mM Na2HPO4, 2.7 mM NaH2PO4 in 0.9% NaCl, pH=7.4)] followed at 3.5 hours by nicotinamide (300 mg/kg dissolved in PBS), and were reassessed on the rotorod 9 and 10 days later using the same paradigm and measures as for baseline determinations; control animals received 2 injections of PBS.
For further rotorod studies with C57BL/6 mice, animals were trained for 5 days (4 trials/day) using an accelerated speed paradigm with a 7 cm diameter spindle to prevent passive rotations, thereby increasing the difficulty of the task. For all training days, animals were placed on the rod at rest with the acceleration rate set to 0.1 revolution/sec. The maximum rotational speed was set to 5 rpm on day 1, to 10 rpm on days 2 and 3, and to 15 rpm on days 4 and 5. Animals were allowed to remain on the rod for a maximum of 300 sec. Baseline performance was measured on days 8 and 9 (4 trials/day with a 180 sec maximum) with the rod rotating at a fixed speed of 10 rpm. On day 10, animals received injections (i.p.) of 3-AP (500 mg/kg) followed at 3.5 hours by nicotinamide (500 mg/kg), and were reassessed on the rotorod 6 and 7 days later using the same paradigm as for baseline determinations; control animals received 2 injections of PBS. To characterize impairment following training, the percent of trials (from a total of 8) for which each animal was able to remain on the rod for 180 sec was determined. Although latency to fall is the most common measure used to assess rotorod performance with decreases characterizing impairment, lesions induced by 3-AP administration produce considerable variability in latency to fall both within trials and across animals, reducing statistical power (Wecker et al., 2013). Therefore, the present study decreased the variability in performance across trials by training mice to a 180 sec criterion prior to impairment and characterizing performance using the percent of trials meeting the 180 sec criterion for each mouse. This approach prevented trial-to-trial fluctuations in the latency to fall from distorting the characterization of the typical performance for each mouse and was better able to capture impairment following the administration of 3-AP by reducing variability and increasing statistical power.
Gait was assessed using an automated treadmill system (DigiGait™ Imaging System, Mouse Specifics, Boston, MA). Mice were trained on the treadmill at a speed of 24 cm/sec for 5 consecutive days with 1 trial/day for a 1 min duration. On the third day after training, baseline performance was assessed on 2 consecutive days with a 1 min trial per day at a treadmill speed of 24 cm/sec. On the day after the second baseline assessment, mice received 2 injections (i.p.) 3.5 hours apart of: PBS + PBS; 200 mg/kg 3-AP + 200 mg/kg nicotinamide; 350 mg/kg 3-AP + 350 mg/kg nicotinamide; or 500 mg/kg 3-AP + 500 mg/kg nicotinamide. Gait was reassessed at weekly intervals for 5 weeks. An individual blinded to the group assignment of each animal, isolated and analyzed video clips for each animal containing an uninterrupted sequence of 12-14 strides. Based on findings from studies with rats (Lambert et al., 2014; Lambert et al., 2015), 4 primary spatial factors and their associated variability (stride length, stance width, paw angle, paw area at peak stance), and 3 primary temporal factors (propulsion, braking and swing time durations), as well as the maximal rate of change of paw area in contact with the treadmill during the braking phase were determined using DigiGait™ Imaging software (version 12.2) as described (Lambert et al., 2014). Parameters were determined for both hindpaws and forepaws. Further, analyses using paired t-tests revealed that the left and right limbs did not differ significantly (p>0.05) on any measure; therefore, left and right limb measurements were combined for both the forelimbs and the hindlimbs.
2.3. Histological analyses
The neuronal marker NeuN was used to determine whether the administration of 3-AP affected the number of neurons in the inferior olive, striatum, hippocampus, motor cortex or pedunculopontine nucleus, brain regions implicated in gait and balance recovery following motor impairment (Holschneider et al., 2013). Studies have demonstrated that NeuN is expressed in the inferior olive in adult mice (Fu and Watson, 2012; Yu et al., 2014) and can be used as a measure of neuronal survival, supported by evidence that the number of NeuN(+) neurons is inversely correlated with the number of Fluoro-Jade labeled dying cells in the inferior olive following 3-AP administration (Sierra et al., 2003).
Brains from mice that were trained and baseline assessed on the rotorod prior to the administration (i.p.) of 3-AP (500 mg/kg) followed at 3.5 hours by nicotinamide (500 mg/kg), and assessed on the rotorod on days 6 and 7 following toxin administration were analyzed. On days 8 or 9, mice were anesthetized with 325 mg/kg pentobarbital (i.p.; Euthasol®, Virbac AH, Fort Worth, TX) and perfused transcardially with filtered normal saline, followed by filtered perfusion solution (0.1 M sodium phosphate buffer containing 4% paraformaldehyde and 4% sucrose; pH=7.2-7.4). Mice were decapitated and the heads stored in this solution in the fume hood overnight at room temperature. Following fixation, whole brains were removed and cryoprotected in filtered PBS containing 10%, 20%, or 30% sucrose over 3 days, respectively. Brains were embedded in gelatin containing 10% sucrose, and placed in 4% paraformaldehyde (in filtered PBS) for 4 hours. Gelatin-embedded brains were stored in 30% sucrose (in filtered PBS) at 4°C until samples were saturated. Gelatin blocks were frozen on dry ice and brains were sectioned (40 μm) in the coronal plane. Sections were stored in cryoprotectant at -20°C until processed for immunohistochemistry.
Free-floating slices were washed to remove cryoprotectant, incubated in borate buffer (pH=8.0) for 30 min in a water bath at 80°C, and rewashed and incubated in PBS containing 3% H2O2 for 20 min at room temperature. All washes were in PBS for 5 min on an orbital shaker with gentle rotation, and were repeated 5 times. Sections were subsequently incubated in PBS containing 10% normal goat serum and 0.3% Triton X-100 for 60 min at room temperature followed by washing. Sections were incubated overnight at 4°C with anti-NeuN antibody (clone EPR12763, 1:5000; ab177487, Abcam, Inc., Cambridge, MA) in PBS containing 3% normal goat serum and 0.1% Triton X-100. After washing, sections were incubated for 60 min at room temperature in PBS containing 3% normal goat serum, 0.1% Triton X-100, and biotinylated goat anti-rabbit antibody (1:300; BA-1000, Vector Laboratories, Inc., Burlingame, CA). The Vectastain Elite ABC kit (Vector Laboratories, Inc.), and SIGMAFAST™ 3, 3’-diaminobenzidine tablets were used to visualize NeuN(+) cells according to manufacturer’s instructions. Sections were mounted, dried overnight, and rehydrated immediately prior to counterstaining with Vector® Hematoxylin QS (Vector Laboratories, Inc.). Following counterstaining, mounted sections were dehydrated and cover slipped.
