Abstract
Background
Alzheimer’s disease (AD) and chronic traumatic encephalopathy (CTE) have long been recognized as sharing some similar neuropathological features, mainly the presence of neurofibrilary tangles and hyperphosphorylated tau, but have generally been described as distinct entities. Evidence indicates that neurotrauma increases the risk of developing dementia and accelerates the progression of disease. Findings are emerging that CTE and AD may be present in the same patients.
Clinical presentation
We present a series of previously unpublished cases with one case demonstrating possible neurotrauma-related AD, one pure CTE, and an example of a case exhibiting features of both AD and CTE. The future significance of this work lies not only in the confirmation of AD-CTE coexistence but more importantly, ways of generating a hypothesis about the possibility that CTE may accelerate AD development. Understanding the relationship between neurotrauma and neurodegenerative disease, will help elucidate how distinct disease entities can co-exist in the same patient. It will ultimately require the use of preclinical animal models and repeat injury paradigms to investigate clinically relevant injury mechanisms. These models should produce a CTE-like phenotype that must be both neuropathologically and behaviorally similar to human disease.
Conclusion
In this case series and review of the literature, we present a discussion of AD and CTE in the context of neurotrauma. We highlight recent work from repetitive neurotrauma models with an emphasis on those exhibiting a CTE-like phenotype. We briefly address potential mechanisms of interest shared amongst AD and CTE and advocate for future experiments to enhance understanding of CTE pathophysiology and the relationship between CTE and AD.
Keywords: Alzheimer’s disease, Chronic Traumatic Encephalopathy, Dementia, Neurotrauma, Traumatic Brain Injury
BACKGROUND
An overview of Alzheimer’s disease
Although chronic traumatic encephalopathy (CTE) and Alzheimer’s disease (AD) share various similarities with regards to tau deposition, the diagnosis of AD has distinct clinical and neuropathological criteria required for diagnosis. The National Institute on Aging has recently published revised guidelines for the neuropathologic assessment of the disease.1,2 It is currently recommended that in cases of suspected AD based on a history of dementia that neuropathological changes be assessed on autopsy.1 In addition, these guidelines can also be applied to mild cognitive impairment (MCI). Primary neuropathological changes that should accompany clinically scored AD include the staining of Aβ/neuritic amyloid plaques in the middle frontal gyrus, the superior and middle temporal gyri, and the inferior parietal lobule in a laminar distribution.1 Secondary pathological considerations with regards to the extent of Aβ deposition should also be made in areas such as the basal ganglia and cerebellum of these patients.1 These neuropathologic changes are usually quite distinct in comparison to those found in the majority of pure CTE cases, which may exhibit few diffuse instead of laminar Aβ plaques or no plaques at all.3–5
In addition to Aβ deposition, the presence of neurofibrillary tangles (NFTs) is considered a primary pathological feature for the diagnosis of typical AD. NFTs composed of intracellular aggregations of hyperphosphorylated tau are laminar in distribution and progress in a distinct fashion consistent with AD. It is recommended that neurpathological staining post-mortem be conducted for NFTs in several brain regions that overlap with Aβ plaque pathology including the middle frontal gyrus, superior and middle temporal gyri, inferior parietal lobule and hippocampus.1 The most commonly accepted method for the scoring of NFT pathology with regards to AD is that of Braak.6 These scoring methods are important in that they give a standardized staging protocol specifically for NFTs in AD that uses the distribution of NFTs as opposed to density measurements. The original scoring method by Braak et al. utilizes stages (I-VI) that correlate with the location of the NFTs and the severity of NFTs with a given stage. In stage I, lesions are in the hippocampus with the distinct absence of cortical involvement. In stage II, NFTs extend into the entorhinal regions of the cortex and CA1 hippocampal regions. Recent evidence suggests that NFTS in the dentate gyrus of the hippocampus may also be present.7 In stage III, NFTs spread into neocortical areas (particularly the temporal cortex) and there is even greater density in the hippocampus. In stage IV, the density of NFTs increases in the neocortex (temporal and insular cortices). In stage V, pathology extends into differentiated areas of the frontal and occipital cortex. In stage VI, there is distinct involvement of striate regions.6 With regards to the recommended scoring of AD severity by the National Institute on Aging, the committee recommends a modified version of the Braak scoring with B0 representing no NFTs, B1 equal to Braak stages I/II, B2 equivalent to Braak stages III/IV, and B3 equivalent to Braak stage V/VI.1
An overview of chronic traumatic encephalopathy
CTE as a diagnosis requires a prior history of neurotrauma, generally repetitive, and the demonstration of specific neuropathological features, namely tauopathy.8 Consequently, CTE has been diagnosed in a multitude of individuals with extensive exposure histories, ranging from traditional impact-acceleration injuries to blast exposure. People at risk include football players, wrestlers, and soldiers.8,9 As the number of concussions and subconcussions in the United States continues to rise, a clear need for improved diagnosis of neurotrauma related neurodegeneration has emerged in order to facilitate the development of better therapeutic regimens.8,10–13 While exciting advances in the use of targeted PET-based imaging compounds have demonstrated the potential for real-time diagnosis and monitoring of CTE,14 our understanding of CTE pathophysiology remains in its infancy. Little progress has been made in the development of preventative or treatment techniques. The following sections highlight CTE pathologic criteria for diagnosis, diagnostic schemes, and neurologic symptoms that are associated with CTE.
CTE pathology
The original description of CTE in boxers by Martland and furthered by Corsellis and colleagues, was actually termed dementia pugilistica (DP) or “punch drunk” syndrome, and was centered upon gross anatomical changes that included cerebral atrophy, enlargement of the ventricles, cerebellar scarring, and a cavum septum pellucidum.15,16 In 2005, Omalu and colleagues identified and provided an updated description of CTE in a retired professional football player as pallor of the substantia nigra, loss of neurons in frontal, temporal, and parietal lobes, and diffuse amyloid plaques with neurofibrillary tau tangles (NFTs) in the neocortex.17 A second reported case, also in a former NFL player, had no amyloid plaques, but did have sporadic NFTs throughout the brain, a cavum septum pellucidum, and pallor of the substantia nigra.18 McKee and colleagues expanded the description of CTE with a broader case series in 2009. In addition to NFTs, they reported widespread astrocytic tangles, neuritic threads, as well as atrophy and tangle involvement in the temporal lobes, thalamus, and mammillary bodies. The pathology was localized primarily to subcortical, perivascular, and periventricular areas in the frontal and temporal lobes.4 In 2010, TDP-43 was added to the list of proteinopathies commonly found in cases of CTE.19 Somewhat surprisingly, a retired professional wrestler had tauopathy that extended into subcortical ganglia and brainstem nuclei.20 A soldier exposed to blast injury as well as other neurotrauma had the additional pathology of cerebral swelling, histocyte infiltration, and fibrillary astrogliosis.21 As the list of pathologic characteristics expanded, the need for key post-mortem diagnostic criteria to differentiate from other tauopathies became a necessity.
Pathologic diagnostic criteria
In 2011, Omalu and colleagues differentiated CTE from other tauopathies by describing key differences in pathologic characteristics. Currently, CTE, as described by Omalu and colleagues, lacked the distinct cerebral cortical atrophy common to frontotemporal lobe dementia (FTLD), AD, and dementia pugilistica. Unlike other tauopathies with focal laminar distribution of NFTs, CTE presented with alternating areas of sparse-to-dense band-shaped, flame-shaped, and globose NFTs. Likewise when amyloid pathology was present, it usually was in a diffuse distribution and α-synucleinopathy was not present. Most cases also had the epsilon 3/epsilon 3 apolipoprotein E genotype.22 McKee and colleagues published a case series in 2013 outlining the stages of pathology seen in the brains of 85 patients with a history of repetitive neurotrauma. A staging system for CTE was thus proposed but has not been widely utilized or endorsed by any advisory bodies, unlike staging in AD. Stage 1 and 2 are characterized by NFTs in brain foci localized to the prefrontal cortex. Stage 3 and 4 consist of high density NFTs at foci throughout the cerebrum that eventually progresses to include the thalamus, mamillary bodies, and brainstem. TDP43 pathology was seen in 85% of cases, and a select group had motor neuron disease in addition to CTE. Axonal loss was reported in deep cortical regions.23 Neuropathological changes observed in CTE, and how these changes differ from other tauopathies such as AD, have also been highlighted to varying degrees of detail elsewhere.4,8,23–26
Recent reports by Omalu et al. and McKee et al. have sought to standardize scoring of NFTs specifically in CTE cases to distinguish from other neurodegenerative disorders such as AD.22,23 Importantly, imaging research currently aimed at the detection and diagnosis of tau pathology in the elderly,27,28 may be applicable for suspected CTE cases in the very near future. This would provide researchers with valuable tools to confirm the presence of CTE and study the progression of neurotrauma related tauopathy in living individuals.14 Prospective studies would then be possible allowing researchers to investigate CTE development, progression, and possible treatment. Ultimately, new imaging modalities such as diffusion tensor imaging, functional magnetic resonance imaging, and magnetic resonance spectroscopy may provide enhanced tools for establishing more complete diagnostic criteria going forward, although none of these have emerged for definitive diagnosis of CTE at this time.26
CTE symptoms
Symptoms reported retrospectively by family, friends, and colleagues, following the neuropathologic diagnosis of CTE, and/or history of repetitive neurotrauma, can be broadly characterized into four overarching domains: mood, behavior, cognition, and motor.29 Mood disturbances such as irritability, depression, and apathy are common in CTE.5,30 Whether these mood changes lead to increased risk for suicide is a topic of open debate.31,32 Behavioral changes are also common including but not limited to impulsivity, aggression, and judgment issues.33,34 Sometimes these behavioral changes are associated with explosive and violent outbursts.35 Cognitive changes can be severely debilitating. Short-term memory loss and learning deficiencies are frequently reported.36 A three-fold increase in severe memory impairment was self-reported in surveys given to former NFL players.24 Motor deficits, particularly in older subjects, vary from decreased reaction time, eye movement disorders, and recurrent falls.37,38 Decreased visual motor speed has been seen in some subjects exposed to repetitive neurotrauma and the suggestion has been made that it may be an early indicator of progressive neurodegeneration.39
Disease onset
The onset of symptoms related to repetitive neurotrauma and the development of CTE are poorly understood at present. Repetitive concussive and subconcussive injuries are believed to accelerate the progression towards CTE.10 This is consistent with previous reports that exposure to TBI increases the risk for future development of a neurodegenerative diseases by ~4 fold.40 As has been previously reported, professional football players have a 3-fold increase in neurodegenerative mortality, and are at the highest risk for developing modern CTE (excluding boxers diagnosed with dementia pugilistica).41,42 In addition, upwards of a quarter of military service personnel are exposed to blast waves during training and active duty.43 The duration of repetitive exposure to head injury is closely correlated to injury severity.44 The average age of diagnosis for CTE is 54 years old with the youngest case at 18 years old.45 Young age of disease onset manifests with mood and behavioral disturbances, while older age at time of onset primarily presents with cognitive decline.46 The latency of onset is variable ranging from a few months to several decades following neurotrauma exposure.47 CTE as a disease has been proposed to be progressive with measurable pathology at each diagnostic stage.48 Symptoms are thought to be progressive as well starting with confusion, loss of oversight, and mood disturbance progressing over time to dementia, speech difficulties, and motor decline.49 Executive dysfunction has been one of the earliest clinical markers of repetitive neurotrauma, and potentially CTE, with changes being observed even in collegiate athletes.50 Future research is needed to delineate why the disease onset is different between individuals and what factors contribute to its progression over time.
