Abstract
After 30 years of characterisation and implementation, fluid percussion injury (FPI) is firmly recognised as one of the best-characterised, reproducible and clinically relevant models of TBI, encompassing concussion through diffuse axonal injury (DAI). Depending on the specific injury parameters (e.g. injury site, mechanical force), FPI can model diffuse TBI with or without a focal component, and may be designated as mild to severe according to the chosen mechanical forces and resulting acute neurological responses. Among FPI models, midline FPI may best represent clinical diffuse TBI, because of the acute behavioural deficits, the transition to late-onset behavioural morbidities, and the absence of gross histopathology. The goal here was to review acute and chronic physiological and behavioural deficits and morbidities associated with diffuse TBI induced by midline FPI. In the absence of neurodegenerative sequelae associated with focal injury, there is a need for biomarkers in the diagnostic, prognostic, predictive and therapeutic approaches to evaluate outcomes from TBI. The current literature suggests that midline FPI offers a clinically-relevant, validated model of diffuse TBI to investigators wishing to evaluate novel therapeutic strategies in the treatment of TBI and the utility of biomarkers in the delivery of healthcare to patients with brain injury.
Keywords: midline, central, diffuse, brain injury, biomarker
Introduction
Experimental models of traumatic brain injury (TBI) have been developed to reproduce many of the clinical sequelae of human TBI, and have traditionally played a crucial role in evaluating and understanding the physiological, behavioural, and histopathological consequences of TBI, with a view towards developing novel therapeutic strategies for this devastating disease. Since human TBI is a markedly heterogeneous disease, no single animal model of TBI can reproduce the entire spectrum of clinical TBI features and symptoms1. Rather, the implementation of distinct, yet complementary, models is necessary to effectively represent the range and types of clinical TBI (e.g. focal, diffuse, multi-focal, haemorrhagic)2. Over the past half-century, experimental TBI models have markedly expanded our insight into the post-traumatic sequelae, particularly with respect to the delayed and progressive secondary cellular and molecular cascades that are initiated by the traumatic event3. Experimental results have led to the continued development of diagnostic tools and treatment strategies, which have either become part of standard clinical practice or remain under intense pre-clinical and clinical investigation. Primarily for the treatment of severe TBI, experimental results have refined the timing of decompressive craniectomy to alleviate intracranial pressure (ICP)4,5. Similarly, experimental studies on injury pathophysiology have informed intensive care unit (ICU) monitoring of mean arterial pressure, intracranial pressure, cerebral blood flow and oxygen and glucose metabolism6–8.
This review provides a succinct overview of one widely used variation of experimental mechanically-induced TBI in whole animals – midline fluid percussion injury (FPI) as a model for diffuse TBI. We make repeated reference to lateral FPI for comparison with midline FPI, since neither model has been completely described, as no model could ever be. Overwhelmingly, rodents have been selected as the species of choice in experimental TBI, due to several overt advantages: small size, accelerated lifespan, modest cost, and extensive normative data. While larger animals have been incorporated in FPI studies, for this review we focus on midline FPI in the rodent.
Considerations in modelling TBI
All TBI begins with the application of mechanical force to the head or brain, which initiates systemic and cellular processes that are hallmarks of the disease9. The applied mechanical forces (hydraulic pressure in the FPI model) are controlled to ensure reproducibility of the device within a specified performance range (see Figure 1). By raising the pendulum height, greater mechanical forces can be applied to the brain that exacerbate the resulting neurological, physiological, behavioural and pathological outcomes. Additionally, the direction or angle of the application of forces can alter these outcomes. Direct relationships between applied mechanical force and outcome measures indicate a continuum of injury severities. Over the years, injury severity for experimental TBI has been categorized as mild, moderate, and severe according to both mechanical parameters of the injury and the magnitude of resulting neuro-behavioural, cellular, molecular, and histopathological changes in an attempt to correlate to the clinical condition, the magnitude of post-traumatic responses, and the rate of recovery. However, severe experimental TBI does not necessarily equate with severe clinical TBI, considering that the need for neurosurgical and critical care is not warranted or possible for rodents. Unlike humans, for all injury severities, rodents resume breathing, gain reflexive responses and ambulate shortly after mild-moderate injury. Within hours, animals are feeding, grooming and require little to no intervention. Rodents with more severe brain injuries may require subcutaneous fluids, softened food, and light husbandry to account for acute post-injury symptoms, but nothing approximating the extent of care required for severe brain-injured humans. Nonetheless, the principal benefit of experimental models of TBI is the reproducibility achieved by employing standardized surgical protocols and the inclusion of uninjured (sham) animals as a control for systemic variables (e.g., anaesthesia, surgical procedure, temperature) as a reference for post-traumatic outcome assessment. These standardized protocols also provide a reference within the same laboratory and inform laboratories at other institutions as to the parameters necessary to reproduce the results.
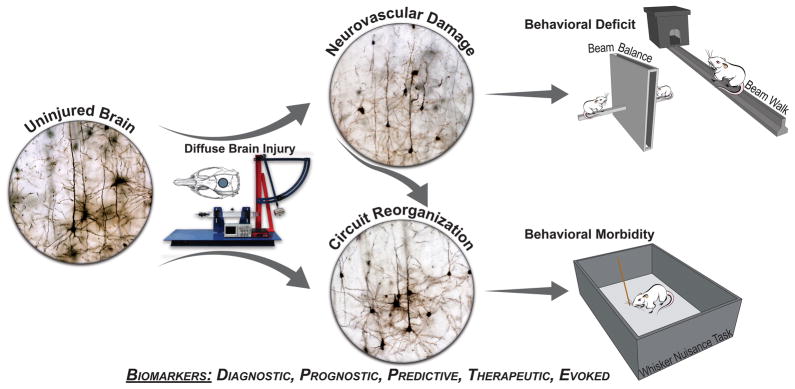
Figure 1.
