Abstract
Background
Opioid use disorder is a common cause of morbidity and mortality among people living with HIV/AIDS. Buprenorphine maintenance treatment (BMT) is an effective means of therapy, but patients with recent criminal justice involvement may need more support during BMT than other patients. We hypothesized that recently incarcerated BMT patients who initiated treatment in primary care would have poorer treatment outcomes than those who were not recently incarcerated.
Methods
We analyzed data from a multi-site cohort study of BMT integrated into HIV care. Patients were stratified by self-reported incarceration in the 30 days before initiation of BMT. The outcomes of interest were 6 and 12-month treatment retention and self-reported opioid use. We used multivariable logistic regression and hierarchical linear model, respectively, to evaluate the association between recent incarceration and these outcomes while adjusting for potential confounding variables.
Results
Among 306 BMT patients living with HIV/AIDS, 39 (13%) reported recent incarceration. Patients with recent incarceration (vs. without) were more likely to be homeless, unemployed, and previously diagnosed with mental illness. Recent incarceration was not significantly associated with differences in 6-month (OR = 0.95; 95% CI = 0.46–1.98) and 12-month treatment retention (OR = 0.57; 95% CI = 0.27–1.18) or in self-reported opioid use (OR = 0.99; 95% CI = 0.51–1.92) after adjustment for potential confounding variables.
Conclusions
Those with incarceration in the 30 days prior to BMT initiation were more likely to be homeless, unemployed, and previously diagnosed with mental illness than those without recent incarceration. However, we did not detect a difference in in self-reported opioid use, 6-month or 12-month retention in treatment between those with and without recent incarceration. Future studies should confirm these findings with larger sample sizes. Encouraging formerly incarcerated individuals with opioid use disorder to initiate evidence-based treatments, including BMT, should be part of efforts to confront the opioid addiction epidemic in the United States.
Keywords: Opiate substitution treatment, Buprenorphine, Opioid-related disorders, Opiate addiction, HIV, Incarceration, Criminal Justice
INTRODUCTION
In 2014, nearly 2.5 million Americans met criteria for an opioid use disorder,1 and nearly 30,000 died from opioid-related overdoses.2 Illicit opioid use also negatively affects quality of life.3 Treatment with buprenorphine or methadone can mitigate these negative effects.4 World Health Organization guidelines highlight pharmacotherapy using either buprenorphine or methadone paired with psychosocial therapy as the most effective means of treating opioid use disorder.5 Buprenorphine is particularly useful because it can be self-administered at home, has low potential for overdose, and may be less stigmatized than methadone.6 Buprenorphine maintenance treatment (BMT) may be offered in primary care or addiction treatment settings with the goal of providing pharmacotherapy and psychosocial counseling over a period of at least months to years.
Among the negative consequences of opioid use disorder is frequent involvement in the criminal justice system. Up to 1/3 of heroin users (200,000 individuals) are incarcerated annually in the United States.7 Initiating pharmacotherapy with buprenorphine or methadone prior to release from incarceration has been feasible and effective in clinical trials.8–12 However, few individuals in the United States actually receive these treatments while incarcerated.13 Due to the chronic relapsing nature of opioid use disorder, even those who abstain from opioid use while incarcerated may still require treatment following release.14 Without pharmacotherapy, rates of relapse to illicit opioid use following release from incarceration may be greater than 80%.15 BMT appears to be highly acceptable among individuals with criminal justice involvement,16 but additional research is necessary to optimize treatment for this high-needs population.
The interrelated problems of opioid use disorder and incarceration can be particularly challenging for people living with HIV/AIDS. One in seven Americans with HIV/AIDS pass through correctional facilities annually.17 A large cohort of formerly incarcerated individuals living with HIV/AIDS demonstrated a prevalence of opioid use disorder of 37%.18 Treatment of opioid use disorder with methadone or buprenorphine reduces illicit opioid use and improves HIV treatment outcomes,19–21 including among those with criminal justice involvement.22,23 Thus, integrating BMT into HIV-treatment settings has been a priority in the United States,24 but patients with criminal justice involvement may have unique treatment needs (e.g., increased counseling or closer supervision).