Alzheimer’s disease & CTE: distinct entities?
Previous reports and studies have considered CTE and AD as distinct entities with little overlap outside of the tau pathology that plays a role in both. Investigators have documented clinical and neuropathological distinctions delineating the two conditions.25 Specifically, the presenting feature of AD is short-term memory deficits whereas patients diagnosed on autopsy with CTE were reported to exhibit depression, mood lability and explosive rage, substance abuse, and occasionally Parkinson-like features later in the disease course. Furthermore, suicide was often implicated as the cause of death in those afflicted with CTE whereas suicide is uncommon in those afflicted with AD. This finding may be inherent to differences in lifestyle or part of unique pathophysiologic processes defining the diseases. Pathologically, AD is characterized by more general cerebral atrophy and the consistent presence of neuritic amyloid plaques, which are present in only a percentage of CTE cases (diffuse senile plaques; found in up to 40% of CTE cases by McKee and colleagues).4 Neurofibrillary tangles within the cortex are seen in both AD and CTE with the difference being in distribution. In CTE, tangles are predominantly in the more superficial layers II and III whereas in AD, tangles are seen in the deeper cortex, namely layers V and VI with a laminar distribution. Likewise in CTE, tangles can be perivascular, another distinguishing feature. Other differences are seen in the hippocampus and parahippocampal gyrus. In CTE, tangles are seen in Ammon’s horn (CA1-CA4) whereas in AD, tangles are found most heavily within CA1. Additionally, neurofibrillary tangles are commonly found in the substantia nigra of those with CTE, but rarely in AD.25 Finally, NFTs may be seen in the entire amygdala, pontine tegmentum even outside the locus ceruleus and raphe nuclei, and thalamus, particularly within the pulvinar region in CTE.24
While CTE and AD are clearly distinct entities based on clinical and neuropathological presentation or findings, a history of neurotrauma has been linked repeatedly to the development of neurodegenerative disease, both with regards to acceleration and increased risk of development.25,49–52 Consequently, McKee and colleagues have documented that CTE may exist in persons of advanced age with AD, thus producing a mixed phenotype of AD and CTE.53 In this work, we present three clinical cases, demonstrating various clinical and pathological features that help delineate the relationship between AD and CTE. In case I, AD in the context of a history of significant previous neurotrauma is shown. In case II, CTE is evident independent of any significant AD changes. Most importantly, case III demonstrates evidence of a mix of AD-CTE pathologies. This case demonstrates the unique characteristics where both AD and CTE may be distinguished in the same patient, raising the possibility that neurotrauma may not only increase the risk of AD development but also possibly accelerate the disease course of CTE.
CLINICAL PRESENTATION
Tissue preparation
Upon arrival at NorthShore University Health System, the brains were fixed in 10% buffered formalin for at least 3 weeks. Sections were taken, paraffin embedded, sliced, and mounted on slides. They were stained according to standard clinical immunohistochemical and H&E staining protocols.54
Immunohistochemistry and Bielschowsky silver staining
A paired helical filament tau antibody (PHF-1), a generous gift from Dr. Peter Davies (Albert Einstein School of Medicine), was used for immunohistochemical staining on an automated IntelliPath (Biocare) at 1:100 dilutions. A standard Bielschowsky silver stain, alpha-synuclein, and TDP-43 immunostaining were also done. The protocols have been utilized and described at length in the CTE literature.4,17,18,20–23,55,56
Clinical cases
Both patient records and interviews with family and friends were used to obtain clinical case data. The study was approved by the Northshore University Health System Institutional Review Board and adhered to ethical guidelines for research with human participants.
Case I: AD in context of a history of significant previous neurotrauma
The patient was a 69 year-old male with a history of waxing and waning cognitive decline. In addition, the patient had visual hallucinations, and significant balance problems with numerous reported falls and a history of a Parkinsonian disorder. He had a limited response to L-Dopa, but had an early good response to Exelon. Past medical history included spinal stenosis, gastroparesis, possible REM sleep disorder, autonomic dysfunction (increased sweating), and kidney stones with numerous kidney infections. Other diseases included myocardial infarction and aspiration pneumonia in 2007. Past social history included playing football in junior high and high school with a history of multiple diagnosed concussions. He also was involved and participated in other contact sports including karate, kick boxing, and was a Golden Gloves boxing coach. In his boxing coach role, he would spar without a helmet, increasing his risk for concussive injury. Finally, the patient was in an automotive accident in 1988 with possible head injury.
On post-mortem examination, the fixed brain weight was 1130 grams. A portion of cerebral dura was available for examination and showed no dural fibrosis and no significant epidural or subdural hemorrhages. There were no old or new subarachnoid hemorrhages. The cerebral gyral pattern was of normal anatomic configuration with moderate cerebral edema, but without masses, vascular abnormalities, or palpable lesions. The left occipital lobe was mildly dusky. There was no evidence of orbital or temporal tip contusions. The cerebral vasculature was ectatic but without atherosclerosis. The circle of Willis had a normal anatomical configuration. The brainstem and cerebellum had a normal external appearance. The spinal dura showed no evidence of epidural hemorrhages and the spinal cord was not distorted by any contained masses or other lesions. Serial coronal sections of the cerebral hemispheres revealed the cortical gray and white matter structures to be without hemorrhages, infarcts, or mass lesions. There was mild atrophy of the anterior hippocampus with moderate atrophy of the posterior hippocampi bilaterally. There was also mild atrophy of the posterior cerebral white matter, which followed the corpus callosum trajectory. The corpus callosum demonstrated mild atrophy as well. The basal ganglia, thalamus, amygdalae, and mamillary bodies bilaterally were of normal size, color and consistency. The ventricular system was moderately dilated with no gradient and was otherwise symmetrical. Serial sections of the brainstem and cerebellum revealed mild superior cerebellar vermal atrophy and mild to moderate cerebellar dentate nucleus atrophy with a dusky gray discoloration. The substantia nigra was thin but did not demonstrate pallor. The locus ceruleus was well pigmented. Serial sections of the spinal cord were symmetrical without discoloration or lesions.
The H&E and Bielschowsky silver stains revealed numerous amyloid plaques of both the diffuse as well as neuritic type in all neocortical areas in laminar arrangement. In addition, there were numerous neurofibrillary tangles, particularly in the pyramidal neurons of layers 3 and 5, but no cortical Lewy bodies were present measured by alpha-synuclein staining (Figure 1). There was marked pallor of the white matter with numerous vessels that were hyalinized. The caudate nucleus and putamen contained diffuse plaques, gliosis, and numerous vessels that were hyalinized. The hippocampus and amygdala contained numerous plaques and neurofibrillary tangles in a laminar distribution. The thalamus contained tangles in the midline nuclei. The substantia nigra was focally gliotic with some neuronal dropout but without evidence of Lewy or pale bodies determined by negative staining for alpha-synuclein. The pons contained tangles in the locus ceruleus and raphe nuclei. The cerebellum contained no diffuse plaques in the molecular layer. Throughout the brain there was retraction artifact surrounding neurons and blood vessels. Immunoperoxidase staining for PHF1/tau revealed numerous neurofibrillary tangles in the cerebral cortex including the temporal lobe, cingulate gyrus, and superior frontal cortex. The overall score for tau changes was at a B3 level (NIA criteria). The PHF/tau also significantly stained the neuritic component of the amyloid plaques and the amyloid plaque score was at a C3 level (NIA criteria). Tau positive tangles were also noted in the raphe and the locus ceruleus. No tau positive tangles were found in the substantia nigra, periaqueductal grey, or in the basis pontis. Ubiquitin stains for Lewy bodies and TDP-43 staining were also negative in the midbrain and pons. The neuropathological findings were consistent with AD Braak stage VI, by the NIA Consensus Criteria.1,2 Therefore, because no brainstem or cortical Lewy bodies were indentified with alpha-synuclein staining, the diagnoses of typical idiopathetic Parkinson’s disease or diffuse Lewy body disease were ruled out. In addition, no neurofibrillary tangles or tufted astrocytes were found in areas that are usually associated with progressive supranuclear palsy (PSP).