Illustration of midline fluid percussion injury (FPI) as a model of diffuse brain injury, in which neurovascular damage to the brain can lead to behavioral deficits, and transition to behavioral morbidity as damaged circuits undergo reorganisation. Golgi-stained neurons from uninjured and braininjured brains show intact neuropil, truncation of neuronal processes, and then a burst of arborisation resulting in circuit reorganisation. Experimental diffuse TBI is induced by midline FPI (device shown) delivered through a craniectomy centered on the sagittal suture. Behavioral data indicate that midline FPI results in acute deficits (e.g. beam balance, beam walk), which transition into chronic morbidities (e.g. sensory sensitivity of the whiskers). Biomarkers across all categories (e.g. diagnostic, prognostic, predictive, therapeutic, evoked) support pre-clinical investigation of the natural course of the disease and evaluation of therapeutic efficacy.
Types of injury
It is generally accepted that human TBI is not a single pathophysiological entity, and in individual cases distinct and heterogeneous radiological lesions may or may not be evident1. The pathophysiology of human TBI ranges from multifocal, microscopic lesions undetectable by conventional imaging (effectively defining diffuse brain injury) to overt haemorrhage and oedema dispersed over circumscribed regions or hemispheres. In this way, human TBI necessarily requires both a quality (e.g. diffuse, focal, haemorrhagic) and severity (e.g. mild, moderate, severe) to capture the accurate diagnosis of a particular patient with TBI. In particular, diffuse brain injuries result from tissue distortion and shear at the moment of injury that are induced by inertial forces exceeding a defined threshold10. As a result, cerebral brain injury emerges from the physiologic disruption, diffuse axonal pathology, and possible petechial haemorrhages scattered throughout the brain parenchyma, depending on injury severity. Pathology typically localizes at tissue interfaces with mismatched biomechanical properties, such as the grey-white matter interface, cerebral blood vessels, and genu or gyri. By their nature, diffuse injuries are difficult to detect in the clinical setting, but occur with the highest prevalence and across a wide range of injury severity in the human population11,12. In contrast, focal TBI involves lacerations and contusions, often, but not always, accompanied by skull fracture and/or hematoma13. At the focal site of mechanical impact to the head or brain, cortical cavitation or atrophy may occur and progress into subcortical structures, particularly at higher magnitudes of injury14. To encompass this range of clinical TBI phenotypes, different injury models and their variations have been developed and characterised. The FPI model affords sufficient flexibility to induce diffuse tissue damage, without overt tissue destruction, by selecting an impact site along the mid-sagittal cranial suture (see Figure 1).
Fluid percussion models of TBI in rodents
FPI is among the most well characterised and commonly used of all TBI pre-clinical models, having originated as early as the 1940s and adapted to rodents in the late 1980s15–18. From its inception to its adaptation to rodents, FPI involved production of a midline injury and was modified shortly thereafter to the lateral injury site in its latest variation in 198719. Using this model, brain injury is induced by a 20 msec fluid pulse delivered onto the intact dura through a craniectomy20, defining this technique as a model of TBI rather than closed head injury. The intended clinical and pathological outcomes from FPI can be produced by varying the placement of the craniectomy, for example. If the craniectomy lies along the midline suture, the result is a diffuse brain injury in both hemispheres21,22, whereas a lateral craniectomy results in a combination of focal brain injury with a diffuse component18,19. Additionally, shifting between parasagittal and lateral craniectomy locations can induce more subtle, but detectable differences, specifically in the presence and size of lesion development, deficits in cognitive performance, hippocampal cell loss and reactive astrocytosis23,24. While FPI procedures necessitate breaching the cranial vault, the skull is sealed to the injury device, recreating a closed system at the time of injury. Midline FPI therefore can successfully model a closed head injury with decompressive craniectomy to accommodate the herniation of the brain through the skull defect, thus controlling intracranial pressure. Most FPI protocols do not include cranioplasty, rather electing to allow for natural fibrosis to occur over the skull defect.
Certain concessions and assumptions exist in studies involving FPI. As expanded upon below, rodents subjected to FPI regain gross neurological function within 2 to 20 minutes depending on injury severity, with little intervention. Therefore, it is generally acknowledged that FPI most closely models mild-moderate TBI, with a Glasgow coma score (GCS) of 9–13, in which patients are generally responsive, but likely disoriented. The magnitude and duration of behavioural performance impairments and histopathological damage depends on the initial injury severity25,26. As with most experimental TBI models, FPI studies have primarily been conducted in male rodents to reflect the 3:1 demographics of TBI in males over females11,12. The focus on male subjects eliminates confounding effects of hormone cycling females, but overlooks any gender-related effects on pathophysiology or therapeutic efficacy27. Sex can differentially affect outcome from TBI, likely due to levels of circulating sex hormones28–31, which encourages inclusion of female rodents in experimental study designs to increase the translational predictability of TBI models. Furthermore, although TBI is the leading cause of death and disability in children in the United States, most TBI related research is conducted in adults and then later translated to the paediatric population. Procedures for FPI can be adapted for juvenile animals, by adjusting craniotomy size and impact forces32,33.
The remainder of this review will focus on the range of acute deficits and chronic morbidities that are evident with midline FPI, in addition to the necessity and utility of biomarkers in study designs using pre-clinical models of TBI.
Acute injury-induced deficits
We define a post-traumatic neurological deficit as a transient consequence of TBI-related pathology that impairs brain activity and behavioural function, including cognitive, motor, and sensory domains, which naturally recover, in part, over time post-injury. Deficits are most pronounced for neurological function subserved by domains within close proximity to the injury. As pathological and reparative processes come into balance, albeit a balance offset from baseline function, neurological function is expected to plateau. Animals subjected to midline FPI express characteristic neurological deficits immediately after injury that typically resolve or plateau with time as a function of injury severity.