To date, studies have been conflicting as to whether criminal justice involvement affects treatment for opioid use disorder. Data from community methadone maintenance treatment programs suggest that criminal justice involvement can negatively affect treatment outcomes.25,26 In contrast, a study of BMT in primary care demonstrated that patients with any prior incarceration for more than three days in their lifetime had similar treatment retention and illicit opioid use to patients without prior incarceration.27 However, it is possible that a recent incarceration may have a greater destabilizing effect on treatment than incarcerations happening more distantly in the past. Another study from primary care demonstrated that BMT patients with criminal charges in the two years prior to initiating treatment had lower treatment retention and opioid abstinence than those without recent criminal charges.28 The challenges of community re-entry after release from incarceration have been documented in numerous qualitative studies, therefore, recent incarceration might also negatively affect BMT outcomes.29–31
To study the interplay between incarceration, BMT and HIV care, we conducted a secondary analysis of data from the Buprenorphine-HIV Evaluation and Support Collaborative (BHIVES). BHIVES was a multi-site, longitudinal cohort study that evaluated whether BMT could be effectively integrated into diverse HIV treatment settings.24 Following the study, BHIVES providers reported challenges with a subset of HIV-positive BMT patients, including those with multi-substance use, cognitive impairments, or “chaotic” life stressors such as incarceration.32
In this analysis, we compared BMT outcomes among HIV patients with and without a history of recent incarceration. We hypothesized that those with recent incarceration would exhibit lower treatment retention and higher opioid use.
METHODS
Setting
The BHIVES collaborative consisted of ten independent sites across the United States, which included seven academic medical centers, two community clinics, and a public hospital. Only one of the sites had prior experience with BMT. Representatives from Yale University provided clinical support and training for implementation. Although sites were given autonomy over how best to integrate BMT into preexisting HIV treatment programs, all provided comprehensive medical and social services to primarily low-income patient populations. Sites had either: all HIV providers prescribe BMT to their own patients (three sites); a few HIV providers who also prescribed BMT to all patients in the practice who needed treatment (six sites); or co-located but separate HIV and BMT specialists. All sites appointed non-physician providers to coordinate medical care with substance use counseling (all sites), case management (all sites), and follow-up outreach (8 sites). Coordinators came from range of disciplines including nurse practitioners, substance use counselors, health educators, and pharmacists. Protocols for BMT prescribing (e.g., frequency of follow-up) were determined by individual sites.32 The study was approved by affiliated institutional review boards.
Patients
Criteria for patient inclusion has been described elsewhere.24 Briefly, patients had to be HIV-infected, diagnosed with the DSM-IV definition of opioid dependence, and greater than 18 years of age. Reasons for exclusion were pregnancy, liver function tests at higher than five times normal levels, benzodiazepine use disorder, alcohol use disorder, suicidal ideations, or severe cognitive impairment. The current analysis only considers patients from the study who received BMT.
Data Collection
The sites developed standardized measures to assess opioid use disorder treatment outcomes. Interviews were collected at baseline (i.e. upon initiating BMT) and then at quarterly intervals for a year. Survey data included sociodemographic information, criminal justice status, and self-reported substance use. Chart abstraction was performed at the end of patient participation in order to ascertain whether or not patients continued to receive BMT during each quarter.
Measures
Incarceration
Patients were asked at baseline “[h]ow many days in the past 30 days were you detained or incarcerated”. We defined “recent incarceration” (dichotomous, yes/no) as whether the patient reported greater than 0 days.
Follow-up intervals
Outcomes were reported at 3 months (60–135 days after baseline), 6 months (135–225 days), 9 months (225–315 days), and 12 months (315–405 days).
BMT retention
BMT assignment was reported from chart abstraction at each follow-up visit. In order to be defined as retained, patients needed to be assigned to BMT for every consecutive interval up to the given one. For example, we defined “12-month retention” as the patient received BMT at 3, 6, 9, and 12 months. Patients who re-initiated BMT after a lapse in treatment were not defined as retained. Retention was considered as an ordinal variable.
Opioid use
Because different study centers had different protocols for urine drug testing, the presence of illicit opioids in the urine was not included as a treatment outcome in this analysis. In baseline and follow-up interviews, patients were asked how many days in the past 30 they had used heroin, methadone, and “other opiates/analgesics” respectively. We defined “illicit opioid use” (dichotomous, yes/no) as any self-report of heroin, methadone, or “other opiate/analgesic” use at each follow-up interview. Therefore, each patient could have up to four follow-up interviews for assessment of this outcome.