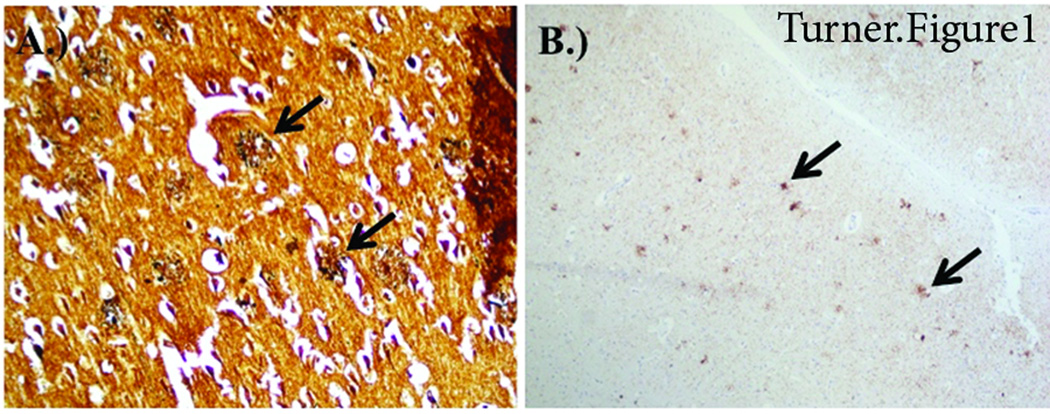
Figure 1.
Temporal lobe staining from AD brain. A) Bielschowsky silver stain demonstrating neuritic plaques and B) PHF-1 stain demonstrating neuritic plaques and neurofibrillary tangles.
Case II: CTE alone
The patient was a 41 year-old male with a history of participation in the National Football League for multiple seasons over the course of years. Upon retirement, the patient began to exhibit forgetfulness that was subsequently followed by progressive agitation, depression, anxiety, and agoraphobia in the years prior to his death.
The fixed brain weighed 1554 grams. A portion of the dura was not available for examination. The leptomeninges were thin and translucent over the convexities but over the base of the brain were fibrotic. There was no cerebral edema, and no evidence of contusions at the base of the brain. There was no evidence of cortical atrophy, infarctions or mass lesions. There was mild cerebral edema. The arteries of the circle of Willis demonstrated no atherosclerosis. There were no aneurysmal dilatations of the cerebral vasculature. Subfalcine, transtentorial, uncal, or tonsillar herniation was not appreciated. The brainstem and cerebellum had a normal external appearance. Serial coronal sectioning through the fixed brain revealed the usual internal architectural features without infarcts or mass lesions. The subcortical nuclei including the basal ganglia and thalamus, as well as mammillary bodies bilaterally were of normal size, color, and consistency. There was no evidence of hippocampal or amygdalae atrophy. The posterior corpus callosum showed mild thinning. The ventricular system was mildly dilated. Serial sections of the brainstem and cerebellum revealed no lesions, but the substantia nigra and the pontine locus ceruleus showed moderate pallor.
The H&E and Bielschowsky silver stains revealed no amyloid plaques of either the diffuse or neuritic type in any of neocortical or subcortical areas examined. However, there were neurofibrillary tangles, particularly in the neurons of the superficial temporal neocortex. No cortical Lewy bodies were identified with alpha-synuclein staining. Neurofibrillary tangles were also noted in entorhinal cortex particularly in the origin of the perforant pathway. The hippocampus and amygdala contained neurofibrillary tangles in a diffuse distribution in the latter. Most of the hippocampal CA fields contained diffuse neurofibrillary tangles. The substantia nigra showed no significant neuronal drop out. Lewy bodies were not readily identified in the substantia nigra. The pons contained tangles in the locus ceruleus. PHF-1 tau staining revealed numerous positive tangles and neuritic neuropil changes in the entorhinal cortex including the neurons in the origin of the perforant pathway. In addition, there was PHF-1 tau positive tangles in the superficial neocortical regions (temporal>mid-frontal>>occipital). The amygdala, hippocampus and thalamus contained PHF-tau positive tangles. The substantia nigra contained a single neuron with surrounding neuritic change, but the locus ceruleus (LC) was severely affected with numerous PHF-tau positive tangles and neuritic change in the neuropil. The raphe and the surrounding pontine tegmentum contained a few scattered phosphorylated tau positive cells (Figure 2).
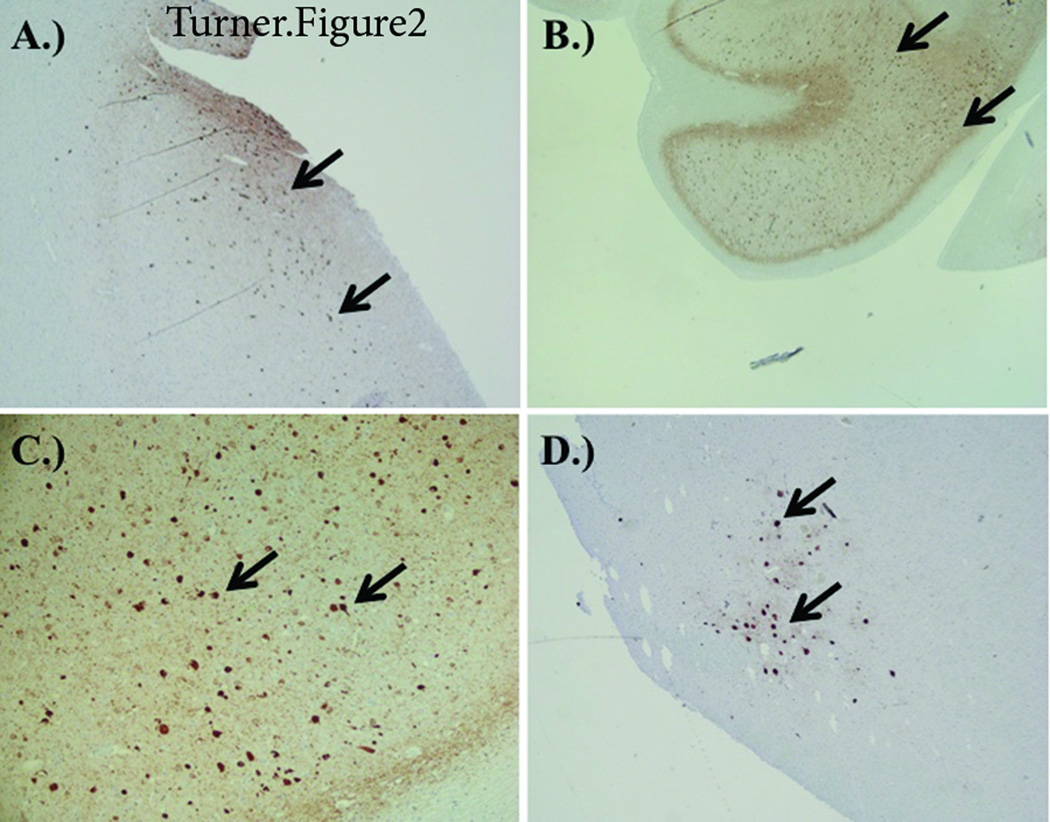
Figure 2.
Staining from CTE brain. A) Entorhinal Cortex PHF-1 staining demonstrating neurofibrillary tau tangles. B) Subiculum/hippocampus PHF-1 staining (low power 20x) demonstrating neurofibrillary tau tangles and neuritic neuropil changes. C) Subiculum/hippocampus PHF-1 staining (high power 63x) demonstrating tau neurofibrillary tau tangles. D) Pontine Tegmentum-Locus Ceruleus PHF-1 staining demonstrating neurofibrillary tau tangles.
Case III: overlapping AD & CTE
The patient was a 74 year-old man at the time of death with a past medical history positive for atrial fibrillation and AD (clinically diagnosed at age 67). His initial symptoms included an inability to compute simple percentage discounts at his furniture store, followed by a gradual progression with variable response to Aricept/memantine, eventually becoming aphasic. Additional neurological history is significant for head trauma in the following instances: breech delivery (as an infant), diving accident (as a child), bicycle accident, warehouse fall (age 60), high school and college football starter with numerous concussions sustained while participating, and two falls while in the nursing home.
The brain weighed 1336 grams. The leptomeninges were fibrotic, including at the base of the brain; there were no exudates. There was no cerebral edema, and no evidence of contusions at the base of the brain. The brain demonstrated evidence of diffuse cerebral cortical atrophy involving the frontal and temporal lobes as well as the cingulate gyrus. The arteries of the circle of Willis demonstrated mild atherosclerosis. The brainstem and cerebellum had a normal external appearance. Serial coronal sectioning revealed the usual internal architectural features without infarcts or mass lesions. The caudate nuclei, however, were flattened bilaterally. The remainder of the basal ganglia and thalamus, and mammillary bodies bilaterally were of normal size, color, and consistency. There was, however, bilateral hippocampal and amygdalae atrophy. The ventricular system was moderately dilated (posteriorly > anteriorly). In addition, a cavum septum pellucidum was found and was most prominent ventrally. Serial sections of the brainstem and cerebellum revealed no lesions with the substantia nigra being well pigmented. However, the pontine locus ceruleus was pale. There was mild superior vermal atrophy in the cerebellum.
