Abstract
In an effort to identify neuronal repair mechanisms of the major pelvic ganglion (MPG), we evaluated changes in the expression of nestin, an intermediate filament protein and neural stem cell marker following cavernous nerve crush injury (CNI). We utilized two groups of Sprague Dawley rats: (i) sham and (ii) bilateral CNI. Erectile responses to cavernous nerve stimulation (CNS) were determined at 48 h in a subset of rats. The MPG was isolated and removed at 48 h after CNI, and nestin immunolocalization, protein levels and RNA expression were evaluated. At 48 h, erectile responses to CNS in CNI rats were substantially reduced (P<0.05; ~70% decrease in intracavernous pressure/mean arterial pressure) compared with sham surgery controls. This coincided with a dramatic 10-fold increase (P<0.05) in nestin messenger RNA expression and protein levels in the MPG of rats with CNI. Immunoflourescence microscopy demonstrated that nestin upregulation after CNI occurred within the ganglion cell bodies and nerve fibers of the MPG. In conclusion, CNI induces nestin in the MPG. These data suggest that nestin may be involved in the regenerative process of the cavernous nerve following crush injury.
Keywords: erectile dysfunction, prostate cancer, radical prostatectomy, stem cell
Introduction
Erectile function is a complex neurovascular event, which is initiated by the stimulation of central and peripheral nervous systems.1 Erectile dysfunction (ED) is a common complication after radical prostatectomy (RP) for the treatment of clinically localized prostate cancer.2,3 The technique of nerve-sparing RP aims at preserving the cavernous nerves, which provide the parasympathetic innervations to the penis and thus regulate normal penile vascular homeostasis.4,5 Albeit difficult to assess, 26–100% of patients undergoing RP will suffer from some degree of sexual dysfunction ranging from mild to severe ED.6 Neuropraxia of the cavernous nerve and resultant ED are common sequalae of nerve-sparing RP.7 Surgical preservation of the neurovascular bundle may vary, and recovery and regeneration of the cavernous nerve may take months. Such a lengthy absence of innervation may lead to permanent pathophysiological and structural changes within the corporal vascular bed.8,9 In order to develop appropriate therapies, it is necessary to discern the molecular pathways that are altered in the major pelvic ganglion (MPG) and cavernous nerve following crush injury. Discovery of novel molecular targets and understanding the mechanisms of nerve recovery may help to improve new ED therapeutic options post RP.
Nestin is a type VI intermediate filament (IF) protein and expressed mostly in the nerve cells where they are implicated in the radial growth of the axon.10–12 It is expressed by many types of cells during development, although its expression is usually transient and does not persist into adulthood.10,13–19 Nestin is also expressed in dividing cells during the early stages of development in the central and peripheral nervous system.10,12,20 Nestin is regarded as an early marker of neural stem cells (NSCs).20,21 During neuro- and gliogenesis, nestin is replaced by cell type-specific IFs, such as glial fibrillary acidic protein (GFAP).20 Interestingly, nestin expression is reinduced in the adult during pathological situations, such as the formation of the glial scar after central nervous system injury and during regeneration of injured muscle tissue.13,20,22–26
Although many studies have documented nestin expression during neural development and under pathological conditions, little information is known about its physiological function. Moreover, there has been no documentation and investigation of nestin activation and expression in nerves responsible for penile innervations and function. The rat model of cavernous nerve crush is well established as a means of studying the functional and structural consequences of cavernous nerve crush injury (CNI).27 The objective of this study was to evaluate transcription, protein expression and localization of nestin in the MPG and cavernous nerves emanating from the MPG.
Materials and methods
Experimental animals
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 150–250 g (7–9 weeks of age) were used (n=17). All experiments were conducted in accordance with the Johns Hopkins University School of Medicine guidelines for animal care and use. Animals were anesthetized with an intraperitoneal injection of a mixture of ketamine/xylazine (100 + 10 mg kg−1). The prostate was exposed via a midline abdominal incision. The cavernous nerve (CN) and MPG were identified posterolateral to the prostate. In order to study the pathophysiology of CNI on molecular signaling in the MPG, the following groups of experimental rats were utilized: i) sham operation with exposure of bilateral CNs and no manipulation of the nerves in age-matched control rats; and ii) exposure of bilateral CNs and associated nerve crush injury. Crush injury was induced by applying Dumont #5 forceps (dimension of forcep tip—0.10 mm×0.06 mm; Fine Science Tools, Foster City, CA, USA) to the nerve 2–3 mm distal to the MPG. The forceps were held to closure three times for 15 s each, causing a moderate crush injury.27–29 All immunofluorescence, molecular and in vivo analyses were determined 48 h after sham operation and bilateral CNI.