Immediate transient deficits
Upon impact from FPI, the neurological reflexes of the injured animal are immediately suppressed, including corneal, pinnae, and righting reflexes. Amongst these, the righting reflex serves as a sensitive, non-invasive evaluation tool in which duration of suppression correlates with injury severity19,34,35. Whether the suppressed reflexes involve a loss of consciousness remains an unresolved argument, since consciousness remains difficult, if not impossible, to assess in rodents. Reflex suppression may be accompanied by apnoea and post-traumatic seizure, where duration and prevalence may also correlate with injury severity, but definitive results are not yet available. More recently, the fencing response, believed to be associated with neurochemical and pathological events in the lateral vestibular nucleus of the brainstem, has been identified as a visible forearm posturing indicative of a TBI that exceeds an injury severity threshold for a mild injury in both experimental (mFPI) and clinical TBI35. In this way, the acute neurological responses to TBI in rodents model the intent of GCS to capture initial neurophysiological function versus injury-induced deficits in the wake of injury, and more faithfully reflect injury severity than the physical parameters and settings of the device itself.
Physiological responses to midline FPI are immediately evident when assessing cerebrovascular changes. Following injury, a severity-dependent pattern of hypertension and elevated intracranial pressure (ICP) has been reported36. The increased ICP is accompanied by an increase in mean arterial pressure (MAP), irregular breathing, and reduced heart rate, known as the Cushing reflex. Furthermore, cerebral blood flow (CBF) after lateral FPI shows an initial global suppression within one hour post-injury and a more persistent focal reduction at the trauma site, both of which resolve within 4–24 hours post-injury,18,37 which have not been demonstrated for midline FPI. Concomitant with physiological alterations, the blood brain barrier (BBB) becomes permeable after midline FPI, initially at the injury site, but possibly progressing to other brain regions, particularly in the cervicomedullary junction38. However, cerebrovascular changes evident early after midline FPI in rodents typically resolve within hours to days post-injury.
After lateral FPI, potassium efflux from mechanically damaged cells releases excitatory amino acids (EAAs), which then activate glutamate receptors and secondary calcium signalling pathways. In re-establishing homeostasis, an initial period of glucose hyper-metabolism that occurs following lateral FPI is followed by a period of glucose hypo-metabolism39,40. Although the time course of glucose utilization may vary between human and animal models, both follow a similar sequence of events41. Sustained post-traumatic glucose hypo-metabolism correlates with injury severity and may render the recovering brain vulnerable to secondary insults42–44. Detailed, acute cerebral metabolic studies have yet to be conducted for midline FPI.
As an extension of physiological deficits, prevailing hypotheses suggest that sleep may aid in cellular repair and be beneficial in recovery following injury. After FPI, sleep significantly increases for up to 6 hours regardless of injury severity or when during the day the injury occurred45. The temporal profile of secondary injury cascades, particularly inflammatory cascades46, may be driving the significant increase in post-traumatic sleep, because inflammatory cytokines may act as sleep-regulatory substances47. Similarly, pyrogenic molecules, which partially overlap with sleep regulatory substances, can induce post-traumatic fever48,49. Post-traumatic sleep and fever could contribute to or confound the natural course of recovery, because their biological functions include restoration, conservation, and adaption of cellular processes50. Further studies are warranted to fully understand the cellular benefit or detriment, if any, of acute post-traumatic physiological responses to TBI51,52.
Acute behavioural deficits
Acute motor deficits, similar to those observed in humans, have been reported in rodents following FPI using tests of neuromotor function such as the beam balance, beam walk, rotarod, and inclined plane tests (see Figure 1)51,53–55. Cognitive deficits, both learning and memory, have been evaluated in rats using tests such as the radial arm maze and the Morris water maze (MWM) task56. Further discrimination of retrograde and anterograde amnesia may be achieved by training animals prior to or following the injury, respectively53. Following midline FPI, these neurobehavioural deficits are most evident in the first few days post-injury, when compared to uninjured control animals. In many cases, behavioural deficits can be magnified when the tasks become more difficult, as exemplified by under-exposure to training trials. Similarly, the severity of TBI can influence the difficulty of the task and extend deficits for months to a year post-injury25. Overall, behavioural performance may improve with time, not necessarily reaching pre-injury levels, and rarely worsening.
Chronic morbidities
We define post-traumatic morbidity as the long-term consequences of injury-related pathological processes that emerge in a delayed manner due to impaired brain circuit function and activation, including problems with cognition, sensory processing, and affective/emotional function57,58. As a corollary to acute behavioural deficits, the behavioural morbidities are minimal or initially absent after injury, and build towards a maximum neurological impairment. In this way, morbidities evolve over time in brain-injured humans (the acute deficits transition to chronic morbidities), and valuable clinically-relevant models of TBI should demonstrate similar injury-specific morbidities with a protracted time course.
Enduring physiological morbidities
The endocrine system is particularly vulnerable to TBI in humans and animal models59–61. However, systematic investigation of the neuroendocrine sequelae in FPI models is limited. Endocrine dysfunction arises from structural damage, leading to hypopituitarism or adrenal insufficiency, which can occur at any time post-injury, and may contribute to, or exacerbate neurological, somatic, and emotional post-traumatic morbidities59,60, particularly in times of stress62. Lateral FPI induces acute increases in the stress response as measured by serum hormone levels61,63,64, but few studies have investigated enduring endocrine dysfunction using lateral or midline FPI.
Epilepsy is a common comorbidity of human TBI, with nearly 50% of TBI cases presenting with epilepsy65. TBI can lead to early seizures, occurring in the first 7 days post-injury, or can develop into post-traumatic epilepsy (PTE). Lateral FPI increases susceptibility to seizure and decreases seizure threshold66. Although evidence for spontaneous electrographic seizures exists, the incidence ranges from 50–92% and can require up to 8 weeks for phenotypic expression67,68. Furthermore, a single lateral FPI has been shown capable of reproducing PTE in the rat, with electrophysiological and structural sequelae paralleling changes seen in human PTE67. It is likely, however, that genetic predisposition or chemical exposure may be necessary to reliably and consistently elicit PTE after FPI. Whether these same results emerge in midline FPI remains to be investigated.