Slices were imaged using a Leica DM2500 (Leica Microsystems, Buffalo Grove, IL) and the number of NeuN(+) neurons from one hemisphere were quantified using Fiji (Schindelin et al., 2012). For the inferior olivary complex, images were obtained at 100X magnification from every third coronal section between -6.76 to -7.96 mm anterior:posterior (A:P) relative to bregma; the entire olive was captured on each section at this magnification. All images were converted to black and white (binary) and “watershed” segmentation was used to separate groups of particles. The 3 major nuclei comprising the inferior olive (medial, principal, and dorsal) and their neuronal subgroups were outlined as defined for C57BL/6J mice (Paxinos and Franklin, 2004), and automated particle quantification was used to include cells in the size range 60-160 μm2 with a circularity of 0.2-1.0. This procedure was validated by comparing the automated results to those counted manually by an observer blinded to the treatment groups using 42 sections from 3 brain regions. Statistical comparison using Cronbach’s α indicated that the automated and manual counting procedures produced nearly identical results (α = 0.999).
For the striatum, motor cortex and pedunculopontine nucleus, images were captured at 200X magnification. For striatum, representative images were obtained from the dorsomedial, dorsolateral, and ventrolateral striatum using a coronal section +0.75 mm (A:P) relative to bregma. For the motor cortex, images from layers 1, 2/3, 5, 6a and 6b were obtained using a coronal section +0.95 mm (A:P) relative to bregma. For the pedunculopontine nucleus, images were obtained from a coronal section -4.28 mm (A:P) relative to bregma. All images were converted to black and white, “watershed” segmentation was used to separate groups of particles, and automated particle quantification was used to include cells with an area ≥72.5 μm2 (250 pixels) and a circularity of 0.2-1.0.
For the hippocampus, images of CA1, CA3 and the dentate gyrus were captured at 400× magnification due to high cell density. NeuN(+) neurons in each of these regions were analyzed from a coronal section +2.26 mm (A:P) relative to bregma. Despite the high magnification, automated particle quantification could not differentiate between individual cells. Therefore, an individual blinded to the group assignment of each animal manually counted the number of NeuN(+) neurons.
2.4. Statistical analyses
All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Significant differences between mouse strains were determined using a two-way analysis of variance (ANOVA) (strain × treatment), and individual group differences determined by the Student t-test. To determine whether 500 mg/kg 3-AP affected balance, data were analyzed by a one-way ANOVA, and individual group differences determined by Tukey’s multiple comparisons. Cell counts from control and 3-AP-injected animals were compared using the Student t-test. Relationships between cell counts in subregions of the inferior olive and latency to fall off the rotorod were assessed using Spearman’s rho (r). Gait parameters were analyzed by a two-way ANOVA (time × treatment) and Dunnett’s test was used to determine significance for alterations across time. In those instances where a significant interaction was present, differences between control and toxin-impaired animals were determined using Fisher’s Least Significant Difference (LSD) test. The criterion for significance was p<0.05 for all comparisons.
3. Results
3.1. Comparison of BALB/c and C57BL/6 mice
Studies in the literature suggest that the dose of 3-AP required to affect balance may differ for different mouse strains. Thus, initial experiments compared the effects of 3-AP on rotorod performance of BALB/c and C57BL/6 mice. These studies used 50 mg/kg 3-AP followed by nicotinamide, which has been reported to impair rotorod performance in C57BL/6 mice (Kotajima et al., 2014), and 70-75 mg/kg followed by nicotinamide, a dose reported to impair rotorod performance in rats (Lambert et al., 2014; Wecker et al., 2013). Only animals who would remain on the rod during familiarization were used for study (8/12 BALB/c and 12/12 C57BL/6 mice). As shown in Fig. 1, performance of BALB/c and C57BL/6 mice differed significantly (p<0.05) at baseline with average latencies for the former of 98.3 ± 4.3 sec, and those for the latter of 134 ± 8.3 sec, 36% longer than the BALB/c mice. Results also indicate that neither dose of 3-AP affected the ability of mice to maintain balance on the rotorod when assessed at 9 and 10 days following toxin administration. Thus, doses of 3-AP that lead to impaired motor behavior in rats do not affect either BALB/c or C57BL/6 mice. Irrespective of this lack of effect of 3-AP, results also indicate that latency on the rotorod differs significantly between these mouse strains [F(1,30)=24.96, p<0.05] with C57BL/6 mice remaining on the rod longer than BALB/c mice. Because C57BL/6 mice performed better than BALB/c mice, they were chosen for further study.
Fig. 1.

Comparison of rotorod performance of BALB/c and C57BL/6 mice prior to and following the administration of 3-AP. Mice from each strain were familiarized to the rotorod for 3 days (using a 3 cm spindle at a fixed speed of 14 rpm, 3 trials/day, 180 sec maximum/trial). Baseline measures (3 trials/day separated by a minimum of 5 min between trials) were obtained on days 4 and 7 using an accelerating paradigm during which the speed of the rod increased from 4 to 40 rpm in 180 sec (at a rate of 0.2 revolutions/sec). On day 8, animals received 2 injections (i.p.) 3.5 hrs apart of: PBS + PBS (Controls); 50 mg/kg 3-AP + 300 mg/kg nicotinamide; or 70-75 mg/kg 3-AP + 300 mg/kg nicotinamide. Mice were reassessed on the rotorod at 9 and 10 days following toxin administration using the same accelerating paradigm as for the baseline measures. The average latency to remain on the rotorod was determined for each animal using the 3 longest times from the 6 trials. Bars represent the mean + s.e.m.; the number of animals in each group is in parentheses. *Significantly (p<0.05) different from corresponding values manifest by BALB/c mice.