The H&E and Bielschowsky silver stains revealed numerous amyloid plaques of both the diffuse as well as neuritic type in all neocortical areas (Figure 3). In addition, there were numerous neurofibrillary tangles seen, particularly in the neocortical pyramidal neurons of layers 3 and 5, but no cortical Lewy bodies were found. The tangles were both laminar in distribution with some punctate perivascular positive staining. The sensory motor-strip and temporal tip showed Congo red positive cortical blood vessels for cerebral amyloid angiopathy. There was pallor of the white matter with numerous hyalinized barrel-shaped small blood vessels. The caudate and putamen contain diffuse plaques, evidence of gliosis, and numerous hyalinized barrel-shaped small blood vessels. Neurofibrillary tangles were also noted in the ventral striatum. The hippocampus and amygdala contained numerous amyloid plaques and neurofibrillary tangles, particularly in the CA1 field. In addition, the amygdala contained Lewy bodies evidenced by positive alpha-synuclein. The thalamus contained neurofibrillary tangles in the midline nuclei. The substantia nigra was gliotic with neuronal drop out with some remaining neurons containing neurofibrillary tangles, but no Lewy bodies were indentified. The pons contained tangles in the locus ceruleus and raphe nuclei. The cerebellum contained diffuse plaques in the molecular layer.
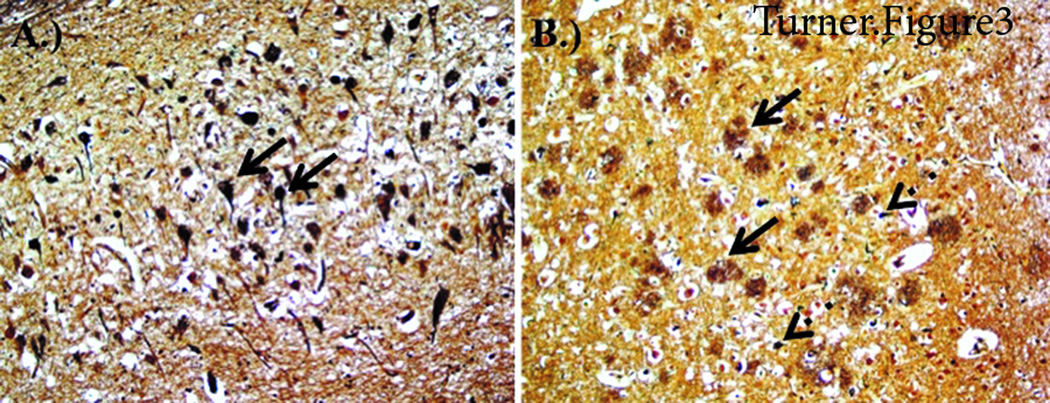
Figure 3.
Staining from CTE-AD brain. A) Hippocampus Bielschowsky Silver staining demonstrating neurofibrillary tau tangles. B) Temporal Cortex Bielschowsky Silver staining demonstrating amyloid plaques and neurofibrillary tau tangles.
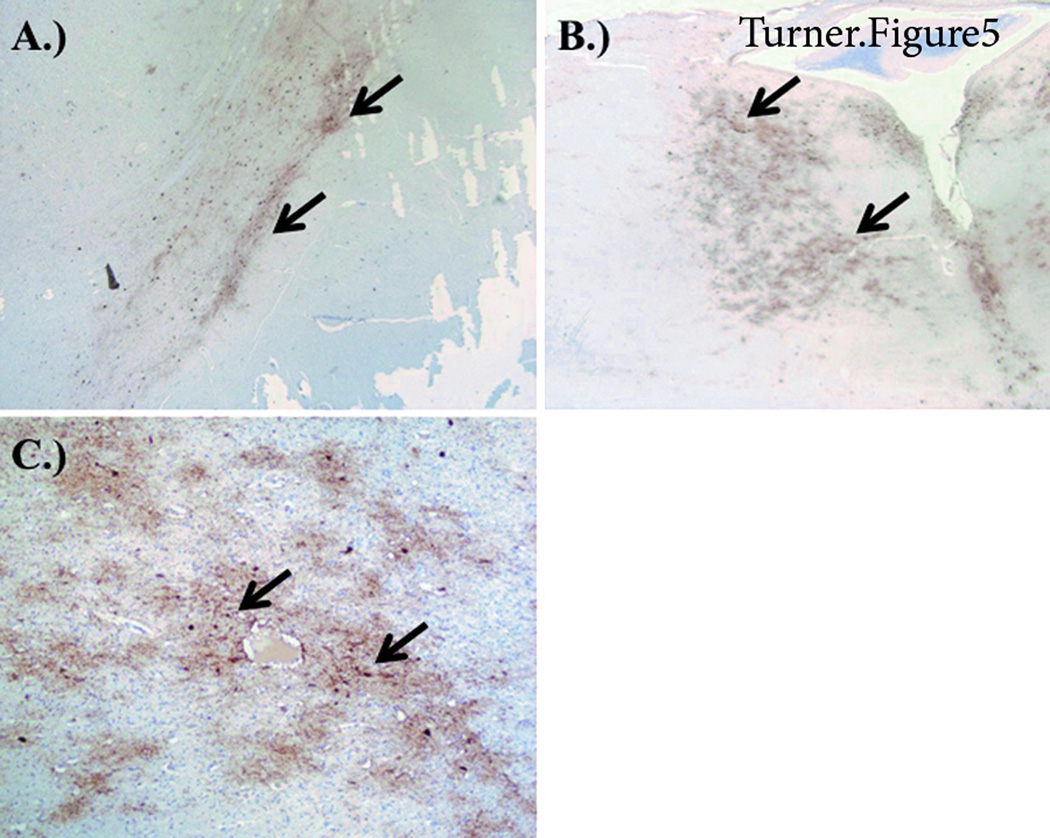
PHF-1 tau staining revealed numerous positive neurofibrillary tangles and neuritic plaques as well as a laminar distribution of neuritic neuropil changes consistent with typical AD as described above. However, there were significant PHF-1 tau tangles and neuritic changes in the following areas: the pontine tegmentum outside the locus ceruleus and raphe, the substantia nigra both laterally and medially, and the lateral thalamic nuclei and pulvinar. Additionally, characteristic of CTE, perivascular PHF was seen at the depths of the sulci in cortical white matter and the indusium griseum (Figures 4 and 5). The neuropathologic findings were consistent with AD neuropathologic change (high level) by the NIA Consensus Criteria 1,2. Each category of scoring – Aβplaque distribution (A score-3), neurofibrillary tangles extent (B score-3), and number of neuritic plaques (C score-3) – received a high grade (A3 B3 C3). When cognitive impairment and behavioral change is apparent clinically, an intermediate or high grade of neuropathologic change strongly substantiates AD as the main disease. The finding of cerebral amyloid angiopathy was typical, as it is rather common in cases with a significant degree of Aβ plaques. The finding of Lewy bodies in the amygdala (Lewy body disease, amygdala-predominant) is also typical for AD, as Lewy body disease in the amygdala typically occurs along with high grade AD neuropathologic change. There was both small and large vessel disease present. Cerebrovascular disease is thought to be a comorbid factor.
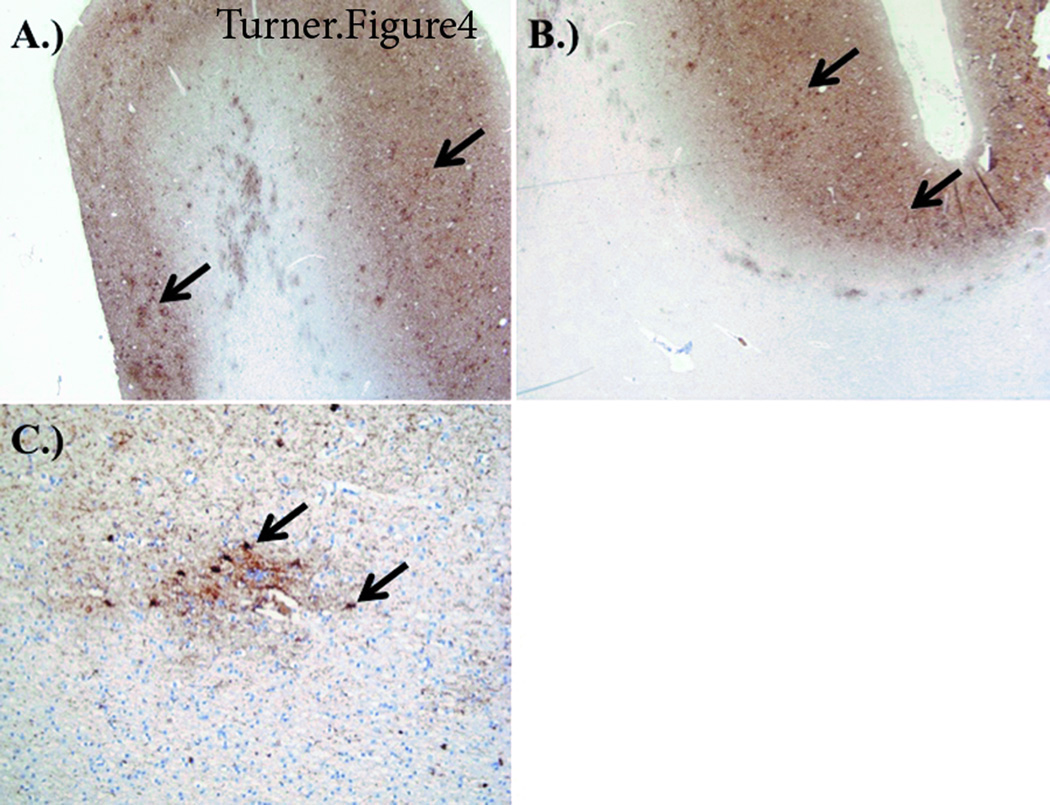
Figure 4.