Behavioural morbidities
Months to years after clinical TBI, neuromotor deficits can transition to chronic morbidities, such as persistent difficulties with coordination, posture, and steadiness of movement18. Alternatively, new morbidities may emerge as acute phases of injury resolve and recovery processes re-establish homeostasis. In recovery, the possibility exists for new circuits to form from collateral sprouting and mediate new functions (see Figure 1). Moreover, once behavioural morbidities emerge from reorganizing circuits, neurobehavioural function may never return to a pre-injury status, because rewired circuits remain. In the experimental setting, rodents demonstrate neuromotor deficits (which can last for months), including poor performance on rotarod, beam walk, catwalk, and rope hang tests, indicating dysfunction in coordination, balance, gait, and grip strength, respectively53. However, few behavioural deficits, if any, establish themselves as late-onset morbidities.
Clinical somatic signs of post-concussion symptoms include sensory sensitivity, particularly to light and sound, which can emerge in a delayed manner and persist for months after injury58. Similarly, midline FPI produces a sensory sensitivity to whisker stimulation in rats69–73. Sensory sensitivity to whisker stimulation is demonstrated by the whisker nuisance task, where brain-injured rodents show an aggravated response to whisker stimulation that develops by 28 days post-injury, while uninjured animals are ambivalent to the same stimulation71.
For midline FPI, the protracted transition of acute behavioural deficits into more chronic behavioural morbidities provides the opportunity for therapeutic interventions to attenuate or prevent persistent neurological symptoms. Achieving this goal requires sensitive and selective tools to identify stages of the symptom transition and precision therapeutics to antagonize the underlying cellular processes. Categories and classes of biomarkers can serve as tools to identify the optimal timing for intervention and evaluate therapeutic efficacy (vide infra). Pre-clinical development of treatments focused on time-sensitive restoration of function, while inhibiting morbidity, likely hold great translational relevance to improve quality of life for those living with TBI.
Anatomical and pathological correlates
Diffuse axonal injury is the primary persisting histological feature of diffuse TBI74, which can only be evaluated in clinical cases resulting in autopsy. The low mortality with mild-moderate TBI precludes post-mortem histological evaluation of human cases. Brains of boxers and football players have been studied for pathology associated with repetitive TBI, where atrophy and degeneration, as well as abundant Tau staining have been described as pathological hallmarks of a newly-described post-traumatic disease, chronic traumatic encephalopathy (CTE)75. The most prominent clinical imaging findings in diffuse TBI, where available, include hematoma. In FPI, subdural hematoma occurs upon impact and generally resolves by one week post-injury. Subarachnoid and intraventricular haemorrhage, both sequelae of fatal TBI, do not occur following the most severe levels of FPI, which highlights the anatomical differences between rodent and human brains and underscores the limitations of experimental models to reproduce all sequelae of human TBI.
In rodent models, histopathological analyses can be used to quantify the magnitude and spatial distribution of molecular and cellular targets. However, the translational importance of these markers is limited, unless they are clinically detectable, as would be the case for physiology, radioligands, or fluid-based biomarkers. Since radioligands present additional patient risks without probable clinical indication, we focus on physiological and fluid-based biomarkers, but leave more comprehensive treatment to other reviews76–79.
Need for biomarkers for the diagnosis and prognosis of TBI
Biomarkers, in general, complement clinical assessments of patient symptoms, mental state, or the impact of a disease on patient quality of life. Whereas clinical history and physical exam may have subjectivity, biomarkers are intended to be objective primary or indicator measurements of normal biologic processes, pathologic processes, or responses to therapeutic intervention. In this way, biomarkers traditionally separate into four categories: diagnostic, prognostic, predictive and therapeutic. Overall, significant challenges arise in validating biomarkers for TBI in pre-clinical models when the disease itself is broadly defined with often antagonistic cellular processes. Pre-clinical investigations into biomarkers can provide the knowledge necessary to advance healthcare delivery, when comprehensively incorporated into study designs. Below, we discuss the need and utility of each biomarker category in experimental studies of diffuse TBI.
Diagnostic biomarkers distinguish patients with a particular disease or disease subset from those without the disease. In an initial evaluation, diagnostic biomarkers would stratify patients based on disease phenotype, in order to assign clinical pathways optimized for a particular disease subset1. Specifically, diagnostic biomarkers may indicate ongoing or cumulative cellular injury or repair processes; however a rapid measurement is essential to capture the dynamics of injury in a clinically meaningful timeframe. Diagnostic biomarkers are similarly valuable in pre-clinical research to stratify research subjects. Stratification of research groups aids in identifying optimal treatments for a refined subset of TBI, rather than the entire disease category. In pursuing diagnoses and treatments for a more precise subset, variability within groups is minimized, thereby increasing the power of the study. For brain-injured animals, righting reflex, neuromotor deficits, and sleep physiology can be incorporated as diagnostic biomarkers of neurological function for stratification to develop uniform animal groups. Potential fluid-based diagnostic biomarkers of injury may include UCH-L1 in the acute phase of injury, whereas GFAP holds utility in the post-acute phase 79.
Prognostic biomarkers inform clinicians and investigators about the clinical course of the disease, with a focus on the emergence and magnitude of neurological deficits and morbidities. The specific measurements themselves are not necessarily clinically meaningful as they often precede specific clinical outcomes, thereby permitting clinicians to forecast disease trajectory. In the laboratory, prognostic biomarkers would predict the onset and magnitude of behavioural impairments, whether deficits or morbidities. For example, circulating levels of stress hormones (e.g. corticosterone) or auto-antibodies following injury could serve as prognostic biomarkers regarding weight gain, anxiety, and immunity.80 To date, few if any, translational studies have investigated prognostic biomarkers in pre-clinical TBI.