3.2. Effects of 3-AP on balance of C57BL/6 mice
Based on studies indicating that a relatively high dose of 3-AP (500 mg/kg 3-AP followed by 500 mg/kg nicotinamide) is necessary to damage neurons in the inferior olive of mice (Katoh et al., 1998; Shutoh et al., 2006), rotorod performance was assessed using these doses. For this experiment, mice were trained for 5 days to reach maximal performance, baseline determined, and performance reassessed 1 week following toxin administration. Only those mice who could maintain their balance on the rod for 180 sec for 5 of the 8 trials at baseline were used for study (14/20 mice). Results (Fig. 2) indicate that 500 mg/kg 3-AP followed by 500 mg/kg nicotinamide at 3.5 hours led to a significant 27% decrease in the % of trials completed by mice, i.e., the number of trials (out of 8) for which mice remained on the rod for 180 sec [F(2,25)=11.48, p<0.05]. Further, a chi-square analysis of the performance of toxin-injected mice on days 6 or 7 post 3-AP indicated no differences in the frequency of completed trials between the first, second, third or fourth trial [χ2(3) = 0.377, p>0.05], evidence that the toxin-induced impairment was not a result of increased fatigue. Thus, the rotorod performance of C57BL/6 mice was significantly impaired following a dose of 3-AP more than 6.5 times the dose necessary to impair rotorod performance in rats (Wecker et al., 2013).
Fig. 2.

Effects of 3-AP on rotorod performance of C57BL/6 mice. Mice were trained on the rotorod for 5 days (4 trials/day; 300 sec maximum/trial) using an accelerating speed paradigm with a 7 cm diameter spindle to prevent passive rotations. For training, animals were placed on the rod at rest, and the acceleration rate was set to 0.1 revolution/sec with a maximum rotational speed set to 5 rpm on day 1, to 10 rpm on days 2 and 3, and to 15 rpm on days 4 and 5. Baseline performance was measured (4 trials/day; 180 sec maximum/trial with a minimum of 5 min between trials) on days 8 and 9 with the rod rotating at a fixed speed of 10 rpm. On day 10, animals received 2 injections (i.p.) 3.5 hrs apart of PBS + PBS (Controls) or 500 mg/kg 3-AP + 500 mg/kg nicotinamide, and were reassessed on the rotorod 6 and 7 days later using the same paradigm as for baseline determinations. The percent of trials (from a total of 8) for which each animal was able to remain on the rod for 180 sec was determined. Bars represent the mean + s.e.m.; the number of animals in each group is in parentheses. *Significantly (p<0.05) different from baseline values.
3.3. Histochemical analyses
The number of NeuN(+) cells in the striatum (dorsomedial, dorsolateral and ventrolateral), pedunculopontine nucleus, hippocampus (CA1, CA3, and dentate gyrus), and motor cortex (layers 1, 2/3, 5, 6a and 6b) from C57BL/6 mice who received 500 mg/kg 3-AP + 500 mg/kg nicotinamide and were assessed for rotorod performance did not differ from controls (Table 1). In contrast, the number of NeuN(+) cells in several subregions within the inferior olive were altered by the toxin. Representative sections from a control mouse with subregions outlined are shown in Fig. 3. The subregions in which significant (p<0.05) differences in NeuN staining between control and toxin-injected mice (Table 2) included the rostral medial nucleus (14% and 26% decreases at depths of -6.76 and -7.00 mm, respectively), dorsomedial cell column (a 76% decrease at a depth of -7.00 mm), cap of Kooy (57% and 72% decreases at depths of -7.36 and -7.60 mm, respectively), ventrolateral protrusion (a 52% decrease at a depth of -7.36 mm), and subnucleus B (an 18% increase at a depth of -7.96 mm). Representative sections from a control and a toxin-injected mouse and bar graphs depicting changes in the number of neurons in regions from toxin-injected animals relative to controls are shown in Fig. 4.
Table 1.
Effects of 3-AP on the number of NeuN(+) cells
| Brain Region | Subregion | Total Cell Counts | |
|---|---|---|---|
| Controls | 3-AP | ||
| Striatum | Dorsomedial | 873 ± 47 | 941 ± 72 |
| Dorsolateral | 961 ± 34 | 928 ± 45 | |
| Ventrolateral | 1122 ± 53 | 1102 ± 54 | |
| Pedunculopontine Nucleus | 602 ± 27 | 650 ± 25 | |
| Hippocampus | CA1 | 602 ± 27 | 650 ± 25 |
| CA3 | 160 ± 16 | 160 ± 12 | |
| Dentate Gyrus | 141 ± 25 | 118 ± 10 | |
| Primary Motor Cortex | Layer 1 | 44.6 ± 3.1 | 54.1 ± 7.0 |
| Layer 2/3 | 855 ± 74 | 983 ± 94 | |
| Layer 5 | 738 ± 30 | 714 ± 47 | |
| Layer 6a | 793 ± 89 | 919 ± 92 | |
| Layer 6b | 172 ± 18 | 190 ± 31 |
Brains obtained from mice assessed for rotorod performance following the administration of 500 mg/kg 3-AP + 500 mg/kg nicotinamide were processed for NeuN immunohistochemistry. The number of NeuN(+) cells in representative samples from each region were analyzed and quantified as described in the methods. Values represent means of determinations in samples from 6-8 mice/group ± s.e.m.
Brains obtained from mice assessed for rotorod performance following the administration of 500 mg/kg 3-AP + 500 mg/kg nicotinamide were processed for NeuN immunohistochemistry. The number of NeuN(+) cells in representative samples from each region were analyzed and quantified as described in the methods. Values represent means of determinations in samples from 6-8 mice/group ± s.e.m.
Fig. 3.

Subregions of the inferior olive in sections from control mice. Representative sections outlined in white from -6.76 to -7.96 A:P mm relative to bregma from a control mouse are shown and labeled as per the nomenclature of Paxinos and Franklin (2004). Sections were processed for NeuN(+) immunohistochemistry using a monoclonal anti-NeuN antibody as described in the Methods.