Key covariates
Interviews also assessed age, race/ethnicity, gender, any history of injecting drugs (dichotomous, yes/no), any history of heroin use (dichotomous, yes/no), addiction severity index score (continuous),33 number of times treated for “drug abuse”, self-reported homelessness (dichotomous, yes/no), education (at least high school graduation or GED, dichotomous, yes/no), employment (works for pay—“on or off the books”, dichotomous, yes/no), self-reported diagnosis of a “serious mental illness” (e.g. depression, anxiety, schizophrenia, bipolar disorder, dementia, or borderline personality, dichotomous, yes/no), and study site location (categorical).
Data analysis
We conducted descriptive analysis of baseline characteristics for all patients. These patients were subsequently stratified by recent incarceration at baseline. We compared baseline characteristics between those who did and did not report recent incarceration using either a Student’s t-test, a Mann-Whitney U-test, a chi-squared test, or a Fisher’s exact test as appropriate.
To examine the association between recent incarceration and retention, we first determined the percentage of patients retained in BMT for each follow-up interval with patients stratified by recent incarceration status at baseline. A chi-squared test was applied to each interval.
Next, we constructed two separate multivariable logistic regression models with recent incarceration at baseline as the main independent variable and retention in treatment at 6 or 12-months as the respective dependent variables (logistic function, Stata 14.1). We included the following variables in all models based on clinical relevance: age, race, gender, and prior injection drug use reported at baseline. We then selected additional covariates for the model that were associated with the independent variable (ɑ ≤ 0.20) in the bivariate testing described above. We then used backwards step-wise subtraction of non-statistically significant variables (ɑ ≤ 0.05) to arrive at the final model.
Because age measurements were missing for 28% of patients, we performed univariate imputation of age with the predictive mean matching method (mi impute pmm function, Stata 14.1). We selected variables to be included in the imputation if they were present in the logistic model of treatment retention above or if they significantly predicted age in simple linear regression.
Finally, we used hierarchical linear modeling (HLM) to determine the association between recent incarceration at baseline and illicit opioid use during follow-up (PROC GLIMMIX, SAS 9.4). Because each patient could have multiple follow-up interviews, this procedure accounted for clustering of follow-up visits within individual patients. Again, recent incarceration was the main independent variable, and self-reported illicit opioid use was the dependent variable. Covariates, which were selected by the same method as above, were considered to be fixed effects because they were derived from baseline interviews. We imputed age as above.
RESULTS
In the entire cohort of 306 individuals, mean age was 45 years, most patients were black (51%) or Hispanic (22%), and male (67%). Thirty-nine (13%) were recently incarcerated. Baseline characteristics did not significantly differ between those with and without recent incarceration. However, those with recent incarceration (vs. those without) were more likely to report homelessness (41% vs. 23%, p < 0.05), unemployment (90% vs. 72%, p < 0.05), and diagnoses of serious mental illness (73% vs. 53%, p < 0.05). Those with recent incarceration also reported a greater median number of times treated for substance use disorders, suggesting more severe addiction in the past (5 vs. 3, p < 0.05) (see Table 1).
Table 1.