Predictive biomarkers have utility in identifying patient populations likely to respond (or not respond) to one or more specific treatments. Predictive biomarkers would indicate levels or responses of specific biological processes that can specifically identify treatment approaches with predictable outcome. To this end, predictive biomarkers epitomize precision and personalized medicine in tailoring treatments to individual patients. Meaningful predictive biomarkers provide the confidence to identify subjects and their disease state for which a particular therapeutic approach will be effective. In the laboratory, the use of genetically similar animals subjected to standardized protocols of TBI models does not facilitate the identification and testing of predictive biomarkers. However, experimental outcomes often differ between species, strain, age, and sex, which indicates that demographics may predict efficacy of therapeutic outcomes relevant to the clinical population.
Therapeutic biomarkers are dynamic assessments that indicate the predicted outcome from a therapeutic intervention has occurred. Ideal therapeutic biomarkers would be periodically measured during the therapeutic intervention to ensure that the therapy was effectively targeting the mechanism of action. As an example, heart rate can be measured during meditation to demonstrate efficacy of the procedure (meditation) on the measured outcome (heart rate). In pre-clinical TBI research, therapeutic biomarkers are infrequently measured; clinical outcomes are compared against homogenous groups of treated and untreated animals. The incorporation of therapeutic biomarkers in pre-clinical study designs could reduce group sizes, since therapeutic efficacy could be measured prospectively. In designing studies, repeated measurements of intracranial pressure, food intake, or neurological function could serve as therapeutic biomarkers depending on the predicted mechanism of action for the therapy.
Evoked biomarkers have not been considered in a systematic manner. Rather than measuring the static or incidental levels of biological molecules or physiological processes, evoked biomarkers would be measured before and after a specific provocative test. This situation is akin to the glucose tolerance test for gestational diabetes and provocative hormone testing for endocrine dysfunction. In the context of TBI, particularly diffuse injury, most neurological functions are within the dynamic range of the general population. However, stress, exercise or other physiological challenge (e.g. hypoxia) may result in exaggerated or suppressed responses, compared to normative values. To a large extent, the current return-to-play guidelines for sport-related concussion suggest an evoked biomarker, where graduated levels of activity are encouraged as long as post-concussive symptoms do not worsen.81 In the laboratory, animals are exposed to numerous measurements and interventions, but typically in a sequential manner, rather than massed to evoke specific symptomatology. An alternate approach may be exposure to a natural stressor (e.g. predator odour) prior to cognitive evaluation, in order to evoke specific symptomatology in brain-injured compared to uninjured animals.
Lastly, none of these biomarkers are effective in isolation, where a refined array of biomarkers (biological and physiological) could improve the diagnosis, add confidence to the prognosis, and follow the course of disease and its response to treatment. Truly personalized medicine will require biomarker arrays to define the specific phenotype or “bio-signature” of the patient with brain injury and refine approaches for their specific condition. However, every potential biomarker requires scrutiny in terms of its own specificity and sensitivity before being incorporated into a biomarker array that can advance clinical care in a meaningful way. Biomarker arrays as a diagnostic strategy extend beyond the clinic to non-health-care settings. Developing these tools could be beneficial at the point of injury (e.g. battlefield, sporting event), as well as rehabilitation and long-term care facilities.
The limited investigation of biomarkers following midline FPI warrants further studies into their bioavailability and utility. Experimental study designs that incorporate biomarkers would permit cross-study comparisons, comparisons between laboratories using the same experimental model, and comparisons between studies that use different experimental models of TBI. Furthermore, biomarkers can benefit the development of current models, which are incorporating mild, repetitive, and paediatric brain injuries. A biomarker platform can represent differences in pathophysiology and outcomes introduced by modifications in model parameters. Readers are directed to current reviews on specific biomarkers, which focus primarily on fluid-based and imaging biomarkers. 77,79,82,83
Limitations of the midline FPI model
Animal research in TBI holds significant validity towards the human condition, but cannot faithfully replicate every feature18. For example, TBI results in chronic sleep disorders in humans, but midline FPI and other rodent models, to date, have failed to reproduce sleep disturbances in the chronic phase52. Additionally, acute and chronic vestibular deficits and morbidities remain challenging to investigate in rodents with prehensile tails, suggesting that vestibular deficits or the difficulty of the test may have to be far greater in the animal model to elicit phenotypic expression. Along these lines, compensatory actions and movements may permit injured rodents to accomplish the behavioural tasks established in the laboratory, while significant underlying changes in circuitry remain undetected84. Similarly, post-traumatic headache remains the single most concerning symptom for those living with TBI85, but strategies to identify the prevalence of and evaluate headache in rodents are limited. Increased attention to detail and refinement of behavioural tests of animal performance are critical to incorporate translationally relevant pre-clinical outcomes in experimental TBI.
All rodent models have general limitations when modelling TBI. Differences in weight, sex, age at injury, and genetic background can influence outcome, including effects on applied mechanical force and secondary injury processes. Similarly, the analgesics and anaesthetics necessary to induce injury can confound the natural course of brain injury (see Rowe et al. 2013 for review)86. The use of anaesthetics during the mFPI model cannot completely be avoided; however, a consistent (drug and dose) intra-study anaesthesia protocol is imperative when conducting TBI studies to reduce variability.