Staining from CTE-AD brain. A) Temporal cortex PHF-1 staining demonstrating tau neuritic plaques (arrows) and neuritic threads. B) Temporal cortex PHF-1 staining (low power 20x) demonstrating perivascular tauopathy. C) Temporal cortex PHF-1 staining (high power 63x) demonstrating perivascular tauopathy.
Figure 5.
Staining from CTE-AD brain. A) Substantia Nigra PHF-1 staining demonstrating neurofibrillary tau tangles and neuritic neuropil changes. B) Pontine tegmentum PHF-1 staining demonstrating neurofibrillary tau tangles and neuritic neuropil changes. C) Thalamus (Pulvinar) PHF-1 staining demonstrating tau neurofibrillary tangles (arrows) and neuritic neuropil changes.
Additional abnormal tau findings in the reticular activating system of the pons, the entire substantia nigra, lateral thalamic nuclei, the pulvinar, and in the perivascular subcortical white matter, suggest a concomitant diagnosis of CTE. CTE has a clinical picture of mood, personality, cognitive change, behavioral change, and motor symptomatology, as described above. Associated histopathologic changes are considered due to an extensive accumulation of phosphorylated tau protein, seen as neurofibrillary and astrocytic tangles. With or without the specific label of CTE, football players with a history of TBI have a higher risk of chronic mood, behavioral, and cognitive issues.3 AD clearly played a significant role in the neurological morbidity but may have been exacerbated by CTE. The deceased patient competed in contact sports at a high level for many years, experiencing multiple concussive events, along with head injuries acquired outside of athletics. Therefore, it is important to consider CTE, in addition to AD, as a separate and/or concomitant contributor in his neurological decline.
DISCUSSION
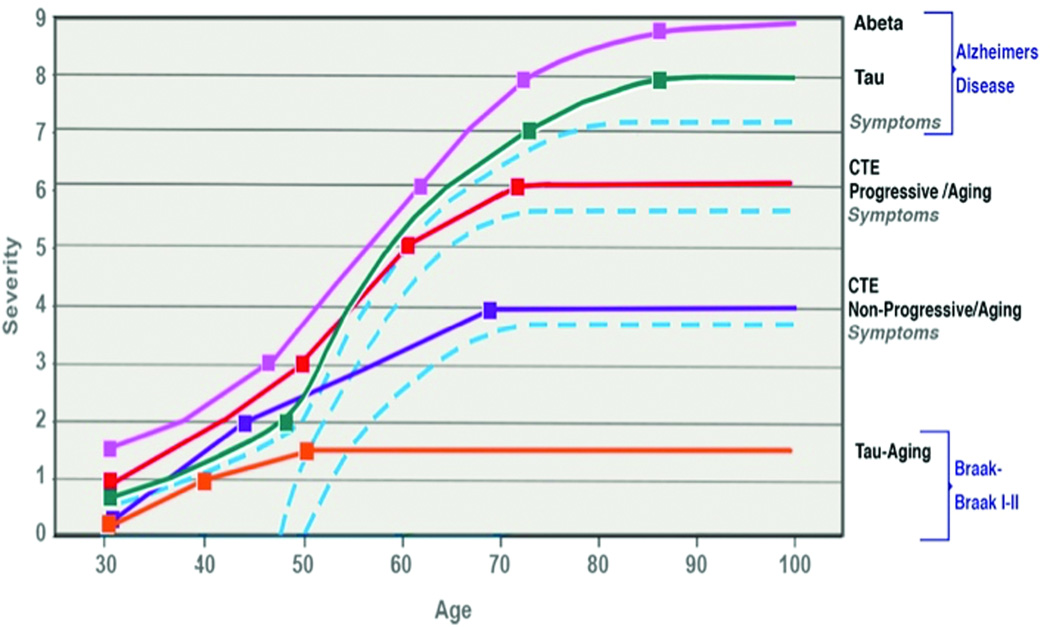
This work represents a case-based presentation of neuropathological evidence indicating important factors related to coexisting AD and CTE in a patient with a prior history of neurotrauma. The key distinctions between these two diseases are highlighted in table 1. The cases of two patients, one with typical AD with a medical history significant for neurotrauma and one with evidence of CTE changes, provides evidence of the distinguishing features between AD and CTE. Interestingly, the context of overlapping AD and CTE has features of both. This work demonstrates a potential relationship between CTE and AD and raises numerous questions regarding disease etiology. Specifically, 1) does CTE accelerate the development of AD, and 2) does CTE exhibit a progressive or non-progressive course with aging? This notion is conceptualized in Figure 6 with curves representing standard AD development that is triggered and accelerates with age, a possible progressive form of CTE in which initial changes induced by head trauma act synergistically with age resulting in a rapid increase in symptoms, and a possible non-progressive form of CTE in which the rate of increase appears independent of the aging process.
Table 1.
Key differences between AD and CTE
| ChronicTraumatic Encephalopathy |
Alzheimer’s Disease | |
|---|---|---|
| Neurofibrillary tau tangles location: |
Layers II and III of cortex, Ammon’s Horn of Hippocampus, and Pulvinar |
Layers V and VI of cortex and CA1 of Hippocampus |
| Clinical Features | Mood disturbances and Parkinson’s like features with tangles in substantia nigra and locus ceruleus and few or no Aβ plaques |
Cognitive impairment with extracellular amyloid pathology in middle frontal, superior and middle temporal, and inferior parietal lobule |
| Anatomy | Perivascular distribution of pathology |
Severe cerebral atrophy |
Figure 6.
Timeline showing natural progression of AD, progressive CTE, non-progressive CTE, and normal aging. The dashed blue lines indicate neurotrauma-associated acceleration. Traumatic brain injuries can accelerate normal aging leading to non-progressive or progressive CTE. CTE may predispose to early onset Alzheimer’s disease. Further epidemiologic studies are warranted.
While the answers to these questions are not clear at present, this case series presented within this manuscript indicates that AD and CTE may be observed within the same patient and therefore, be mechanistically and temporally linked. How to best address this link and answer the questions posed above is a subject of debate but some context and suggestions for directions forward are provided in the subsequent sections.
Understanding the possible link between AD & CTE: role of preclinical models
Improving the understanding of CTE development
To address questions regarding the relationship of CTE and AD, the fundamentals of CTE pathophysiology and development of clinical symptoms must be better understood. CTE has been documented in athletes and soldiers alike with a history of concussion exposure. Recent evidence indicates that subconcussive injury, a less severe and generally undetectable form of injury, may still result in the development of CTE. As such, it is clear that numerous questions remain that must be addressed to further understand the origin and pathophysiology of CTE. These include the role of impact severity, the time between impacts, the age at which impacts occur, the total number of impacts, the location of impacts, character of impacts, and genetic makeup of the individual being studied.
Preclinical studies are only just beginning to address these questions with the development or repurposing of prior models of neurotrauma that produce characteristic behavioral deficits or replicate hallmark neuropathological characteristics seen in CTE. Importantly, no model currently in use has been well characterized in terms of not only behavioral or functional but also biochemical or immunohistochemical findings to be fully representative of CTE. Petraglia and colleagues have developed a model of repetitive neurotrauma that replicates many of the behavioral features seen in CTE when 42 impacts are administered over a 7-day period (6 impacts per day).34 Specifically, animals exhibited long-term depressive-like and risk-taking behaviors while also demonstrating deficits in spatial learning and memory.34 Notably, a single impact using this model resulted in short-term behavioral abnormalities which resolved with time, representative of a post-concussive state, but the repetitive injury paradigm produced persistent deficits that lasted months after impact 34. Furthermore, studies were completed in unanesthetized mice, increasing the clinical relevance of the study.34 Work by Mouzon and colleagues performed both behavioral and neuropathological assessments on animals receiving either single or repeat injuries (5 injuries spaced 48 hours apart) and determined that repetitive injury results in persistent cognitive deficits, diminished learning capabilities, and altered rotarod and elevated-plus maze performance. Importantly, these deficits were documented in the context of neuropathological changes that included persistent neuroinflammation and white matter degradation as far out as 12 months post-injury. However, no changes in amyloid beta or tau phosphorylation were observed at 6 or 12 months post-injury.57 Luo, et al developed a model of closed head repeat injury in mice with a total of three impacts and a 24-hour interval between impacts. When animals were assessed months after injury, cognitive deficits persisted. Importantly, neuropathological analysis revealed an increase in phosphorylated tau as well following repeat injury months after the neurotrauma. Surprisingly, no changes in anxiety-like behavior were seen in this study.58 Models employing a single injury have been shown in some cases to produce the prolonged persistence of tau oligomers as soon as 4 hours post-injury out to at least 2 weeks post-injury.59 This work demonstrates the ability of neurotrauma to induce CTE-like neuropathology in rodents, following even a single injury, however it is clear that more work is needed to fully characterize preclinical rodent models aiming to replicate CTE.
Does repetitive neurotrauma increase risk and accelerate AD development?