Immunofluorescence
The MPG and CN were excised, and fixed in 10% formalin, and paraffin embedded as described.30,31 Sections were deparafinized, and endogenous peroxidases were quenched with 3% H2O2. Nonspecific binding of IgGs was blocked using normal goat serum 1:50 in 0.1% bovine serum albumin in phosphate-buffered saline. Sections were incubated overnight at room temperature with primary antibodies for neuronal nitric oxide synthase (nNOS 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), protein gene product 9.5 (PGP 9.5 1:1000; Abcam, Cambridge, MA, USA), GFAP (1:1000; Abcam) and nestin (1:200; Abcam). For simultaneous demonstration of two antigens, primary antibodies were incubated as cocktails as previously described.29,32 In brief, MPG and CN sections were then double immunostained with mouse anti-nestin/rabbit anti-GFAP at a dilution of 1:200 for nestin and 1:1000 for GFAP. After rinsing in phosphate-buffered saline (30 min), appropriate species-directed secondary antibodies (1:600; Alexa Fluor, Molecular Probes, Leiden, The Netherlands) were applied to the sections (60 min). Secondary antibodies were fluorescein isothiocyanate-conjugated horse anti-mouse IgG and rhodamine-conjugated donkey anti-rabbit IgG at a dilution of 1:600. Sections were analyzed using a laser fluorescence microscope with single and double filter settings at 488 and 522 nm (Olympus, Osaka, Japan). Images were acquired using Viewfinder Lite version 2.0 (Pixera, Egham, UK). Control staining in the absence of primary antibodies was performed to evaluate unspecific staining by the secondary antibodies.
Western blot analysis
MPG and CN were excised and homogenized together in a buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM EDTA, 2 mM EGTA, 150 mM NaCl, 50 mM NaF, 10% glycerol, 10 μg ml−1 leupeptin, 2 μg ml−1 aprotinin, 10 μg ml−1 trypsin inhibitor, 1 mM phenylmethylsulfornyl fluoride and 1 mM Na3VO4.30 Six separate whole MPG cellular fractions from six sham-operated and CNI rats were isolated for nestin western blot analysis. Protein concentration was determined by the BCA kit (Pierce, Rockford, IL, USA), and equal amounts of protein (30 mg) were loaded to 4–20% Tris-HCl gel (Bio-Rad, Hercules, CA, USA). After their separation by SDS-PAGE, the proteins were transferred to polyvinylidene fluoride membranes and incubated with primary antibodies (nestin 1:1000 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 1:10 000) overnight at 4 °C. The membranes were incubated with a horseradish peroxidase-linked secondary antibody and visualized using an enhanced chemiluminescence kit (Amersham Biosciences Corp., Piscataway, NJ, USA). The densitometry results were normalized by GAPDH expression. Nestin expression was calculated as the ratio of nestin to GAPDH protein. The intensities of the resulting bands were quantified by using Software Image J 1.43s NIH (NIH, Bethesda, MD, USA).
Quantitative PCR (qPCR)
Real-time qPCR was used to determine relative expression of nestin in MPG and CN from sham and CNI rats. Frozen MPG was homogenized and total RNA purified using the RNeasy system (Qiagen, Hilden, Germany), quantified and then reverse transcribed using Superscript II (Life Technologies, Carlsbad, CA, USA). Real-time qPCR was performed using the StepOnePlus™ system (Applied Biosystems, Foster City, CA, USA).33 TaqMan gene expression assays for nestin (Rn00564394_m1) and GAPDH (Rn99999916_s1) were used, with GAPDH serving as an endogenous control (Applied Biosystems). All experiments were performed on four separate whole MPG cellular fractions from four sham-operated and CNI rats with quadruplicate technical replicate PCR reactions per sample.