Conclusion and future directions
After 30 years of characterisation and implementation, midline FPI is recognised as a clinically relevant model of TBI, particularly mild, diffuse TBI, such as concussion. This model remains valuable for translational research, and can be analysed across time post-injury at different levels of analysis. Additionally, TBI, whether associated with a fall, motor vehicle accident, sports injury, or assault, often occurs concomitantly with other injuries. While studies have begun to investigate combined injuries such as TBI and bone fractures87–89, future opportunities exist to expand this research and study combined injuries that often occur in the clinical setting, such as TBI with hypoxia, and TBI with hypoglycemia. Furthermore, future opportunities exist to expand the mFPI model to repetitive head injury studies. Repetitive TBI, common in sports-related injury, has yet to be routinely modelled specifically in mFPI, but a growing number of studies have successfully administered repetitive TBI with other FPI animal models90–94. Additionally, age-at-injury has been largely overlooked in studies involving the FPI model32,95 where continued investigation is warranted. It is essential that females gain greater representation in brain-injury research to address anatomical and physiological differences between genders. Lastly, pre-clinical study designs would benefit from and provide additional translational value by incorporating multiple biomarker categories into the study design. It remains clear that midline FPI continues to complement other experimental models of TBI to aid in the understanding and search for treatments of the clinical condition.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. Sources of funding include: U.S. Department of Health and Human Services, National Institutes of Health grants R21 NS072611, RO1 NS-065052, and Science Foundation Arizona, Bisgrove Scholar Fellowship.
References
- 1.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther. 2011;130(2):106–13. doi: 10.1016/j.pharmthera.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Walker AE. The physiological basis of concussion: 50 years later. J Neurosurg. 1994;81(3):493–4. doi: 10.3171/jns.1994.81.3.0493. [DOI] [PubMed] [Google Scholar]
- 4.Zweckberger K, Eros C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006;23(7):1083–93. doi: 10.1089/neu.2006.23.1083. [DOI] [PubMed] [Google Scholar]
- 5.Friess SH, Lapidus JB, Brody DL. Decompressive craniectomy reduces white matter injury after controlled cortical impact in mice. J Neurotrauma. 2015;32(11):791–800. doi: 10.1089/neu.2014.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda T, Lee SM, Hovda DA. Restoration of cerebral vasoreactivity by an L-type calcium channel blocker following fluid percussion brain injury. J Neurotrauma. 2005;22(7):763–71. doi: 10.1089/neu.2005.22.763. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, McArthur DL, Alger JR, Etchepare M, Hovda DA, Glenn TC, Huang S, Dinov I, Vespa PM. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cereb Blood Flow Metab. 2010;30(4):883–94. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wee HY, Lim SW, Chio CC, Niu KC, Wang CC, Kuo JR. Hyperbaric oxygen effects on neuronal apoptosis associations in a traumatic brain injury rat model. J Surg Res. 2015;197(2):382–9. doi: 10.1016/j.jss.2015.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–40. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Yoganandan N, Pintar FA, Gennarelli TA. Brain strains in vehicle impact tests. Annu Proc Assoc Adv Automot Med. 2006;50:1–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Faul MXL, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 12.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–6. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 13.Graham DI, Adams JH, Nicoll JA, Maxwell WL, Gennarelli TA. The nature, distribution and causes of traumatic brain injury. Brain Pathol. 1995;5(4):397–406. doi: 10.1111/j.1750-3639.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 14.Levine B, Kovacevic N, Nica EI, Cheung G, Gao F, Schwartz ML, Black SE. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70(10):771–8. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- 15.Groat RA, Windle WF, Magoun HW. Functional and structural changes in the monkey’s brain during and after concussion. J Neurosurg. 1945;2(1):26–35. [Google Scholar]
- 16.Lindgren S, Rinder L. Experimental studies in head injury. I. Some factors influencing results of model experiments. Biophysik. 1965;2(5):320–9. [PubMed] [Google Scholar]
- 17.Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO. Fluid-percussion model of mechanical brain injury in the cat. J Neurosurg. 1976;45(5):521–34. [PubMed] [Google Scholar]
- 18.Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22(1):42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28(1):233–44. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 20.Lifshitz J. Fluid Percussion Injury. In: Chen JZX, Xu X-M, Zhang J, editors. Animal Models of Acute Neurological Injuries. Totowa, NJ: The Humana Press Inc; 2008. [Google Scholar]
- 21.Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67(1):110–9. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh TK, Noble L, Andrews B, Faden AI. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4(2):119–34. doi: 10.1089/cns.1987.4.119. [DOI] [PubMed] [Google Scholar]
- 23.Vink R, Mullins PG, Temple MD, Bao W, Faden AI. Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. J Neurotrauma. 2001;18(8):839–47. doi: 10.1089/089771501316919201. [DOI] [PubMed] [Google Scholar]
- 24.Floyd CL, Golden KM, Black RT, Hamm RJ, Lyeth BG. Craniectomy position affects morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma. 2002;19(3):303–16. doi: 10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- 25.Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87(2):359–69. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 26.Smith DH, Chen XH, Pierce JE, Wolf JA, Trojanowski JQ, Graham DI, McIntosh TK. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14(10):715–27. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- 27.Stein DG. Sex differences in brain damage and recovery of function: experimental and clinical findings. Prog Brain Res. 2007;161:339–51. doi: 10.1016/S0079-6123(06)61024-8. [DOI] [PubMed] [Google Scholar]
- 28.Naderi V, Khaksari M, Abbasi R, Maghool F. Estrogen provides neuroprotection against brain edema and blood brain barrier disruption through both estrogen receptors alpha and beta following traumatic brain injury. Iran J Basic Med Sci. 2015;18(2):138–44. [PMC free article] [PubMed] [Google Scholar]