The suggestion has been previously made that the onset of AD may be accelerated following repetitive concussions. This suggestion was based on clinical evidence indicating that not only do retired NFL players with a history of three concussions have a five-fold greater prevalence of mild cognitive impairment but also an earlier onset of AD was observed in retired players than in the general American male population.60 In a population of military veterans, similar findings were observed with a TBI diagnosis at baseline equating to a 60% increase in likelihood of developing dementia in the subsequent nine years. Besides just increasing the likelihood of a dementia diagnosis, the age of dementia onset appeared to be accelerated by approximately 2 years.61 Similar findings have been reported in other studies with neurotrauma being associated with an increased risk of AD onset,62–64 or accelerated development.65
To more definitively address the role of neurotrauma as a risk factor for AD or as a synergistic factor leading to acceleration of AD development, preclinical models may play an important role. Work by the Brody group in a mouse model of Alzheimer’s disease (3xTg-AD) showed that neurotrauma resulted in acceleration of intraxonal Aβ accumulation and the hyperphosphorylation of tau, indicating that neurotrauma does at the very least accelerate the development of AD changes in predisposed rodents.66 This work and others raises further questions regarding the distinctions between dementia associated with AD and CTE independently and perhaps most importantly, the question of whether overlapping features may exist and whether individuals may suffer from both diseases simultaneously.25,66 Importantly, other work from the Brody group regarding modulation of tau hyperphosphorylation via Jun N-terminal kinase (JNK) in this same animal model has shown that administration of a JNK inhibitor prevents tau hyperphosphorylation and represents a possible therapeutic approach going forward for these conditions related to neurotrauma exposure.67
As such, further studies are required to determine the relationship between neuropathological features/mechanisms associated with CTE and AD and the possibility of overlapping features/phenotypes existing. Specific questions relating to the temporality of AD development in the context of CTE and/or neurotrauma remain unanswered (Figure 6). These include: does AD develop simultaneously with CTE or does CTE lead to an earlier expression of AD? What is the role of impact history in development of these conditions? Do genetics play a role? What mechanisms may be implicated and shared across conditions?
Mechanisms implicated in neuropathology of AD & CTE
Endoplasmic reticulum stress
A prominent theory for the progression of chronic neurodegenerative tauopathies and amyloidopathies is a disruption of calcium homeostasis,68 ultimately leading to the development of granulovacuolar degeneration and endoplasmic reticulum (ER) stress, eventually causing a cessation of normal protein folding.69,70 Similarly, the upregulation of amyloid precursor protein expression, which is a prominent feature of TBI,71–73 is also known to activate the endoplasmic reticulum stress response.74 In AD, ER stress is particularly pronounced in early stages of the disease process.75 ER stress has also been postulated to contribute to CTE progression.76,77 The three arms of the ER stress response are mediated by PKR-like endoplasmic reticulum eIF2α kinase (PERK), inositol requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6).78 The PERK arm can activate caspase 12 leading to apoptosis.79 Apoptosis signal regulating kinase 1 interacts with caspase 12 to contribute to chronic neurodegeneration once the PERK pathway is initiated.80 Inhibiting the PERK pathway alleviates memory deficits in an AD rodent model.81 The IRE1α arm activates x-box binding protein 1 (XBP-1) and upregulates a disintegrin and metalloproteinase 10 (ADAM10) gene expression.82 ADAM10 has been intimately linked with β-amyloid pathology.83 Targeting ADAM10 following TBI reduces amyloid β accumulation over time.84 Hyperphosphorylated tau increases the cleavage of ATF6,85 which subsequently results in the ATF6-CREBH inducer contributing to neuroinflammation.86 Shifting balance towards the first arm of the ER stress response following TBI limits neuroinflammatory damage from ATF6.87 Continued research is required to fully understand how ER stress influences chronic neurodegenerative disease progression but may represent a promising avenue going forward in neurotrauma research.
Mitochondrial dysfunction
Mitochondrial function or dysfunction may play an important role in AD and CTE pathology.88 Reactive oxygen species can damage mitochondria leading to axonal degeneration following TBI.89 Cells that survive the primary insult are subject to tau-mediated mitochondrial damage, which occurs over an extended period of time.90 Tau can increase glutathione leading to increased permeability of mitochondrial membranes. In AD, damaged mitochondria can no longer generate ATP leaving neuronal cells susceptible to metabolic crisis.91 In CTE, mitochondrial damage accelerates the cell stress response and has the potential for further neurodegeneration.92 Additionally, amyloid-β accumulation is increased at the mitochondria-endoplasmic reticulum interface.93 Mitochondria specific antioxidants have also proved promising in transgenic models of AD.94 Ongoing research is needed to investigate therapeutic strategies targeting mitochondria. Mitochondrial biomarkers might also offer improved diagnostic potential for both AD and CTE.95 Recent evidence has confirmed that mutated mitochondrial DNA are a clear indicator of future neurodegenerative pathology.96 The question remains as to how acute mitochondrial changes lead to progressive decline over time. The answer is likely due to mitochondria heteroplasmy and a shift past threshold as the brain ages.97
Neuroinflammation
Reactive gliosis following neural injury can lead to chronic inflammation and neurodegeneration.98 CD36 receptors triggered by reactive astrocytes initiate a robust innate immune response.99 In addition, neuroinflammatory genes are increased following injury.100 Chronic inflammation disrupts neural networks impeding effective transynaptic communication.101 Neurodegerative diseases, such as CTE and AD, can confound recovery by perpetually activating microglia.102 A new treatment strategy focuses on neuroregeneration through stem cell management,103 theorizing that induction or placement of stem cells or stem cell-related products may result in inflammation modulation. Other effective strategies under investigation include cyclin-dependent kinase inhibitors; decay accelerating factor inducers, and CD integrin inhibitors.104–106
Oxidative stress
Oxidative stress can occur as early as 3 hours following TBI.107 Oxygen free radicals can damage lipid, proteins, and DNA.108 Most importantly oxidative stress leads to mitochondrial dysfunction and contributes to chronic neurodegeneration.109 The mitochondrial transition pore can become damaged leading to an efflux of free radicals.110 Over time oxidative stress triggers neuronal apoptosis.111 Additionally, oxidative stress contributes to hippocampal synaptic protein loss.112 Oxidative stress has been associated with post-traumatic stress disorder, cognitive decline, and CTE.113 DNA damage induced by oxidative stress is common in AD.114 Not surprisingly, a single TBI increases the risk for early onset AD by 30% partly due to oxidative damage.115 An endogenous glutathione anti-oxidant response is often insufficient to prevent oxidative damage following TBI.116 Puerarin administered post-TBI has produced promising preclinical findings showing reduced oxidative stress.117 Further research is needed to develop effective anti-oxidant solutions for neurotrauma treatment.
CONCLUSION
The emergence of neuropathological findings of CTE as a disease affecting a significant subset of former athletes and military veterans has raised substantial interest in the scientific and lay communities regarding neurotrauma exposure in adults and children alike. Increasing evidence has implicated neurotrauma, both of the concussive and subconcussive severities, in not only the development of CTE but also the possible subsequent development of AD and other neuropsychiatric disease. In this work, we demonstrate a case of overlapping AD and CTE. This case provides evidence that a single individual may possess neuropathological features of both conditions. The existence of a dual diagnosis has significant public health relevance as it provides a potential link between neurotrauma and neurodegeneration development while raising numerous potential questions with regards to how impact severity, age at time of impact, and inter-injury interval may affect the likelihood of disease development. These questions and potential mechanisms for answering them were discussed. Important features of new preclinical models were highlighted in addition to potential mechanisms of interest for understanding not only disease pathophysiology but also identification of therapeutic targets. These findings demonstrate a clear need for further investigation into CTE and AD, particularly with the potential of overlapping disease entities.
Acknowledgments
An American Medical Association Foundation Seed Grant, an American Foundation of Pharmaceutical Education Pre-doctoral Fellowship, and a Neurosurgery Research and Education Foundation Medical Student Summer Research Fellowship were awarded to support Brandon Lucke-Wold. Ryan Turner was supported by a NIH Training Grant. Research reported in this publication was supported by the NIGMS of the National Institutes of Health under award number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DECLARATIONS OF INTEREST
The authors report no competing financial interests exist.