Table 2.
Effects of 3-AP on the number of NeuN(+) cells in the inferior olive
| Section Depth | Total Cell Counts
|
||
|---|---|---|---|
| A:P from Bregma (mm) | Inferior Olive Subregion | Controls | 3-AP |
| -6.76 | Medial Nucleus | 61.5 ± 3.4 | 52.7 ± 2.8* |
| Principal Nucleus | 54.3 ± 1.0 | 57.6 ± 6.3 | |
| Dorsal Nucleus | 58.6 ± 4.0 | 63.3 ± 2.5 | |
| -7.00 | Medial Nucleus | 79.3 ± 2.3 | 59.0 ± 7.6* |
| Principal Nucleus | 136.7 ± 3.8 | 144.8 ± 7.4 | |
| Dorsal Nucleus | 109.3 ± 2.8 | 112.5 ± 5.7 | |
| Dorsomedial Cell Group | 51.7 ± 4.5 | 51.1 ± 2.5 | |
| Dorsomedial Cell Column | 11.1 ± 3.4 | 2.7 ± 2.6* | |
| -7.36 | Principal Nucleus | 79.5 ± 7.3 | 66.0 ± 7.7 |
| Dorsal Nucleus | 108.8 ± 8.2 | 101.8 ± 8.2 | |
| Subnucleus B | 54.4 ± 3.5 | 62.6 ± 6.1 | |
| Subnucleus C | 37.8 ± 4.3 | 42.2 ± 3.0 | |
| Ventrolateral Protrusion | 17.0 ± 2.4 | 8.2 ± 1.6* | |
| Beta Subnucleus | 47.3 ± 5.8 | 49.3 ± 4.8 | |
| Cap of Kooy | 31.0 ± 9.1 | 13.2 ± 3.6* | |
| -7.60 | Dorsal Nucleus | 19.8 ± 2.7 | 16.7 ± 0.8 |
| Subnucleus B | 52.3 ± 10.2 | 52.5 ± 4.3 | |
| Subnucleus C | 157.2 ± 9.2 | 144.1 ± 4.1 | |
| Beta Subnucleus | 35.3 ± 7.1 | 24.4 ± 6.6 | |
| Cap of Kooy | 11.5 ± 3.3 | 3.2 ± 1.1* | |
| Subnucleus A | 61.6 ± 4.1 | 66.1 ± 5.3 | |
| -7.96 | Subnucleus B | 59.2 ± 2.8 | 69.8 ± 4.9* |
| Subnucleus C | 10.0 ± 2.7 | 6.2 ± 0.8 | |
| Subnucleus A | 70.8 ± 11.0 | 71.0 ± 4.8 | |
Brains obtained from mice assessed for rotorod performance following the administration of 500 mg/kg 3-AP + 500 mg/kg nicotinamide were processed for NeuN immunohistochemistry. The number of NeuN(+) cells in the major nuclei comprising the inferior olive (medial, principal, and dorsal) and their neuronal subgroups were quantified using Fiji (Schindelin et al., 2012). Values represent means of determinations from samples from 4-8 mice/group ± s.e.m.
The asterisks denote significant (p<0.05) differences from controls determined by the Student t-test.
Fig. 4.
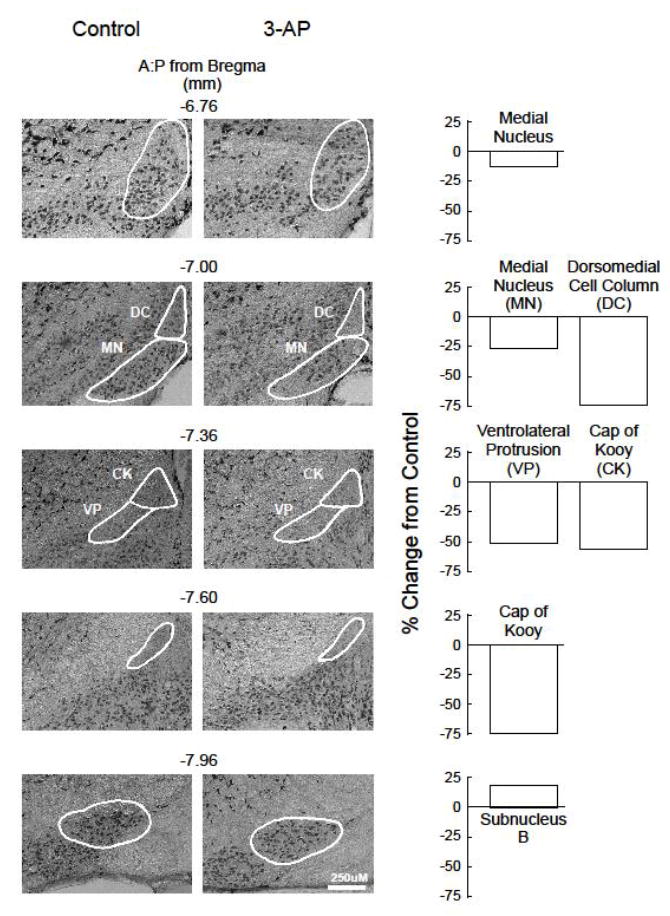
Effects of 3-AP on NeuN(+) immunohistochemistry in subregions of the inferior olive. Brains from control and toxin-injected mice (500 mg/kg 3-AP) were obtained 1-2 days following assessment of rotorod performance (see Fig. 2 legend), and sections were processed for NeuN(+) immunohistochemistry using a monoclonal anti-NeuN antibody. Representative sections from control and toxin-injected animals are shown for those subregions demonstrating significant (p<0.05) differences between groups, with group means from toxin-injected animals expressed as % change from controls depicted in the bar graphs. NeuN(+) cell counts in each subregion from the inferior olive are presented in Table 2.