Measurement of erectile responses in vivo
Rats were anesthetized with an intraperitoneal injection of ketamine/xylazine (100 + 10 mg kg−1) and placed on a thermoregulated surgical table and a standard in vivo experimental protocol was conducted.29,32,34 Briefly, the CN was stimulated (CNS) distal to the crush injury with a square pulse stimulator (Grass Instruments, Quincy, MA, USA) at a frequency of 15 Hz and pulse width of 30 s. The application of 2, 4, 6 and 8 volts was used to achieve a significant and consistent erectile response. The duration of stimulation was 1 min with rest periods of 5–10 min between subsequent stimulations. Total erectile response or total intracavernous pressure (ICP) was determined by the area under the erectile curve (AUC; mm Hg·sec) from the beginning of CNS until the ICP pressure returned to baseline or pre-stimulation pressures. The ratio between the maximal ICP and mean arterial pressure (MAP) obtained at the peak of erectile response was calculated to normalize for variations in systemic blood pressure. During tumescence, the time for the ICP to reach 80% of peak ICP (T80) was recorded and evaluated. These methods have been previously described.29,34
Statistical analysis
Data were expressed as mean±s.e.m. Differences between multiple groups were compared by analysis of variance followed by a Tukey’s multiple comparisons test. Two-group analysis was performed by t-test (paired or unpaired as appropriate). P-value of <0.05 was used as criteria for statistical significance.
Results
In vivo erectile responses
In order to evaluate the effect of CNI on erectile hemodynamics, in vivo erectile responses to CNS were studied 48 h after nerve crush injury, and these data are summarized in Figure 1. There was a significant decline (P<0.01) in erectile function in CNI rats when compared with age-matched sham-operated rats (Figure 1). The peak ICP (mm Hg) and ratio of ICP/MAP after CNS in CNI rats was significantly lower (P<0.01) than the sham-operated animals at all levels of stimulation (Figures 1a and b). The total ICP (area under the erectile curve; mm Hg·sec) of penile erection was significantly lower (P<0.01) in CNI rats at all voltage settings (Figure 1c). The rise in ICP as measured by the T80 (mm Hg sec−1) was greater (P<0.05) in the bilateral CNI rats when compared with the sham-operated rats (Figure 1d). These data demonstrate that severe neurogenic-ED occurred after CNI and verifies that CNI causes impaired erectile responses to CNS.
Figure 1.
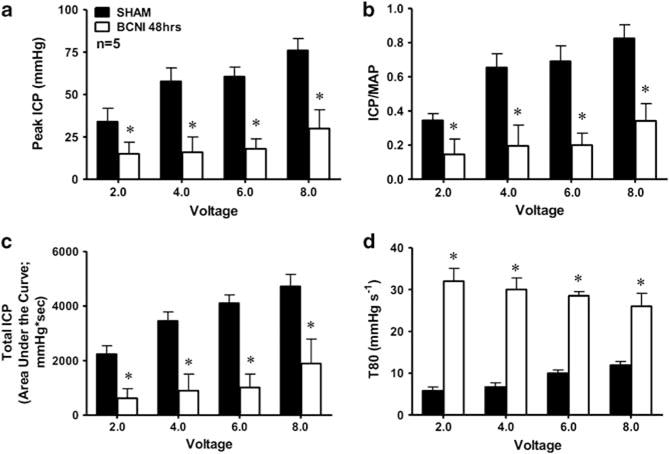
Bar graph depicting the voltage-dependent erectile response as measured by the peak ICP (a), intracavernous pressure (ICP)/mean arterial pressure (MAP) ratio (b), total ICP (area under the erectile curve; c), and rise in intracavernosal pressure as measured by T80 (d) after CNS for 1 min in age-matched sham-operated and bilateral cavernous nerve (CN) crush injury rats. In vivo erection experiments were conducted 48 h after cavernous nerve crush injury (CNI). n indicates number of experiments; *(P<0.05) response significantly different compared with sham rats.