Baseline characteristics of HIV-infected patients receiving buprenorphine treatment for opioid use disorder:
| All (N = 305) |
Not recently incarcerated (N = 266) |
Recently incarcerated (N = 39) |
P-value* | |
|---|---|---|---|---|
| Agea, mean years +/− SD | 44.6 +/− 8.5 | 44.6 +/− 8.4 | 44.4 +/− 9.3 | 0.93 |
| Race/Ethnicityb, n (%): | ||||
| Non-Hispanic White | 68 (22.3%) | 61 (22.9%) | 7 (18.0%) | 0.56 |
| Non-Hispanic Black | 156 (51.2%) | 133 (50.0%) | 23 (59.0%) | 0.47 |
| Hispanic | 67 (22.0%) | 59 (22.2%) | 8 (20.5%) | 0.71 |
| Non-Hispanic Other | 10 (3.3%) | 9 (3.4%) | 1 (2.6%) | 1.00 |
| Male, n (%) | 205 (67.2%) | 179 (67.3%) | 26 (66.7%) | 0.94 |
| Ever injected drugs, n (%) | 241 (79.0%) | 207 (77.8%) | 34 (87.2%) | 0.18 |
| Ever used heroin, n (%) | 292 (95.7%) | 253 (95.1%) | 39 (100%) | 0.16 |
| Drug addiction severity indexc, mean +/− SD | 25.7 +/− 11.7 | 26.3 +/− 11.4 | 21.7 +/− 12.7 | 0.04 |
| Times treated for drug abused, median (IQR) | 3 (2–6) | 3 (2–6) | 5 (3–7) | 0.03 |
| Homelessness, n (%) | 76 (24.9%) | 60 (22.6%) | 16 (41.0%) | 0.01 |
| High school diploma or equivalencye, n (%) | 174 (57.1%) | 157 (59.0%) | 17 (43.6%) | 0.17 |
| Employed, n (%) | 78 (25.6%) | 74 (27.8%) | 4 (10.3%) | 0.02 |
| Diagnosed with mental illnessf, n (%) | 167 (54.8%) | 140 (52.6%) | 27 (69.2%) | 0.02 |
Chi-square, Fisher’s Exact Test, Mann-Whitney Test, or T-Test as appropriate
Missing 84 observations
Missing 4 observations
Missing 5 observations
Missing 3 observations
Missing 1 observation
Missing 5 observations
Both those with and without recent incarceration showed decreased retention over time. Sixty-six percent of patients were retained in BMT at 6 months regardless of incarceration status; at 12 months, 39% of those with recent incarceration at baseline were retained in BMT versus 50% of those without (p = 0.19) (see Figure 1).
FIGURE 1. Consecutive months of retention in buprenorphine maintenance treatment of HIV-infected patients stratified by recent incarceration at baseline.
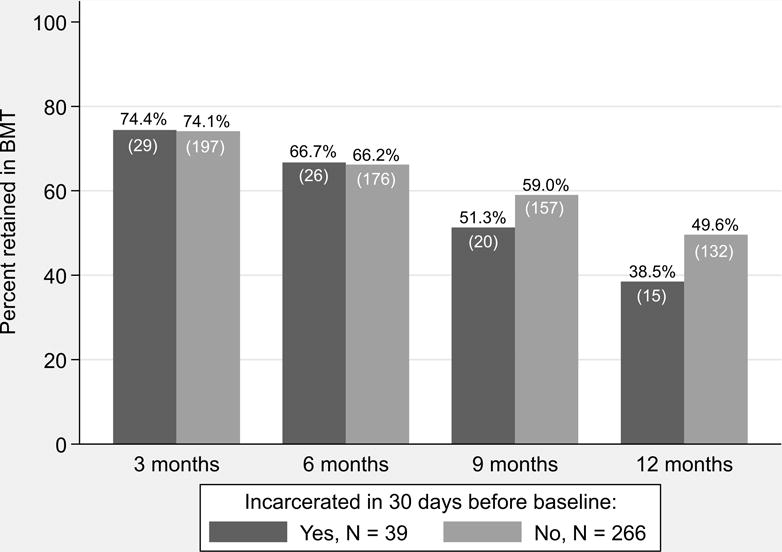
Chi-square analysis: all comparisons non-significant | Buprenorphine retention = received any buprenorphine during given quarter and all previous quarters | N = sample size.
After adjustment for age, race, gender, and history of injection drug use, recent incarceration at baseline still was not significantly associated with 6-month (OR = 0.95; 95% CI = 0.46–1.98) or 12-month retention (OR = 0.57; 95% CI = 0.27–1.18). However, in the final multivariable model, male gender decreased and age increased predicted odds of 12-month retention significantly (see Table 2).
Table 2.