To ascertain whether the number of cells in these regions was related to rotorod performance, a correlational analysis was performed between NeuN(+) cell counts and the latency to fall off the rotorod using data from all animals. Significant (p<0.05) relationships were observed between neurons in the rostral medial nucleus at section depths of -6.76 and -7.00 mm (r=0.50 and 0.53, respectively), the ventrolateral protrusion at a depth of -7.36 mm (r=0.57), and the cap of Kooy at a depth of -7.60 mm (r=0.54); no significant relationships were noted between the number of NeuN(+) cells in either the dorsomedial cell column at a depth of -7.00 (r=0.41) or in subnucleus B at a depth of -7.96 mm (r=-0.53).
3.4. Effects of 3-AP on gait of C57BL/6 mice
An automated treadmill system was used to determine whether the administration of 3-AP affected spatial or temporal gait parameters. Experiments investigated the effects of 200, 350 and 500 mg/kg 3-AP followed by corresponding doses of nicotinamide; only mice capable of completing 12-14 uninterrupted strides at baseline were used for study (38/44). Mice who received the two lower doses of 3-AP followed by nicotinamide did not exhibit any significant alterations in any gait parameter. In contrast, the administration of 500 mg/kg 3-AP (followed by 500 mg/kg nicotinamide), which led to the loss of NeuN(+) cells in several subregions within the inferior olive, led to significant changes in both spatial and temporal parameters. Spatial gait parameters are shown in Fig.5. For hindpaw stride length, a two-way ANOVA indicated a significant interaction between treatment and time [F(5,140)=3.993, p<0.05] with controls exhibiting significant (p<0.05) 5-6% increases at weeks 3-5; stride length did not change significantly over time for toxin-injected animals. In addition, there were no significant differences in stride length between groups. Hindpaw stride length variability decreased throughout the 5 weeks for both groups, but this decrease was not statistically significant and no differences were noted between groups. Hindpaw stance width increased significantly over time for control and 3-AP-injected mice [F(5,140)=9.133, p<0.05], with a significant (p<0.05) 8% increase at weeks 4 and 5 for controls, and significant (p<0.05) 10-14% increases at weeks 1-5 for toxin-injected mice; no significant differences were apparent between groups. Further, hindpaw stance width variability was irregular and did not differ between groups. Hindpaw angle was very stable across 5 weeks for the controls, whereas the administration of 3-AP led to a significant [F(5,140)=9.713, p<0.05] 33-44% increase relative to baseline, and paw angle manifest by toxin-injected mice was significantly (p<0.05) different from controls at all times after baseline. Similarly, hindpaw angle variability manifest by the controls did not change over time, but increased significantly [F(5,140)=2.838, p<0.05] by 26-51% following the administration of 3-AP. The maximal hindpaw area at peak stance increased significantly over time by 8-14% at weeks 2-5 for the controls [F(5,140)=3.696, p<0.05]. In contrast, the maximal hindpaw area at peak stance for toxin-injected mice exhibited significant (p<0.05) 6-8% decreases at weeks 1, 3 and 4. All hindpaw areas at peak stance for toxin-injected mice were significantly (p<0.05) less than the controls at all times following baseline. Hindpaw area variability for the controls did not change significantly over time, whereas variability for 3-AP-injected mice increased significantly [F(5,140)=4.306, p<0.05] by 25-34% beginning 2 weeks post toxin administration.
Fig. 5.
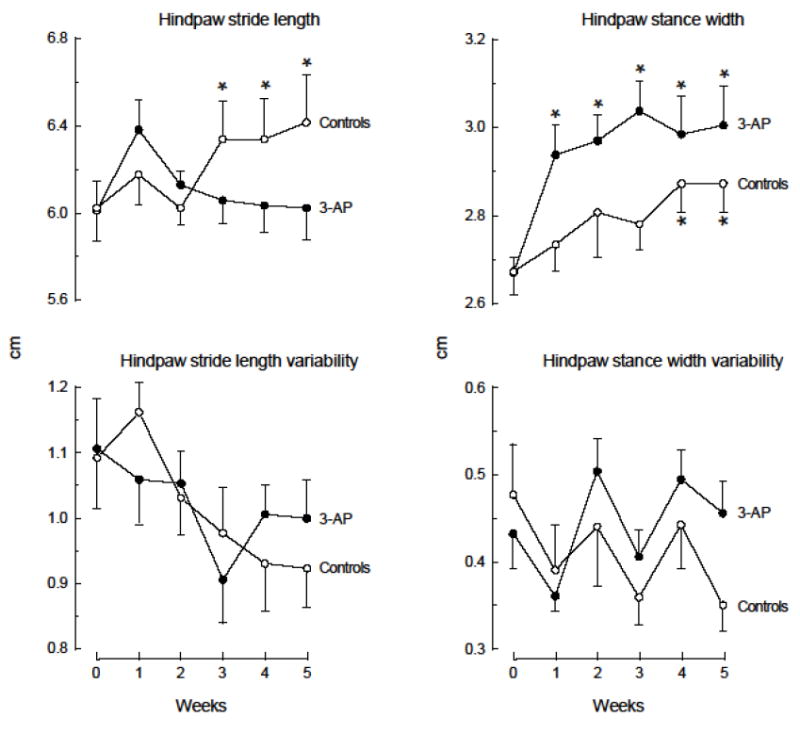
Effects of 3-AP on spatial gait parameters of C57BL/6 mice. Mice were trained on the treadmill at a speed of 24 cm/sec for 5 consecutive days (1 trial/day, 1 min/trial). On the third day after training (week 0), baseline performance was determined for 2 consecutive days using the same paradigm as for training. The day following the second baseline assessment, mice received 2 injections (i.p.) 3.5 hrs apart of PBS + PBS (Controls) or 500 mg/kg 3-AP + 500 mg/kg nicotinamide (3-AP). Mice were reassessed on the treadmill at weekly intervals for 5 weeks using the same paradigm. Values represent the mean +/- s.e.m. of determinations from 13 (Controls) or 17 (3-AP) mice per group. *Significantly (p<0.05) different from control values at baseline (week 0). **Significantly (p<0.05) different from corresponding control group values.