Immunolocalization of nestin in the MPG
Immunoreactivity (IR) for nestin and GFAP was evaluated in the MPG of sham-operated rats and 48 h after CNI as shown in Figure 2. Nestin IR was not visualized in the sham-operated rat MPG (Figure 2a). After 48 h CNI, nestin IR was markedly enhanced in the injured MPG (Figures 2d and g) when compared with the IR of nestin in sham-operated rat MPG (Figure 2a). Nestin and GFAP IR was shown to be co-expressed (arrow) within the ganglion cell bodies and nerve fibers in the rat MPG after CNI (Figures 2f and i). There was no qualitative difference in increased expression of the nestin IR for the ganglion cell bodies vs the nerve fibers in response to CNI. All cell bodies and nerve fibers expressed GFAP (Figures 2b, e and h) demonstrating neuronal origin of the tissue samples studied.
Figure 2.
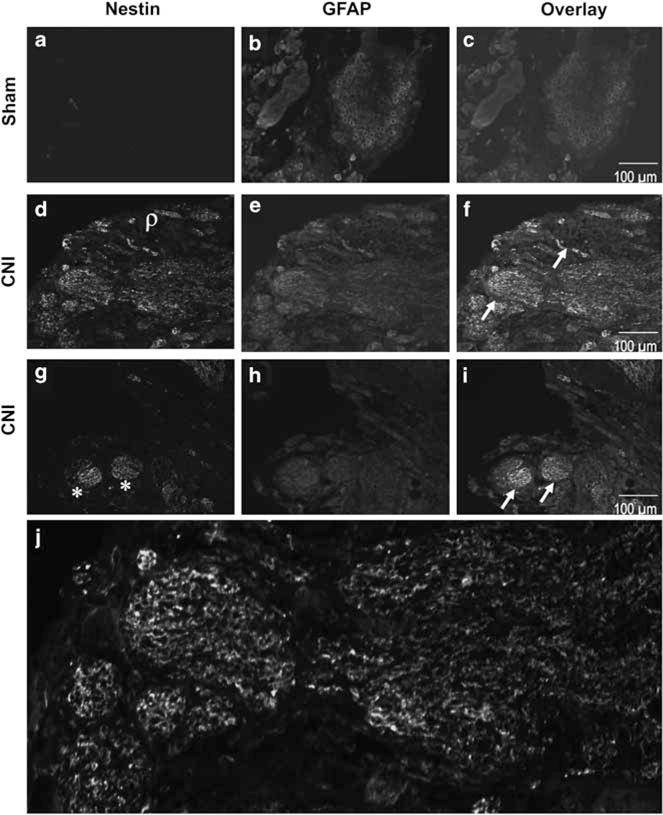
Immunoreactivity (IR) of nestin (a, d, g) and glial fibrillar acidic protein (GFAP; b, e, h) in the major pelvic ganglion (MPG) and cavernous nerve (CN) in age-matched control sham-operated rats (a–c) and bilateral cavernous nerve crush injury (CNI; d–i) rats. Overlay images (c, f, i) represent co-localization of nestin and GFAP in sham and CNI MPG. Arrow represents co-expression (yellow staining) of nestin and GFAP. Asterisk (*) represents nestin expression in the ganglion cell bodies and rho (ρ) represents nestin expression in nerve fibers. Photomicrographs are representative of four experiments. Magnification ×10. (j) Nestin expression in the CN at higher magnification (×20).
Western blot analysis and qPCR of nestin in the MPG
Nestin protein and gene expression was measured in MPG of control sham-operated rats and 48 h after CNI, and these data are summarized in Figure 3. Nestin protein levels (western blot) were significantly higher in the CNI rat MPG (n=6) when compared with sham-operated rats (n=6) 48 h after CNI (Figure 3a). When nestin levels were analyzed by densitometry, nestin protein levels were significantly elevated (P<0.01) in the CNI rat MPG when compared with sham rat MPG 48 h after CNI (Figure 3b). Nestin gene expression (qPCR) was significantly higher (~10-fold; P<0.05) in the CNI rat MPG (n=4) when compared with sham-operated rats (n=4) 48 h after CNI (Figure 3c). These data demonstrate increased protein and gene expression of nestin 48 h after CNI in the MPG of the rat.
Figure 3.