REFERENCES
- 1.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavett BE, Cantu RC, Shenton M, Lin AP, Nowinski CJ, McKee AC, Stern RA. Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Curr Opin Neurol. 2011;24(6):525–531. doi: 10.1097/WCO.0b013e32834cd477. [DOI] [PubMed] [Google Scholar]
- 4.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartaglia MC, Hazrati LN, Davis KD, Green RE, Wennberg R, Mikulis D, Ezerins LJ, Keightley M, Tator C. Chronic traumatic encephalopathy and other neurodegenerative proteinopathies. Front Hum Neurosci. 2014;8:30. doi: 10.3389/fnhum.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seifan A, Marder KS, Mez J, Noble JM, Cortes EP, Vonsattel JP, Honig LS. Hippocampal Laminar Distribution of Tau Relates to Alzheimer’s Disease and Age of Onset. J Alzheimers Dis. 2014 doi: 10.3233/JAD-140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1):179–188. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 Suppl):S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg. 2013;119(5):1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- 11.Dashnaw ML, Petraglia AL, Bailes JE. An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg Focus. 2012;33(6):1–9. doi: 10.3171/2012.10.FOCUS12284. [DOI] [PubMed] [Google Scholar]
- 12.Faul M, Xu L, Wald MM, Coronado VG. Trauamtic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Services UDoHaH. 2010 [Google Scholar]
- 13.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Small GW, Kepe V, Siddarth P, Ercoli LM, Merrill DA, Donoghue N, Bookheimer SY, Martinez J, Omalu B, Bailes J, et al. PET scanning of brain tau in retired national football league players: preliminary findings. Am J Geriatr Psychiatry. 2013;21(2):138–144. doi: 10.1016/j.jagp.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 16.Martland HS. Punch drunk. JAMA. 1928;91:1103–1107. [Google Scholar]
- 17.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 128–34. [DOI] [PubMed] [Google Scholar]
- 18.Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. discussion 1092–3. [DOI] [PubMed] [Google Scholar]
- 19.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69(9):918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6(3):130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 21.Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, Webster G, Fitzsimmons RP. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus. 2011;31(5):E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 22.Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69(1):173–183. doi: 10.1227/NEU.0b013e318212bc7b. discussion 183. [DOI] [PubMed] [Google Scholar]
- 23.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol. 2014;127(1):29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. 2012;69(10):1245–1251. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner RC, Lucke-Wold BP, Robson MJ, Omalu BI, Petraglia AL, Bailes JE. Repetitive traumatic brain injury and development of chronic traumatic encephalopathy: a potential role for biomarkers in diagnosis, prognosis, and treatment? Front Neurol. 2012;3:186. doi: 10.3389/fneur.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villemagne VL, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Hodges J, Harada R, Yates P, Piguet O, Pejoska S, Dore V, et al. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2014;41(5):816–826. doi: 10.1007/s00259-013-2681-7. [DOI] [PubMed] [Google Scholar]
- 28.Villemagne VL, Okamura N. In vivo tau imaging: Obstacles and progress. Alzheimers Dement. 2014;10(3 Suppl):S254–S264. doi: 10.1016/j.jalz.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Baugh CM, Robbins CA, Stern RA, McKee AC. Current understanding of chronic traumatic encephalopathy. Curr Treat Options Neurol. 2014;16(9):306. doi: 10.1007/s11940-014-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banks SJ, Mayer B, Obuchowski N, Shin W, Lowe M, Phillips M, Modic M, Bernick C. Impulsiveness in professional fighters. J Neuropsychiatry Clin Neurosci. 2014;26(1):44–50. doi: 10.1176/appi.neuropsych.12070185. [DOI] [PubMed] [Google Scholar]
- 31.Randolph C. Is chronic traumatic encephalopathy a real disease? Curr Sports Med Rep. 2014;13(1):33–37. doi: 10.1097/OPX.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 32.Wortzel HS, Shura RD, Brenner LA. Chronic traumatic encephalopathy and suicide: a systematic review. Biomed Res Int. 2013;2013:424280. doi: 10.1155/2013/424280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Love S, Solomon GS. Talking With Parents of High School Football Players About Chronic Traumatic Encephalopathy: A Concise Summary. Am J Sports Med. 2014 doi: 10.1177/0363546514535187. [DOI] [PubMed] [Google Scholar]
- 34.Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma. 2014;31(13):1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep. 2013;13(12):407. doi: 10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther. 2014;6(1):4. doi: 10.1186/alzrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A, Javien H, Merchant-Borna K, Abar B, Blackman EG. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9(4):e94734. doi: 10.1371/journal.pone.0094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling H, Kara E, Revesz T, Lees AJ, Plant GT, Martino D, Houlden H, Hardy J, Holton JL. Concomitant progressive supranuclear palsy and chronic traumatic encephalopathy in a boxer. Acta Neuropathol Commun. 2014;2(1):24. doi: 10.1186/2051-5960-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen HA, Ferraro FR, Himle M, Schultz C, Poolman M. Neuropsychological factors related to college ice hockey concussions. Am J Alzheimers Dis Other Demen. 2014;29(3):201–204. doi: 10.1177/1533317513517036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chauhan NB. Chronic neurodegenerative consequences of traumatic brain injury. Restor Neurol Neurosci. 2014;32(2):337–365. doi: 10.3233/RNN-130354. [DOI] [PubMed] [Google Scholar]
- 41.Lehman EJ. Epidemiology of neurodegeneration in American-style professional football players. Alzheimers Res Ther. 2013;5(4):34. doi: 10.1186/alzrt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowinski C. Hit parade: the future of the sports concussion crisis. Cerebrum. 2013;2013:2. [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol. 2013;12(9):882–893. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- 44.Bernick C, Banks S. What boxing tells us about repetitive head trauma and the brain. Alzheimers Res Ther. 2013;5(3):23. doi: 10.1186/alzrt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin B, Bhardwaj A. Chronic traumatic encephalopathy: a critical appraisal. Neurocrit Care. 2014;20(2):334–344. doi: 10.1007/s12028-013-9931-1. [DOI] [PubMed] [Google Scholar]
- 46.Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, Fritts NG, Stamm JM, Robbins CA, McHale L, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol. 2013;9(4):192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakis N, Corona RJ, Toshkezi G, Chin LS. Chronic traumatic encephalopathy - neuropathology in athletes and war veterans. Neurol Res. 2013;35(3):290–299. doi: 10.1179/1743132813Y.0000000177. [DOI] [PubMed] [Google Scholar]
- 49.Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. 2013;9(4):222–230. doi: 10.1038/nrneurol.2013.33. [DOI] [PubMed] [Google Scholar]
- 50.Seichepine DR, Stamm JM, Daneshvar DH, Riley DO, Baugh CM, Gavett BE, Tripodis Y, Martin B, Chaisson C, McKee AC, et al. Profile of self-reported problems with executive functioning in college and professional football players. J Neurotrauma. 2013;30(14):1299–1304. doi: 10.1089/neu.2012.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broglio SP, Eckner JT, Paulson HL, Kutcher JS. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc Sport Sci Rev. 2012;40(3):138–144. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sayed N, Culver C, Dams-O’Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma. 2013;30(13):1117–1122. doi: 10.1089/neu.2012.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanaan NM, Cox K, Alvarez VE, Stein TD, Poncil S, McKee AC. Characterization of Early Pathological Tau Conformations and Phosphorylation in Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol. 2015 doi: 10.1093/jnen/nlv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner RC, Naser ZJ, Logsdon AF, DiPasquale KH, Jackson GJ, Robson MJ, Gettens RT, Matsumoto RR, Huber JD, Rosen CL. Modeling clinically relevant blast parameters based on scaling principles produces functional & histological deficits in rats. Exp Neurol. 2013;248:520–509. doi: 10.1016/j.expneurol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134):134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omalu B. Chronic traumatic encephalopathy. Prog Neurol Surg. 2014;28:38–49. doi: 10.1159/000358761. [DOI] [PubMed] [Google Scholar]
- 57.Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol. 2014;75(2):241–254. doi: 10.1002/ana.24064. [DOI] [PubMed] [Google Scholar]
- 58.Luo J, Nguyen A, Villeda S, Zhang H, Ding Z, Lindsey D, Bieri G, Castellano JM, Beaupre GS, Wyss-Coray T. Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol. 2014;5:12. doi: 10.3389/fneur.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkins BE, Krishnamurthy S, Castillo-Carranza DL, Sengupta U, Prough DS, Jackson GR, DeWitt DS, Kayed R. Rapid accumulation of endogenous tau oligomers in a rat model of traumatic brain injury: possible link between traumatic brain injury and sporadic tauopathies. J Biol Chem. 2013;288(23):17042–17050. doi: 10.1074/jbc.M113.472746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719–726. doi: 10.1093/neurosurgery/57.4.719. discussion 719–26. [DOI] [PubMed] [Google Scholar]
- 61.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013;8(5):e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, Sano M, Stern Y, Mayeux R. Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62(2):119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang HK, Lin SH, Sung PS, Wu MH, Hung KW, Wang LC, Huang CY, Lu K, Chen HJ, Tsai KJ. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83(11):1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 65.Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149(1):32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- 66.Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31(26):9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran HT, Sanchez L, Brody DL. Inhibition of JNK by a peptide inhibitor reduces traumatic brain injury-induced tauopathy in transgenic mice. J Neuropathol Exp Neurol. 2012;71(2):116–129. doi: 10.1097/NEN.0b013e3182456aed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tillement JP, Papadopoulos V. Subcellular injuries in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2014;13(4):593–605. doi: 10.2174/18715273113126660197. [DOI] [PubMed] [Google Scholar]