Association of baseline patient characteristics with BMT outcomes in multivariable models:
| 6-month treatment retention | 12-month treatment retention | Self-reported opioid use | ||||
|---|---|---|---|---|---|---|
| Variable | Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI |
| Recent incarceration | 0.95 | 0.46–1.98 | 0.57 | 0.27–1.18 | 0.99 | 0.51–1.92 |
| Age | 1.02 | 0.98–1.05 | 1.04 | 1.01–1.07 | 0.99 | 0.96–1.02 |
| White | 0.71 | 0.39–1.28 | 0.57 | 0.31–1.04 | 0.91 | 0.54–1.55 |
| Male | 1.13 | 0.67–1.90 | 0.56 | 0.34–0.94 | 0.89 | 0.57–1.40 |
| Inject drugs | 0.70 | 0.37–1.34 | 0.80 | 0.43–1.48 | 1.42 | 0.82–2.46 |
| Addiction severity index | * | * | * | * | 1.03 | 1.01–1.05 |
| Homelessness | * | * | * | * | 0.57 | 0.34–0.96 |
= dropped from final model
After adjustment for age, race, gender, history of injection drug use, addiction severity index score, and homelessness, recent incarceration was not significantly associated with the odds of opioid use at any follow-up visit (OR = 0.99; 95% CI = 0.51–1.92). However, in the final multivariable model, homelessness decreased and addiction severity increased the predicted odds of opioid use significantly (see Table 2).
DISCUSSION
In this study assessing BMT outcomes in patients living with HIV/AIDS, those with incarceration in the 30 days prior to BMT initiation were more likely to be homeless, unemployed, and previously diagnosed with mental illness than those without recent incarceration. However, we did not detect significant differences in self-reported opioid use, 6-month, or 12-month retention in BMT, including after adjustment for potential confounding factors. Our findings suggest that patients in the community who seek treatment for opioid use disorder can achieve standard outcomes with BMT in spite of histories with recent incarceration.
Our work confirms others findings and expands their applicability to patients who are recruited from the community and/or are living with HIV/AIDS. Wang et al. analyzed data from a randomized controlled trial of BMT and counseling in primary care and similarly did not find a difference in treatment retention or opioid use between primary care patients stratified by any history of incarceration.27 Lee et al. assessed the effects of more recent incarceration by comparing patients connected to community-based BMT after release from jail with patients recruited directly from the community.34 They also found similar rates of treatment retention and opioid use between groups. However, the majority of incarcerated patients had initiated BMT before release, thereby providing continuity of care during the period of community reentry. A pilot study comparing pre-release vs. post-release initiation of BMT demonstrated superior treatment retention among prisoners initiating treatment prior to release.35 Therefore, our findings may be more applicable than those of Lee et al. for primary care providers looking to initiate BMT for patients in the community who have recently been incarcerated. Two other studies also examined BMT outcomes among individuals on probation or parole, many of whom may have been recently incarcerated. Mitchell et al. found no difference in treatment retention or opioid use in a randomized controlled trial of buprenorphine and outpatient counseling when comparing patients who were on probation or parole to those who were not.36 Gordon et al. examined a cohort of BMT patients on probation or parole and found a slightly lower rate of 3-month retention (67%) than our study, but there was no comparison group of patients who were not on probation or parole.37 Taken together, these studies suggest that criminal justice status does not absolutely dictate poor BMT outcomes, at least in large urban areas similar to the ones where these studies took place.
We were surprised that we could not establish a difference in BMT outcomes between those who had and had not recently been incarcerated. We had expected that recent incarceration would be more acutely destabilizing. It is possible that we failed to detect any negative effects of recent incarceration due to a lack of power. The point estimate for the odds ratio of retention at 12 months was 0.57, which could represent a clinically meaningful difference. It is also possible that factors unique to HIV-positive populations, such as availability of special benefits or supportive services, protected study patients from destabilization following release from incarceration. These findings could be different in an HIV-negative population. Additionally, being under criminal justice supervision (i.e. probation or parole) might have also influenced outcomes; however, adjusting for probation or parole status did not change the association between recent incarceration and treatment retention or opioid use (data not shown). Recent incarceration may have provided motivation or increased urgency to stop illicit opioid use; however, our data does not include a measure of motivation for treatment. Future studies examining BMT outcomes among individuals with recent incarceration should account for age, gender, addiction severity, and homelessness, as these factors were associated with BMT outcomes in our study. Other unidentified factors may also be important.