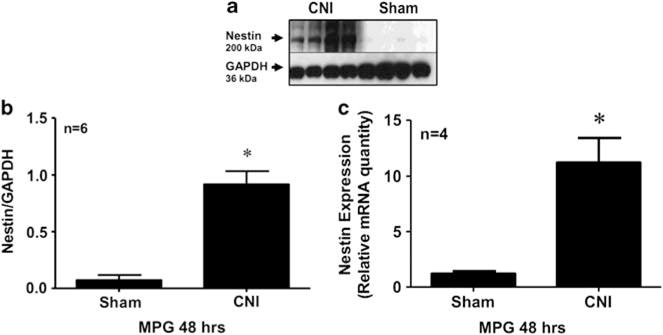
Western blot analysis (a, b) and quantitative PCR (qPCR; c) demonstrating expression of nestin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the major pelvic ganglion (MPG) of age-matched control sham-operated rats and bilateral cavernous nerve crush injury (CNI) rats. Densitometry analysis (b) shows the ratio of nestin to GAPDH protein expression in CNI (lanes 1–4) and sham (lanes 5–8) MPG. Analyses were conducted 48 h after CNI. n indicates number of tissue samples; *(P<0.05) when compared with sham.
Discussion
In the present study, we evaluated the activation and expression of nestin, an IF protein and NSC marker, in the MPG after CNI in an effort to identify novel gene expression changes that may be involved in early preservation and regeneration of the CN in response to crush injury. The in vivo erectile responses results demonstrated that 48 h after bilateral CNI there is a profound decline in cavernous nerve-mediated erectile responses. The results of the present study demonstrate for the first time increased expression of nestin in the autonomic nerve supply of the penis after CNI. The localization of nestin expression was found in both the ganglion cell bodies and nerve fibers. These findings implicate that nestin may influence cavernous nerve function potentially by influencing cavernous nerve regeneration and/or neuroprotection after crush injury.
There is a need for a better understanding of the mechanisms of neuronal regeneration and structures such as stem cells in MPG and CNs after iatrogenic injury. The animal model chosen for this study was CNI as this type of injury is designed to mimic the partial nerve damage that occurs during nerve-sparing RP.27 Similar to the results of our previous study, the model of bilateral CNI causes a decreased erectile response in bilateral CNI group when compared with age-matched sham-operated rats.29 Using this model, we observed that nestin expression increased markedly in the MPG and nerve fibers after CNI.
Many structures directly involve the cytoskeleton. The cytoskeleton is formed by three types of filamentous structures: microtubuli, microfilaments and IFs.13 IFs represent the least understood but are perhaps the most important components of the cytoskeleton.13,33 These 10-nm thin structures constitute a cage-like network within the cell cytoplasm that regulates cytoskeletal remodeling during cellular development and maturation.22,33,35 More than 50 IF proteins are now known, and divided into six classes.10 Nestin belongs to class VI of IFs, which is produced in stem/progenitor cells in the mammalian central nervous system during development and is a marker of proliferating and migrating cells.10–12
Nestin was discovered in 1985 as an antigen recognized by monoclonal antibody Rat-401 and its gene was characterized in 1990.10,36 The nestin protein consists of >1600 amino acids and it is structurally similar to other IFs proteins. The regulatory mechanisms are still not fully understood, but phosphorylation of nestin by cdc2 kinase may have a role in connecting the three components of the cytoskeleton and coordinate changes in cell dynamics.35,37 The short N-terminal of nestin can form heterodimers and heterotetramers by binding with other IF proteins, particularly the class 3 proteins vimentin and α-internexin.33,38 Although little is known about nestin’s physiological role in the nervous system, most authors regard nestin-expressing cells as NSCs, because of their features including multipotency, self renewal and regeneration.20,21 Accordingly, nestin is mainly expressed at the early stages of CNS where it can be detected in both neuronal and glial cells.20 It has also been reported that the dominant expression of nestin protein in the prenatal- and postnatal-developing central nervous system of mammals reflects the differentiation or proliferative state of neural precursors.36,39,40 Nestin expression progressively decreases during the postnatal period, and it is generally not detected in the brains of adult animals.13 In the present study, we saw very little immunoexpression of nestin in the MPG in sham-operated rats while there was a significant increase in immunoexpression in response to CNI. This increase in nestin expression was only observed at 48 h and was not present at 14 days after CNI in the MPG (data not shown). These data suggest that nestin is activated in the MPG in response to crush injury similar to results obtained in the central nervous system.