- 69.Kohler C, Dinekov M, Gotz J. Granulovacuolar degeneration and unfolded protein response in mouse models of tauopathy and Abeta amyloidosis. Neurobiol Dis. 2014;71C:169–179. doi: 10.1016/j.nbd.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Montague K, Malik B, Gray AL, La Spada AR, Hanna MG, Szabadkai G, Greensmith L. Endoplasmic reticulum stress in spinal and bulbar muscular atrophy: a potential target for therapy. Brain. 2014;137(Pt 7):1894–1906. doi: 10.1093/brain/awu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itoh T, Satou T, Nishida S, Tsubaki M, Hashimoto S, Ito H. Expression of amyloid precursor protein after rat traumatic brain injury. Neurol Res. 2009;31(1):103–109. doi: 10.1179/016164108X323771. [DOI] [PubMed] [Google Scholar]
- 72.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11(5):361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mills JD, Bailes JE, Turner RC, Dodson SC, Sakai J, Maroon JC. Anabolic steroids and head injury. Neurosurgery. 2012;70(1):205–209. doi: 10.1227/NEU.0b013e3182250918. discussion 209–10. [DOI] [PubMed] [Google Scholar]
- 74.Placido AI, Pereira CM, Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Oliveira CR, Moreira PI. The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: Implications for Alzheimer’s disease. Biochim Biophys Acta. 2014;1842(9):1444–1453. doi: 10.1016/j.bbadis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Lin L, Yang SS, Chu J, Wang L, Ning LN, Zhang T, Jiang Q, Tian Q, Wang JZ. Region-Specific Expression of Tau, Amyloid-beta Protein Precursor, and Synaptic Proteins at Physiological Condition or Under Endoplasmic Reticulum Stress in Rats. J Alzheimers Dis. 2014;41(4):1149–1163. doi: 10.3233/JAD-140207. [DOI] [PubMed] [Google Scholar]
- 76.Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surg Neurol Int. 2011;2:107. doi: 10.4103/2152-7806.83391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lucke-Wold BP, Turner RC, Logsdon AF, Bailes JE, Huber JD, Rosen CL. Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development. J Neurotrauma. 2014;31(13):1129–1138. doi: 10.1089/neu.2013.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Y, Li J, Zhang YQ, Jiang LH, Zhang YN, Yan CQ. Protein kinase C delta mediated cytotoxicity of 6-Hydroxydopamine via sustained extracellular signal-regulated kinase 1/2 activation in PC12 cells. Neurol Res. 2014;36(1):53–64. doi: 10.1179/1743132813Y.0000000267. [DOI] [PubMed] [Google Scholar]
- 79.Lu X, Li Y, Wang W, Chen S, Liu T, Jia D, Quan X, Sun D, Chang AK, Gao B. 3 beta-hydroxysteroid-Delta 24 reductase (DHCR24) protects neuronal cells from apoptotic cell death induced by endoplasmic reticulum (ER) stress. PLoS One. 2014;9(1):e86753. doi: 10.1371/journal.pone.0086753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song J, Park KA, Lee WT, Lee JE. Apoptosis Signal Regulating Kinase 1 (ASK1): Potential as a Therapeutic Target for Alzheimer’s Disease. Int J Mol Sci. 2014;15(2):2119–2129. doi: 10.3390/ijms15022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flight MH. Neurodegenerative diseases: New kinase targets for Alzheimer’s disease. Nat Rev Drug Discov. 2013;12(10):739. doi: 10.1038/nrd4132. [DOI] [PubMed] [Google Scholar]
- 82.Reinhardt S, Schuck F, Grosgen S, Riemenschneider M, Hartmann T, Postina R, Grimm M, Endres K. Unfolded protein response signaling by transcription factor XBP-1 regulates ADAM10 and is affected in Alzheimer’s disease. FASEB J. 2014;28(2):978–997. doi: 10.1096/fj.13-234864. [DOI] [PubMed] [Google Scholar]
- 83.Marcello E, Saraceno C, Musardo S, Vara H, de la Fuente AG, Pelucchi S, Di Marino D, Borroni B, Tramontano A, Perez-Otano I, et al. Endocytosis of synaptic ADAM10 in neuronal plasticity and Alzheimer’s disease. J Clin Invest. 2013;123(6):2523–2538. doi: 10.1172/JCI65401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zohar O, Lavy R, Zi X, Nelson TJ, Hongpaisan J, Pick CG, Alkon DL. PKC activator therapeutic for mild traumatic brain injury in mice. Neurobiol Dis. 2011;41(2):329–337. doi: 10.1016/j.nbd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Liu XA, Song J, Jiang Q, Wang Q, Tian Q, Wang JZ. Expression of the hyperphosphorylated tau attenuates ER stress-induced apoptosis with upregulation of unfolded protein response. Apoptosis. 2012;17(10):1039–1049. doi: 10.1007/s10495-012-0744-z. [DOI] [PubMed] [Google Scholar]
- 86.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubovitch V, Shachar A, Werner H, Pick CG. Does IGF-1 administration after a mild traumatic brain injury in mice activate the adaptive arm of ER stress? Neurochem Int. 2011;58(4):443–446. doi: 10.1016/j.neuint.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 88.De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 89.Bros H, Millward JM, Paul F, Niesner R, Infante-Duarte C. Oxidative damage to mitochondria at the nodes of Ranvier precedes axon degeneration in ex vivo transected axons. Exp Neurol. 2014;261C:127–135. doi: 10.1016/j.expneurol.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 90.Saman S, Lee NC, Inoyo I, Jin J, Li Z, Doyle T, McKee AC, Hall GF. Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J Alzheimers Dis. 2014;40(Suppl 1):S47–S70. doi: 10.3233/JAD-132135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khatri N, Man HY. Synaptic activity and bioenergy homeostasis: implications in brain trauma and neurodegenerative diseases. Front Neurol. 2013;4:199. doi: 10.3389/fneur.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naviaux RK. Metabolic features of the cell danger response. Mitochondrion. 2014;16:7–17. doi: 10.1016/j.mito.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 93.Schreiner B, Hedskog L, Wiehager B, Ankarcrona M. Amyloid-beta Peptides are Generated in Mitochondria-Associated Endoplasmic Reticulum Membranes. J Alzheimers Dis. 2014 doi: 10.3233/JAD-132543. [DOI] [PubMed] [Google Scholar]
- 94.Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Rachel W, Grela A, Zyss T, Zieba A, Piekoszewski W. [Biomarkers of Alzheimer disease] Przegl Lek. 2014;71(2):98–101. [PubMed] [Google Scholar]
- 96.Hroudova J, Singh N, Fisar Z. Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer’s disease. Biomed Res Int. 2014;2014:175062. doi: 10.1155/2014/175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coon KD, Valla J, Szelinger S, Schneider LE, Niedzielko TL, Brown KM, Pearson JV, Halperin R, Dunckley T, Papassotiropoulos A, et al. Quantitation of heteroplasmy of mtDNA sequence variants identified in a population of AD patients and controls by array-based resequencing. Mitochondrion. 2006;6(4):194–210. doi: 10.1016/j.mito.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 98.Avila-Munoz E, Arias C. When astrocytes become harmful: Functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res Rev. 2014;18C:29–40. doi: 10.1016/j.arr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 99.Diamandis T, Gonzales-Portillo C, Gonzales-Portillo GS, Staples M, Borlongan MC, Hernandez D, Acosta S, Borlongan CV. Diabetes insipidus contributes to traumatic brain injury pathology via CD36 neuroinflammation. Med Hypotheses. 2013;81(5):936–939. doi: 10.1016/j.mehy.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, Eiferman DS, Godbout JP. Methylene Blue Attenuates Traumatic Brain Injury-associated Neuroinflammation and Acute Depressive-like Behavior in Mice. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol. 2014;10(3):156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 102.Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73(1):14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dela Pena I, Sanberg PR, Acosta S, Tajiri N, Lin SZ, Borlongan CV. Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J Neurosurg Sci. 2014;58(3):145–149. [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Chavko M, Slack JL, Liu B, McCarron RM, Ross JD, Dalle Lucca JJ. Protective effects of decay-accelerating factor on blast-induced neurotrauma in rats. Acta Neuropathol Commun. 2013;1(1):52. doi: 10.1186/2051-5960-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo T, Wu J, Kabadi SV, Sabirzhanov B, Guanciale K, Hanscom M, Faden J, Cardiff K, Bengson CJ, Faden AI. Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology. 2013;119(6):1370–1388. doi: 10.1097/ALN.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 106.Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J Neuroinflammation. 2013;10:26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25(5):513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 108.Huang Y, Huang X. [Course of acute oxidative damage after traumatic brain injury: problem and strategy] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39(2):195–198. doi: 10.11817/j.issn.1672-7347.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 109.Wang F, Franco R, Skotak M, Hu G, Chandra N. Mechanical stretch exacerbates the cell death in SH-SY5Y cells exposed to paraquat: mitochondrial dysfunction and oxidative stress. Neurotoxicology. 2014;41:54–63. doi: 10.1016/j.neuro.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mazzeo AT, Beat A, Singh A, Bullock MR. The role of mitochondrial transition pore, and its modulation, in traumatic brain injury and delayed neurodegeneration after TBI. Exp Neurol. 2009;218(2):363–370. doi: 10.1016/j.expneurol.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 111.Itoh T, Imano M, Nishida S, Tsubaki M, Mizuguchi N, Hashimoto S, Ito A, Satou T. Increased apoptotic neuronal cell death and cognitive impairment at early phase after traumatic brain injury in aged rats. Brain Struct Funct. 2013;218(1):209–220. doi: 10.1007/s00429-012-0394-5. [DOI] [PubMed] [Google Scholar]
- 112.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45(4):443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kochanek PM, Dixon CE, Shellington DK, Shin SS, Bayir H, Jackson EK, Kagan VE, Yan HQ, Swauger PV, Parks SA, et al. Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J Neurotrauma. 2013;30(11):920–937. doi: 10.1089/neu.2013.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith JA, Park S, Krause JS, Banik NL. Oxidative stress, DNA damage, and the telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem Int. 2013;62(5):764–775. doi: 10.1016/j.neuint.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36(5):1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 116.Potts MB, Rola R, Claus CP, Ferriero DM, Fike JR, Noble-Haeusslein LJ. Glutathione peroxidase overexpression does not rescue impaired neurogenesis in the injured immature brain. J Neurosci Res. 2009;87(8):1848–1857. doi: 10.1002/jnr.21996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang JW, Wang HD, Cong ZX, Zhou XM, Xu JG, Jia Y, Ding Y. Puerarin ameliorates oxidative stress in a rodent model of traumatic brain injury. J Surg Res. 2014;186(1):328–337. doi: 10.1016/j.jss.2013.08.027. [DOI] [PubMed] [Google Scholar]