- 29.Maghool F, Khaksari M, Siahposht Khachki A. Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 2013;1497:61–72. doi: 10.1016/j.brainres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Soltani Z, Khaksari M, Shahrokhi N, Mohammadi G, Mofid B, Vaziri A, Amiresmaili S. Effect of estrogen and/or progesterone administration on traumatic brain injury-caused brain edema: the changes of aquaporin-4 and interleukin-6. J Physiol Biochem. 2015 doi: 10.1007/s13105-015-0453-5. [DOI] [PubMed] [Google Scholar]
- 31.Khaksari M, Abbasloo E, Dehghan F, Soltani Z, Asadikaram G. The brain cytokine levels are modulated by estrogen following traumatic brain injury: Which estrogen receptor serves as modulator? Int Immunopharmacol. 2015;28(1):279–87. doi: 10.1016/j.intimp.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003;20(2):123–37. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 33.Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res. 1996;95(2):272–82. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- 34.Dixon CE, Lighthall JW, Anderson TE. Physiologic, histopathologic, and cineradiographic characterization of a new fluid-percussion model of experimental brain injury in the rat. J Neurotrauma. 1988;5(2):91–104. doi: 10.1089/neu.1988.5.91. [DOI] [PubMed] [Google Scholar]
- 35.Hosseini AH, Lifshitz J. Brain injury forces of moderate magnitude elicit the fencing response. Med Sci Sports Exerc. 2009;41(9):1687–97. doi: 10.1249/MSS.0b013e31819fcd1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lafrenaye AD, McGinn MJ, Povlishock JT. Increased intracranial pressure after diffuse traumatic brain injury exacerbates neuronal somatic membrane poration but not axonal injury: evidence for primary intracranial pressure-induced neuronal perturbation. J Cereb Blood Flow Metab. 2012;32(10):1919–32. doi: 10.1038/jcbfm.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22(1):3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma. 1993;10(4):415–30. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- 39.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561(1):106–19. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 40.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergsneider M, Hovda DA, Lee SM, Kelly DF, McArthur DL, Vespa PM, Lee JH, Huang SC, Martin NA, Phelps ME, et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma. 2000;17(5):389–401. doi: 10.1089/neu.2000.17.389. [DOI] [PubMed] [Google Scholar]
- 42.Ip EY, Zanier ER, Moore AH, Lee SM, Hovda DA. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J Cereb Blood Flow Metab. 2003;23(8):900–10. doi: 10.1097/01.WCB.0000076702.71231.F2. [DOI] [PubMed] [Google Scholar]
- 43.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30(1):33–48. vii–iii. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Prins ML, Alexander D, Giza CC, Hovda DA. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma. 2013;30(1):30–8. doi: 10.1089/neu.2012.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe RK, Striz M, Bachstetter AD, Van Eldik LJ, Donohue KD, O’Hara BF, Lifshitz J. Diffuse Brain Injury Induces Acute Post-Traumatic Sleep. PLoS One. 2014;9(1):e82507. doi: 10.1371/journal.pone.0082507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raghupathi R, McIntosh TK, Smith DH. Cellular responses to experimental brain injury. Brain Pathol. 1995;5(4):437–42. doi: 10.1111/j.1750-3639.1995.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 47.Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clin. 2007;2(2):161–169. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson HJ, Hoover RC, Tkacs NC, Saatman KE, McIntosh TK. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J Cereb Blood Flow Metab. 2005;25(2):163–76. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- 49.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12(3):163–73. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 50.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 2014;76(7):575–84. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe RK, Harrison JL, O’Hara BF, Lifshitz J. Diffuse brain injury does not affect chronic sleep patterns in the mouse. Brain Inj. 2014;28(4):504–10. doi: 10.3109/02699052.2014.888768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma. 2001;18(11):1207–16. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- 54.Bachstetter AD, Rowe RK, Kaneko M, Goulding D, Lifshitz J, Van Eldik LJ. The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci. 2013;33(14):6143–53. doi: 10.1523/JNEUROSCI.5399-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison JL, Rowe RK, Ellis TW, Yee NS, O’Hara BF, Adelson PD, Lifshitz J. Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav Immun. 2015;47:131–40. doi: 10.1016/j.bbi.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136(4):971–89. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 57.Chen AJ, D’Esposito M. Traumatic brain injury: from bench to bedside to society. Neuron. 2010;66(1):11–4. doi: 10.1016/j.neuron.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 58.McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am. 1992;15(2):395–413. [PubMed] [Google Scholar]
- 59.Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79(7):753–9. doi: 10.1136/jnnp.2007.132837. [DOI] [PubMed] [Google Scholar]
- 60.Dusick JR, Wang C, Cohan P, Swerdloff R, Kelly DF. Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary. 2012;15(1):2–9. doi: 10.1007/s11102-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grundy PL, Harbuz MS, Jessop DS, Lightman SL, Sharples PM. The hypothalamo-pituitary-adrenal axis response to experimental traumatic brain injury. J Neurotrauma. 2001;18(12):1373–81. doi: 10.1089/08977150152725669. [DOI] [PubMed] [Google Scholar]
- 62.Griesbach GS, Hovda DA, Tio DL, Taylor AN. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–58. doi: 10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griesbach GS, Tio DL, Vincelli J, McArthur DL, Taylor AN. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J Neurotrauma. 2012;29(7):1426–33. doi: 10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griesbach GS, Tio DL, Nair S, Hovda DA. Recovery of stress response coincides with responsiveness to voluntary exercise after traumatic brain injury. J Neurotrauma. 2014;31(7):674–82. doi: 10.1089/neu.2013.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh CC, Chen TL, Hu CJ, Chiu WT, Liao CC. Risk of epilepsy after traumatic brain injury: a retrospective population-based cohort study. J Neurol Neurosurg Psychiatry. 2013;84(4):441–5. doi: 10.1136/jnnp-2012-302547. [DOI] [PubMed] [Google Scholar]