The strengths of our study include the diverse treatment settings, multiple study sites from across the United States, and adjustment for multiple potential confounding variables. However, there were also multiple limitations. As with any observational study, we cannot establish clear causative relationships between incarceration and buprenorphine treatment outcomes. Our sample size for those with recent incarceration was small, which limited our ability to perform some multivariable analyses and may have led to Type II error. The cohort was HIV positive, so generalizability to HIV-negative individuals is challenging; repeating this analysis in an HIV-negative population may uncover differences in outcomes. Most treatment sites were in urban areas, and we do not know if these findings would generalize to other geographic areas. Finally, our study relied heavily on self-reported factors.
In conclusion, our study provides more evidence that BMT is an effective opioid use disorder treatment for individuals with and without criminal justice involvement. Treatment retention and self-reported opioid use among recently incarcerated patients was not significantly worse than in patients without such a history. Encouraging formerly incarcerated individuals with opioid use disorder to initiate evidence-based treatments, including buprenorphine maintenance treatment, should be part of efforts to confront the opioid addiction epidemic in the United States.
Acknowledgments
We thank the participants and investigators of the Buprenorphine-HIV Evaluation and Support Collaborative for performing the original study. We also thank Linda Weiss and Bert Chantarat from the New York Academy of Medicine for providing data.
FUNDING
This study was supported by NIH K23DA034541 (Principal Investigator [PI]: Fox); NIH K24DA036955 (PI: Cunningham); the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519); NIH R25DA023021 (PI: Arnsten); and the David E. Rogers Fellowship Program of the New York Academy of Medicine (Riggins). These sources had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Footnotes
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to research conception and design. D.P.R., Y.N., and A.D.F. performed data analysis. D.P.R. wrote the first draft of the manuscript, and all authors contributed to revisions.
References
- 1.Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2015. http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf. [Google Scholar]
- 2.Number and Age-Adjusted Rates of Drug-Poisoning Deaths Involving Opioid Analgesics and Heroin: United States, 2000–2014. Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. http://www.cdc.gov/nchs/data/health_policy/AADR_drug_poisoning_involving_OA_Heroin_US_2000-2014.pdf. [Google Scholar]
- 3.Aden B, Dunning A, Nosyk B, Wittenberg E, Bray JW, Schackman BR. Impact of Illicit Drug Use on Health-Related Quality of Life in Opioid Dependent Patients Undergoing HIV Treatment. J Acquir Immune Defic Syndr 1999. 2015 Jul; doi: 10.1097/QAI.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. World Health Organization; 2009. [PubMed] [Google Scholar]
- 6.Mauger S, Fraser R, Gill K. Utilizing buprenorphine–naloxone to treat illicit and prescription-opioid dependence. Neuropsychiatr Dis Treat. 2014;10:587–598. doi: 10.2147/NDT.S39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutwell AE, Nijhawan A, Zaller N, Rich JD. Arrested on heroin: a national opportunity. J Opioid Manag. 2007;3(6):328–332. doi: 10.5055/jom.2007.0021. [DOI] [PubMed] [Google Scholar]
- 8.Garcia CA, Correa GC, Viver ADH, et al. Buprenorphine-naloxone Treatment for Pre-release Opioid-dependent Inmates in Puerto Rico. J Addict Med. 2007;1(3):126–132. doi: 10.1097/ADM.0b013e31814b8880. [DOI] [PubMed] [Google Scholar]
- 9.Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O’Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: prison outcomes and community treatment entry. Drug Alcohol Depend. 2014;142:33–40. doi: 10.1016/j.drugalcdep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37(3):277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie M, Zaller N, Dickman SL, et al. A randomized trial of methadone initiation prior to release from incarceration. Subst Abuse. 2012;33(1):19–29. doi: 10.1080/08897077.2011.609446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich JD, McKenzie M, Larney S, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet Lond Engl. 2015;386(9991):350–359. doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105(1–2):83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandler RK, Fletcher BW, Volkow ND. Treating Drug Abuse and Addiction in the Criminal Justice System: Improving Public Health and Safety. JAMA J Am Med Assoc. 