Increased nestin expression is also seen in the adult organism under certain pathological conditions such as brain injury, ischemia, inflammation and neoplastic transformation.13,20,22–26 For instance, nestin expression has been observed in the reactive astrocytes of the glial scar in experimental models of central nervous system injury.13,23,33,40 In addition, a recent study that examined nestin activation in response to peripheral nerve injury showed that nestin-expressing cells increase in the dorsal horn in response to nerve injury providing evidence that nestin may be involved in repair mechanisms in the peripheral nervous system.41 In the present study, we observed very little expression of nestin in the MPG of sham-operated rats while there was an ~10-fold increase in nestin gene expression in the MPG in response to CNI with resultant increased protein expression 48 h post crush injury. These data suggest that nestin is activated relatively early and in a transient fashion in the MPG in response to crush injury. The exact mechanism by which nestin contributes to regenerating peripheral CNs has not been studied to date, but one can hypothesize that nestin may become reactivated along with other embryonic pathways to protect the CN from degeneration or contribute to CN proliferation.
There are a number of limitations that are worth noting from this study. We have only used one time point for detailed quantitative expression of nestin in the MPG after CNI. However, the goal of this study was to evaluate early response genes expressed in the MPG after crush injury in an attempt to identify important signaling pathways involved in CN function. Additionally, the biological importance of increased nestin expression was not investigated and is the focus of future research.
Conclusion
The results of the present study demonstrate that bilateral crush injury to the cavernous nerves significantly impairs neurogenic-mediated erectile function in vivo at 48 h post crush injury and causes marked upregulation of nestin messenger RNA and protein in the MGP as well as the CN. The results of this study showed that the NSC marker nestin proliferate early in the MPG and CN in response to peripheral nerve crush injury. Further studies are of interest to understand the role of nestin activation in the MPG and CN after crush injury and to assess the involvement of nestin in neuroprotection and regeneration after CNI.
Acknowledgments
The present study was funded in part from an AUA Foundation Astellas USA Foundation MD/PhD grant to TJB, K08 National Institute of Health Career Development Award to TJB, from the European Society of Sexual Medicine (ESSM) Grant for Medical Research to CG and a grant from the German Society of Andrology to CG.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- 2.Meuleman EJ, Mulders PF. Erectile function after radical prostatectomy: a review. Eur Urol. 2003;43:95–101. doi: 10.1016/s0302-2838(02)00546-8. discussion 101–102. [DOI] [PubMed] [Google Scholar]
- 3.Mulhall JP. Penile rehabilitation following radical prostatectomy. Curr Opin Urol. 2008;18:613–620. doi: 10.1097/MOU.0b013e3283136462. [DOI] [PubMed] [Google Scholar]
- 4.Walsh PC. The discovery of the cavernous nerves and development of nerve sparing radical retropubic prostatectomy. J Urol. 2007;177:1632–1635. doi: 10.1016/j.juro.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Ahlering TE, Kaplan AG, Yee DS, Skarecky DW. Prostate weight and early potency in robot-assisted radical prostatectomy. Urology. 2008;72:1263–1268. doi: 10.1016/j.urology.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AL, Aus G, Canby-Hagino ED, Cookson MS, D’Amico AV, Dmochowski RR, et al. Erectile function outcome reporting after clinically localized prostate cancer treatment. J Urol. 2007;178:597–601. doi: 10.1016/j.juro.2007.03.140. [DOI] [PubMed] [Google Scholar]
- 7.Mulhall J. Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur Urol. 2007;51:1488–1489. doi: 10.1016/j.eururo.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Rambhatla A, Kovanecz I, Ferrini M, Gonzalez-Cadavid NF, Rajfer J. Rationale for phosphodiesterase 5 inhibitor use post-radical prostatectomy: experimental and clinical review. Int J Impot Res. 2008;20:30–34. doi: 10.1038/sj.ijir.3901588. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AL. Erectile dysfunction following radical prostatectomy. JAMA. 2005;293:2648–2653. doi: 10.1001/jama.293.21.2648. [DOI] [PubMed] [Google Scholar]
- 10.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 12.Dell’Albani P. Stem cell markers in gliomas. Neurochem Res. 2008;33:2407–2415. doi: 10.1007/s11064-008-9723-8. [DOI] [PubMed] [Google Scholar]