- 66.Pitkanen A, Bolkvadze T. Head Trauma and Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): 2012. [Google Scholar]
- 67.D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127(Pt 2):304–14. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23(2):241–61. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- 69.Johnstone VP, Yan EB, Alwis DS, Rajan R. Cortical hypoexcitation defines neuronal responses in the immediate aftermath of traumatic brain injury. PLoS One. 2013;8(5):e63454. doi: 10.1371/journal.pone.0063454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alwis DS, Yan EB, Morganti-Kossmann MC, Rajan R. Sensory cortex underpinnings of traumatic brain injury deficits. PLoS One. 2012;7(12):e52169. doi: 10.1371/journal.pone.0052169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNamara KC, Lisembee AM, Lifshitz J. The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J Neurotrauma. 2010;27(4):695–706. doi: 10.1089/neu.2009.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Learoyd AE, Lifshitz J. Comparison of rat sensory behavioral tasks to detect somatosensory morbidity after diffuse brain-injury. Behav Brain Res. 2012;226(1):197–204. doi: 10.1016/j.bbr.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas TC, Hinzman JM, Gerhardt GA, Lifshitz J. Hypersensitive glutamate signaling correlates with the development of late-onset behavioral morbidity in diffuse brain-injured circuitry. J Neurotrauma. 2012;29(2):187–200. doi: 10.1089/neu.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma. 2011;28(5):701–9. doi: 10.1089/neu.2010.1733. [DOI] [PubMed] [Google Scholar]
- 75.Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep. 2013;13(12):407. doi: 10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mondello S, Tortella FC. Brain Injury Markers: Where are We? Front Neurol. 2014;5:145. doi: 10.3389/fneur.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papa L, Ramia MM, Edwards D, Johnson BD, Slobounov SM. Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J Neurotrauma. 2015;32(10):661–73. doi: 10.1089/neu.2014.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forde CT, Karri SK, Young AM, Ogilvy CS. Predictive markers in traumatic brain injury: opportunities for a serum biosignature. Br J Neurosurg. 2014;28(1):8–15. doi: 10.3109/02688697.2013.815317. [DOI] [PubMed] [Google Scholar]
- 79.Jeter CB, Hergenroeder GW, Hylin MJ, Redell JB, Moore AN, Dash PK. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma. 2013;30(8):657–70. doi: 10.1089/neu.2012.2439. [DOI] [PubMed] [Google Scholar]
- 80.Raad M, Nohra E, Chams N, Itani M, Talih F, Mondello S, Kobeissy F. Autoantibodies in traumatic brain injury and central nervous system trauma. Neuroscience. 2014;281c:16–23. doi: 10.1016/j.neuroscience.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 81.McCrory P, Meeuwisse WH, Aubry M, Cantu RC, Dvorak J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS, Raftery M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport, Zurich, November 2012. J Athl Train. 2013;48(4):554–75. doi: 10.4085/1062-6050-48.4.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carpenter KL, Czosnyka M, Jalloh I, Newcombe VF, Helmy A, Shannon RJ, Budohoski KP, Kolias AG, Kirkpatrick PJ, Carpenter TA, et al. Systemic, local, and imaging biomarkers of brain injury: more needed, and better use of those already established? Front Neurol. 2015;6:26. doi: 10.3389/fneur.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smitherman E, Hernandez A, Stavinoha PL, Huang R, Kernie SG, Diaz-Arrastia R, Miles DK. Predicting Outcome after Pediatric Traumatic Brain Injury by Early Magnetic Resonance Imaging Lesion Location and Volume. J Neurotrauma. 2016;33(1):35–48. doi: 10.1089/neu.2014.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol. 2014;10(3):156–66. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 85.Lew HL, Poole JH, Guillory SB, Salerno RM, Leskin G, Sigford B. Persistent problems after traumatic brain injury: The need for long-term follow-up and coordinated care. J Rehabil Res Dev. 2006;43(2):vii–x. doi: 10.1682/jrrd.2006.05.0054. [DOI] [PubMed] [Google Scholar]
- 86.Rowe RK, Harrison JL, Thomas TC, Pauly JR, Adelson PD, Lifshitz J. Using anesthetics and analgesics in experimental traumatic brain injury. Lab Anim (NY) 2013;42(8):286–91. doi: 10.1038/laban.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shultz SR, Sun M, Wright DK, Brady RD, Liu S, Beynon S, Schmidt SF, Kaye AH, Hamilton JA, O’Brien TJ, et al. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J Cereb Blood Flow Metab. 2015;35(8):1339–47. doi: 10.1038/jcbfm.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maegele M, Sauerland S, Bouillon B, Schafer U, Trubel H, Riess P, Neugebauer EA. Differential immunoresponses following experimental traumatic brain injury, bone fracture and “two-hit”-combined neurotrauma. Inflamm Res. 2007;56(8):318–23. doi: 10.1007/s00011-007-6141-3. [DOI] [PubMed] [Google Scholar]
- 89.Chen B, Mutschler M, Yuan Y, Neugebauer E, Huang Q, Maegele M. Superimposed traumatic brain injury modulates vasomotor responses in third-order vessels after hemorrhagic shock. Scand J Trauma Resusc Emerg Med. 2013;21:77. doi: 10.1186/1757-7241-21-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeRoss AL, Adams JE, Vane DW, Russell SJ, Terella AM, Wald SL. Multiple head injuries in rats: effects on behavior. J Trauma. 2002;52(4):708–14. doi: 10.1097/00005373-200204000-00017. [DOI] [PubMed] [Google Scholar]
- 91.Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J Neuroinflammation. 2013;10:26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2012;29(2):281–94. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- 93.Wang T, Van KC, Gavitt BJ, Grayson JK, Lu YC, Lyeth BG, Pichakron KO. Effect of fish oil supplementation in a rat model of multiple mild traumatic brain injuries. Restor Neurol Neurosci. 2013;31(5):647–59. doi: 10.3233/RNN-130316. [DOI] [PubMed] [Google Scholar]
- 94.Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab. 2014;34(7):1223–32. doi: 10.1038/jcbfm.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hawkins BE, Cowart JC, Parsley MA, Capra BA, Eidson KA, Hellmich HL, Dewitt DS, Prough DS. Effects of trauma, hemorrhage and resuscitation in aged rats. Brain Res. 2013;1496:28–35. doi: 10.1016/j.brainres.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]