The effects of 3-AP on hindpaw temporal gait parameters are shown in Fig.6. Hindpaw propulsion, which represents the major component of stance time, was significantly altered over time for both control and toxin-injected mice, albeit in opposite directions [F(5,140)=6.795, p<0.05]. Propulsion manifest by the controls increased significantly (p<0.05) by approximately 10% at weeks 3-5, whereas propulsion manifest by 3-AP-injected mice decreased significantly (p<0.05) by 5% at weeks 2 and 4. Further, propulsion at weeks 3-5 differed significantly (p<0.05) between groups. There was a significant time effect for hindpaw swing [F(5,140)=3.870, p<0.05) with controls exhibiting a significant (p<0.05) 9% increase at week 5, and toxin-injected mice exhibiting significant (p<0.05) 8-14% increases at all times after baseline. Hindpaw braking decreased significantly over time for the controls [F(5,140)=5.357, p<0.05] with significant (p<0.05) 10-12% decreases at weeks 3-5; toxin-injected mice did not exhibit any significant alterations in hindpaw braking relative to baseline, but there was a significant (p<0.05) difference in brake time between toxin-injected mice and controls at 2 weeks. The maximal rate of change of paw contact with the treadmill during the braking phase, commonly referred to as limb loading maximum, was significantly altered in both groups of mice over time [F(5,140)=6.314, p<0.05]. Controls exhibited significant (p<0.05) 10-15% increases at weeks 2-5, whereas toxin-injected mice exhibited significant (p<0.05) 8-16% decreases beginning at week 1 after toxin injection. Further, the limb loading maximum was significantly (p<0.05) different between the controls and toxin-injected mice at all times after baseline. No significant changes were noted for either temporal or spatial forepaw gait parameters.
Fig. 6.
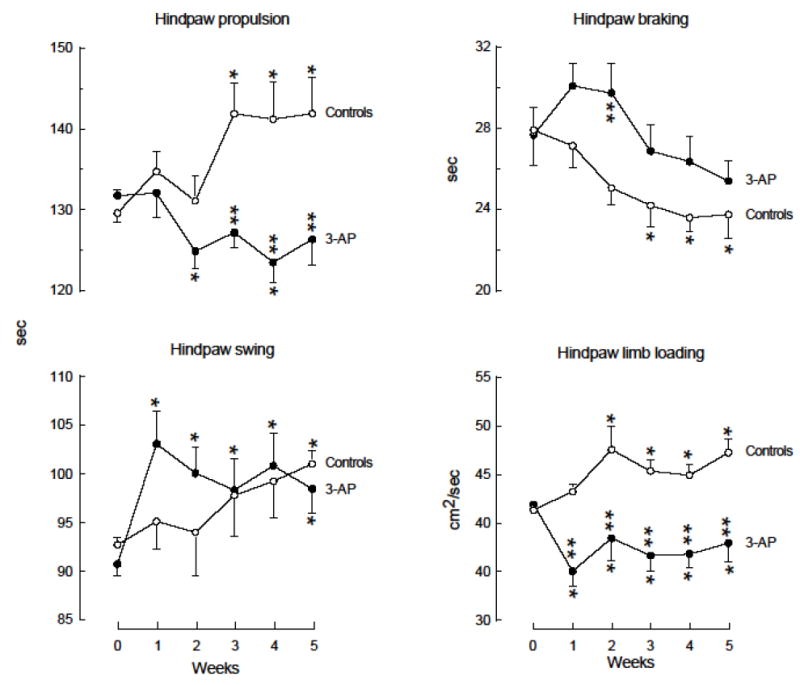
Effects of 3-AP on temporal gait parameters of C57BL/6 mice. Mice were trained and assessed on the treadmill, and received injections of 3-AP as described for Fig. 5. Values represent the mean +/- s.e.m. of determinations from 13 (Controls) or 17 (3-AP) mice per group. *Significantly (p<0.05) different from control values at baseline (week 0). **Significantly (p<0.05) different from corresponding control group values.
Discussion
The goals of this study were to determine the effects of 3-AP on balance and neuronal integrity of several brain regions involved in motor coordination in mice, determine whether impaired balance was related to the loss of neurons in a specific brain region, and characterize the effects of the toxin on gait. Results indicate that the administration of 3-AP to C57BL/6 mice led to deficits in both rotorod performance and gait that were similar to, but of a smaller magnitude than those reported for rats at a dose that was 6.5 times greater than that used for rats. In addition, this dose of 3-AP led to a significant (p<0.05) loss of NeuN(+) neurons in the rostral medial nucleus, dorsomedial cell column, ventrolateral protrusion, and cap of Kooy, with cell number in the rostral medial nucleus, ventrolateral protrusion and cap of Kooy, but not the dorsomedial cell column, correlated with the ability to maintain balance on the rotorod.
Although the original goal of this study was to extend findings that a relatively low dose of 3-AP (50 mg/kg) led to ataxia and lesions in BALB/c mice (Sillitoe et al., 2003), we could not detect alterations in rotorod performance using this dose of 3-AP, either alone (data not shown) or followed by nicotinamide. In addition, based on evidence that this dose of 3-AP led to total impairment of rotorod performance and a loss of cells in the inferior olive in C57BL/6 mice (Kotajima et al., 2014), we began using this strain of mice, but again, could not detect any alterations in rotorod performance. Thus, although the initial intent of these studies was not to compare mouse strains, results underscore differences in baseline rotorod behavior between C57BL/6 and BALB/c mice. The finding that C57BL/6 mice were better able to maintain their balance on the rod than BALB/c mice is supported by studies indicating that the former are also better than many other inbred strains on this task including C3HeB/FeJ, DBA/2J and 129/SnlmJ mice (Tarantino et al., 2000) and A/J, BALC/cByJ, C3H/HeJ, CBA/J, DBA/2J and FVB/NJ mice (McFadyen et al., 2003). This knowledge is critical for the design and interpretation of any study investigating motor behavior in mice.