- 13.About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol. 2000;157:287–295. doi: 10.1016/S0002-9440(10)64539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohno H, Sakai T, Kitahara K. Induction of nestin, Ki-67, and cyclin D1 expression in Muller cells after laser injury in adult rat retina. Graefes Arch Clin Exp Ophthalmol. 2006;244:90–95. doi: 10.1007/s00417-005-0030-7. [DOI] [PubMed] [Google Scholar]
- 15.Medina RJ, Kataoka K, Takaishi M, Miyazaki M, Huh NH. Isolation of epithelial stem cells from dermis by a three-dimensional culture system. J Cell Biochem. 2006;98:174–184. doi: 10.1002/jcb.20757. [DOI] [PubMed] [Google Scholar]
- 16.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H, Yamada Y, Watanabe R, Mukai E, Hosokawa M, Takahashi A, et al. Nestin-positive cells in adult pancreas express amylase and endocrine precursor cells. Pancreas. 2005;31:126–131. doi: 10.1097/01.mpa.0000172564.80921.f7. [DOI] [PubMed] [Google Scholar]
- 18.Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li H, et al. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448:485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]
- 19.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–249. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- 21.Ernst C, Christie BR. Nestin-expressing cells and their relationship to mitotically active cells in the subventricular zones of the adult rat. Eur J Neurosci. 2005;22:3059–3066. doi: 10.1111/j.1460-9568.2005.04499.x. [DOI] [PubMed] [Google Scholar]
- 22.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838:1–10. doi: 10.1016/s0006-8993(99)01502-4. [DOI] [PubMed] [Google Scholar]
- 24.Itoh T, Satou T, Hashimoto S, Ito H. Isolation of neural stem cells from damaged rat cerebral cortex after traumatic brain injury. Neuroreport. 2005;16:1687–1691. doi: 10.1097/01.wnr.0000183330.44112.ab. [DOI] [PubMed] [Google Scholar]
- 25.Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103(Pt 2):589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- 26.Strojnik T, Rosland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. discussion 143–134. [DOI] [PubMed] [Google Scholar]
- 27.Canguven O, Burnett A. Cavernous nerve injury using rodent animal models. J Sex Med. 2008;5:1776–1785. doi: 10.1111/j.1743-6109.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 28.Bivalacqua TJ, Guzzo TJ, Schaeffer EM, Gebska MA, Champion HC, Burnett AL, et al. Application of Evicel to cavernous nerves of the rat does not influence erectile function in vivo. Urology. 2008;72:1169–1173. doi: 10.1016/j.urology.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Gratzke C, Strong TD, Gebska MA, Champion HC, Stief CG, Burnett AL, et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J Urol. 2010;184:2197–2204. doi: 10.1016/j.juro.2010.06.094. [DOI] [PubMed] [Google Scholar]
- 30.Sezen SF, Blackshaw S, Steiner JP, Burnett AL. FK506 binding protein 12 is expressed in rat penile innervation and upregulated after cavernous nerve injury. Int J Impot Res. 2002;14:506–512. doi: 10.1038/sj.ijir.3900919. [DOI] [PubMed] [Google Scholar]
- 31.Allaf ME, Hoke A, Burnett AL. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J Urol. 2005;174:2060–2064. doi: 10.1097/01.ju.0000176808.94610.dd. [DOI] [PubMed] [Google Scholar]
- 32.Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278–H1290. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- 33.Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, et al. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- 34.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665–671. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- 36.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, et al. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456–16463. doi: 10.1074/jbc.M009669200. [DOI] [PubMed] [Google Scholar]
- 38.Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111(Pt 14):1951–1961. doi: 10.1242/jcs.111.14.1951. [DOI] [PubMed] [Google Scholar]
- 39.Wei LC, Shi M, Chen LW, Cao R, Zhang P, Chan YS. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res Dev Brain Res. 2002;139:9–17. doi: 10.1016/s0165-3806(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience. 2002;114:905–916. doi: 10.1016/s0306-4522(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura S, Takagi K, Okuda-Ashitaka E, Lu J, Naritsuka H, Yamaguchi M, et al. Characterization of nestin expression in the spinal cord of GFP transgenic mice after peripheral nerve injury. Neuroscience. 2010;170:942–953. doi: 10.1016/j.neuroscience.2010.07.034. [DOI] [PubMed] [Google Scholar]