2009;301(2):183–190. doi: 10.1001/jama.2008.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addict Abingdon Engl. 2008;103(8):1333–1342. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates’ experiences with buprenorphine or methadone maintenance. J Psychoactive Drugs. 2010;42(3):339–346. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among Inmates of and Releasees from US Correctional Facilities, 2006: Declining Share of Epidemic but Persistent Public Health Opportunity. PLoS ONE. 2009;4(11) doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Paola A, Altice FL, Powell ML, Trestman RL, Springer SA. A comparison of psychiatric diagnoses among HIV-infected prisoners receiving combination antiretroviral therapy and transitioning to the community. Health Justice. 2014;2(11) doi: 10.1186/s40352-014-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palepu A, Tyndall MW, Joy R, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84(2):188–194. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Fiellin DA, Weiss L, Botsko M, et al. Drug Treatment Outcomes among HIV-Infected Opioid Dependent Patients Receiving Buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(01) doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr 1999. 2011;56(Suppl 1):S22–S32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health Bull N Y Acad Med. 2010;87(4):592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PloS One. 2012;7(5):e38335. doi: 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss L, Egan JE, Botsko M, Netherland J, Fiellin DA, Finkelstein R. The BHIVES collaborative: organization and evaluation of a multisite demonstration of integrated buprenorphine/naloxone and HIV treatment. J Acquir Immune Defic Syndr 1999. 2011;56(Suppl 1):S7–S13. doi: 10.1097/QAI.0b013e3182097426. [DOI] [PubMed] [Google Scholar]
- 25.Koehn JD, Bach P, Hayashi K, et al. Impact of incarceration on rates of methadone use in a community recruited cohort of injection drug users. Addict Behav. 2015;46:1–4. doi: 10.1016/j.addbeh.2015.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addict Abingdon Engl. 2005;100(6):820–828. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang EA, Moore BA, Sullivan LE, Fiellin DA. Effect of Incarceration History on Outcomes of Primary Care Office-based Buprenorphine/Naloxone. J Gen Intern Med. 2010;25(7):670–674. doi: 10.1007/s11606-010-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris EE, Jacapraro JS, Rastegar DA. Prior criminal charges and outcomes among individuals initiating office-based buprenorphine treatment. Health Justice. 2013;1(1):2. doi: 10.1186/2194-7899-1-2. [DOI] [Google Scholar]
- 29.Binswanger IA, Nowels C, Corsi KF, et al. Return to drug use and overdose after release from prison: a qualitative study of risk and protective factors. Addict Sci Clin Pract. 2012;7(1):3. doi: 10.1186/1940-0640-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox AD, Maradiaga J, Weiss L, Sanchez J, Starrels JL, Cunningham CO. Release from incarceration, relapse to opioid use and the potential for buprenorphine maintenance treatment: a qualitative study of the perceptions of former inmates with opioid use disorder. Addict Sci Clin Pract. 2015;10:2. doi: 10.1186/s13722-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Olphen J, Freudenberg N, Fortin P, Galea S. Community reentry: perceptions of people with substance use problems returning home from New York City jails. J Urban Health Bull N Y Acad Med. 2006;83(3):372–381. doi: 10.1007/s11524-006-9047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss L, Netherland J, Egan JE, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr 1999. 2011;56(Suppl 1):S68–S75. doi: 10.1097/QAI.0b013e31820a8226. [DOI] [PubMed] [Google Scholar]
- 33.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 34.Lee JD, Grossman E, Truncali A, et al. Buprenorphine-Naloxone Maintenance Following Release from Jail. Subst Abuse. 2012;33(1):40–47. doi: 10.1080/08897077.2011.620475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaller N, McKenzie M, Friedmann PD, Green TC, McGowan S, Rich JD. Initiation of buprenorphine during incarceration and retention in treatment upon release. J Subst Abuse Treat. 2013;45(2):222–226. doi: 10.1016/j.jsat.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell SG, Gryczynski J, Kelly SM, et al. Treatment Outcomes of African American Buprenorphine Patients by Parole and Probation Status. J Drug Issues. 2014;44(1):69–82. doi: 10.1177/0022042613491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon MS, Kinlock TW, Schwartz RP, et al. Buprenorphine Treatment for Probationers and Parolees. Subst Abuse. 2015;36(2):217–225. doi: 10.1080/08897077.2014.902787. [DOI] [PMC free article] [PubMed] [Google Scholar]