Evidence that a dose of 500 mg/kg 3-AP impaired the balance of C57BL/6 mice on the rotorod and led to lesions within the inferior olive extends findings of others reporting that this dose of 3-AP led to a 35% loss of neurons in the medial nuclei cell groups in the vicinity of the cap of Kooy (Katoh et al., 1998; Shutoh et al., 2006). The present study demonstrated a loss of cells in the rostral medial nucleus, dorsomedial cell column, cap of Kooy and ventrolateral protrusion. These regions are involved with limb movement and the integration of vestibular and optokinetic information (Leonard et al. 1988; Barmack et al., 1993; Kaufman, 1996; De Zeeuw et al., 1998; Cerminara and Apps, 2011; Lee et al., 2014). Not surprisingly, with the exception of the dorsomedial cell column, a reduction in cell number in these regions was associated with reduced performance on the rotorod. These findings do not agree with the report of a 50% loss of cells in the inferior olive and a total loss of balance following the administration of 50 mg/kg 3-AP (Kotajima et al., 2014). Further, additional experiments (data not shown) assessed rotorod performance of C57BL6 mice after the administration of 100-150 mg/kg 3-AP followed 3 hours later by harmaline (15 mg/kg) to increase blood flow or followed by harmaline and nicotinamide (300 mg/kg) 90 minutes later; however, neither regimen affected rotorod performance. It is difficult to reconcile these findings with either those in the present study or others in the literature indicating that much higher doses of 3-AP are needed to cause neuronal loss in the brain of mice (Caddy and Vozeh, 1997; Katoh et al., 1998; Shutoh et al., 2006). Perhaps an error was made in either calculating or reporting the dose of 3-AP used (Kotajima et al., 2014). It is also possible that the behavioral paradigm used may be responsible for the divergent results. Kotajima at al. (2014) habituated mice to the rotorod for 120 seconds before testing acquisition of the task, implying that motor learning may have been involved in determining performance and that the lower dose of 3-AP impaired such. In contrast, for the current study, mice were trained extensively prior to toxin administration and mice who could not meet a defined criterion were not included for further study; thus, no learning was involved in performance.
In addition to alterations in rotorod performance, 3-AP also led to both spatial and temporal gait changes in mice. This is the first study to report gait alterations in mice as a consequence of 3-AP-induced lesions of the inferior olive. In general, although changes in gait in mice following the administration of 3-AP paralleled those observed in rats with significant increases in stance width and paw angle and decreases in paw area at peak stance and propulsion, the magnitude of these changes was much less in mice than rats, despite the administration of a higher dose (Lambert et al., 2014). These results underscore differences in the sensitivity of rats and mice to the neurotoxic effects of 3-AP, even though both species exhibit lesions of the inferior olive following administration of the toxin.
A noteworthy difference between gait measurements in rats and mice following administration of 3-AP is that rats exhibit a 12% decrease in swing duration (Lambert et al., 2014; Lambert et al., 2015) while mice exhibit a 15% increase in swing duration. Control rats and mice both exhibit increased swing over time, likely reflecting the increased stride length that occurs with experience on the treadmill. Although the decreased swing manifest by toxin-injected rats is accompanied by decreased stride length, this is not true for toxin-injected mice who exhibit increased swing and no changes in stride length. These findings suggest that rats adapt differently than mice to the effects of 3-AP. Further experiments investigating possible relationships between gait impairment and lesion location will determine whether gait and balance deficits are mediated via the same circuitry or cerebellar modules in rats and mice (Cerminara and Apps, 2011).
Highlights.
3-acetylpyridine leads to changes in both balance and gait in mice
mice are less sensitive to the neurotoxic effects of 3-acetylpyridine than rats
3-acetylpyridine led to a loss of neurons several subregions within the inferior olive
rotorod performance was related to neuronal loss in the rostral medial nucleus, ventrolateral protrusion, and cap of Kooy
Acknowledgments
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS072114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors contributed in a significant manner to the studies reported and have read and approved the final manuscript.
Abbreviations
- 3-AP
3-acetylpyridine
- i.p
intraperitoneal
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barmack NH, Fagerson M, Fredette BJ, Mugnaini E, Shojaku H. Activity of neurons in the beta nucleus of the inferior olive of the rabbit evoked by natural vestibular stimulation. Exp Brain Res. 1993;94:203–225. doi: 10.1007/BF00230288. [DOI] [PubMed] [Google Scholar]
- Caddy KW, Vozeh F. The effect of 3-acetylpyridine on inferior olivary neuron degeneration in Lurcher mutant and wild-type mice. Eur J Pharmacol. 1997;330:139–142. doi: 10.1016/s0014-2999(97)01030-3. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Apps R. Behavioural significance of cerebellar modules. Cerebellum. 2011;10:484–494. doi: 10.1007/s12311-010-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Maclean PD. Hippocampal lesions following administration of 3-acetylpyridine. Proc Soc Exp Biol Med. 1958;98:687–689. doi: 10.3181/00379727-98-24152. [DOI] [PubMed] [Google Scholar]
- Desclin JC, Escubi J. Effects of 3-acetylpyridine on the central nervous system of the rat, as demonstrated by silver methods. Brain Res. 1974;77:349–364. doi: 10.1016/0006-8993(74)90627-1. [DOI] [PubMed] [Google Scholar]
- DeZeeuw CI, Simpson JI, Hoogenraad CC, Galjart N, Koekkoek SKE, Ruigrok TJH. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- Fu YH, Watson C. The arcuate nucleus of the C57BL/6J mouse hindbrain is a displaced part of the inferior olive. Brain Behav Evol. 2012;79:191–204. doi: 10.1159/000335032. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Pompili A, Pacitti C, Cicirata F. Comparative effects of lesions to the ponto-cerebellar and olivo-cerebellar pathways on motor and spatial learning in the rat. Neuroscience. 2003;116:1131–1140. doi: 10.1016/s0306-4522(02)00780-7. [DOI] [PubMed] [Google Scholar]
- Hicks SP. Pathologic effects of antimetabolites. I. Acute lesions in the hypothalamus, peripheral ganglia, and adrenal medulla caused by 3-acetyl pyridine and prevented by nicotinamide. Am J Pathol. 1955;31:189–199. [PMC free article] [PubMed] [Google Scholar]
- Hodgson E, Roe RM, Mailman RB, Chambers JE. In: Dictionary of Toxicology. Hodgson ERR, Mailman RB, Chambers JE, editors. Elsevier; London: 2015. pp. 4–5. [Google Scholar]
- Holschneider DP, Guo Y, Wang Z, Roch M, Scremin OU. Remote brain network changes after unilateral cortical impact injury and their modulation by acetylcholinesterase inhibition. J Neurotrauma. 2013;30:907–919. doi: 10.1089/neu.2012.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Morita I, Murota S, Sekiguchi F, Yuasa T, Miyatake T. Cerebellar nitric oxide synthase activity is reduced in nervous and Purkinje cell degeneration mutants but not in climbing fiber-lesioned mice. Neurosci Lett. 1993;155:148–150. doi: 10.1016/0304-3940(93)90694-g. [DOI] [PubMed] [Google Scholar]
- Jones N, Le Marec N, Stelz T, Caston J. Effect of administration of 3-acetylpyridine followed by niacinamide injection on survival, extent of the inferior olivary complex lesion, and response to harmaline in the young rat. Brain Res. 1994;656:257–262. doi: 10.1016/0006-8993(94)91468-0. [DOI] [PubMed] [Google Scholar]
- Kaplan NO, Goldin A, Humphreys SR, Ciotti MM, Venditti JM. Significance of enzymatically catalyzed exchange reactions in chemotherapy. Science. 1954;120:437–440. doi: 10.1126/science.120.3116.437. [DOI] [PubMed] [Google Scholar]
- Katoh A, Kitazawa H, Itohara S, Nagao S. Dynamic characteristics and adaptability of mouse vestibulo-ocular and optokinetic response eye movements and the role of the flocculo-olivary system revealed by chemical lesions. Proc Natl Acad Sci U S A. 1998;95:7705–7710. doi: 10.1073/pnas.95.13.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman GD. Activation of immediate early genes by vestibular stimulation. Ann N Y Acad Sci. 1996;781:437–442. doi: 10.1111/j.1749-6632.1996.tb15718.x. [DOI] [PubMed] [Google Scholar]
- Kotajima H, Sakai K, Hashikawa T, Yanagihara D. Effects of inferior olive lesion on fear-conditioned bradycardia. Neuroreport. 2014;25:556–561. doi: 10.1097/WNR.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CS, Philpot RM, Engberg ME, Johns BE, Kim SH, Wecker L. Gait analysis and the cumulative gait index (CGI): Translational tools to assess impairments exhibited by rats with olivocerebellar ataxia. Behav Brain Res. 2014;274:334–343. doi: 10.1016/j.bbr.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CS, Philpot RM, Engberg ME, Johns BE, Wecker L. Analysis of gait in rats with olivocerebellar lesions and ability of the nicotinic acetylcholine receptor agonist varenicline to attenuate impairments. Behav Brain Res. 2015;291:342–350. doi: 10.1016/j.bbr.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RX, Huang J-J, Huang C, Tsai M-L, Yen C-T. Collateral projections from vestibular nuclear and inferior olivary neurons to lobules I/II and IX/X of the rat cerebellar vermis: a double retrograde labelling study. Eur J Neurosci. 2014;40:2811–2821. doi: 10.1111/ejn.12648. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Simpson JI, Graf W. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. I. Typology of inferior olive neurons of the dorsal cap of Kooy. J Neurophysiol. 1988;60:2073–2090. doi: 10.1152/jn.1988.60.6.2073. [DOI] [PubMed] [Google Scholar]
- McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Christan EL. Pathologic effects of antimetabolites on the hippocampus of diet controlled mice. Brain Res Bull. 1976;1:255–259. doi: 10.1016/0361-9230(76)90095-2. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Murakami TH, Shimada M. Learning deficits on avoidance task and hippocampal lesions in area CA3 following intraperitoneal administration of 3-acetylpyridine. J Neurosci Res. 1983;10:425–435. doi: 10.1002/jnr.490100409. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain. Academic Press; London, U.K: 2004. [Google Scholar]
- Rondi-Reig L, Delhaye-Bouchaud N, Mariani J, Caston J. Role of the inferior olivary complex in motor skills and motor learning in the adult rat. Neuroscience. 1997;77:955–963. doi: 10.1016/s0306-4522(96)00518-0. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane A, Apps R, Balbuena E, Herrero L, Llorens J. Differential effects of trans-crotononitrile and 3-acetylpyridine on inferior olive integrity and behavioural performance in the rat. Eur J Neurosci. 2005;22:880–894. doi: 10.1111/j.1460-9568.2005.04230.x. [DOI] [PubMed] [Google Scholar]
- Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Sierra A, Azcoitia I, Garcia-Segura L. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocrine. 2003;21:43–51. doi: 10.1385/endo:21:1:43. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Benson MA, Blake DJ, Hawkes R. Abnormal dysbindin expression in cerebellar mossy fiber synapses in the mdx mouse model of Duchenne muscular dystrophy. J Neurosci. 2003;23:6576–6585. doi: 10.1523/JNEUROSCI.23-16-06576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Hillman DE, Zamora AJ, Llinas R. Climbing fiber deafferentation: its action on Purkinje cell dendritic spines. Brain Res. 1975;98:574–581. doi: 10.1016/0006-8993(75)90374-1. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome. 2000;11:555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kinoshita K, Koguchi A, Yamamura M. A new method for evaluation of motor deficits in 3-acetylpyridine-treated rats. J Neurosci Methods. 1997;77:25–29. doi: 10.1016/s0165-0270(97)00104-0. [DOI] [PubMed] [Google Scholar]
- Wecker L, Engberg ME, Philpot RM, Lambert CS, Kang CW, Antilla JC, Bickford PC, Hudson CE, Zesiewicz TA, Rowell PP. Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia. Neuropharmacology. 2013;73:75–86. doi: 10.1016/j.neuropharm.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fu Y, Watson C. The inferior olive of the C57BL/6J mouse: a chemoarchitectonic study. Anat Rec (Hoboken) 2014;297:289–300. doi: 10.1002/ar.22866. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Forster C, Milner TA, Iadecola C. Attenuation of activity-induced increases in cerebellar blood flow by lesion of the inferior olive. Am J Physiol Heart Circ Physiol. 2003;285:H1177–1182. doi: 10.1152/ajpheart.00240.2003. [DOI] [PubMed] [Google Scholar]


