Abstract
Purpose of review
Diarrhea-associated hemolytic uremic syndrome (HUS) caused by Shiga toxin-producing Escherichia coli (STEC) continues to be an important public health threat worldwide. Specific therapies are lacking and patient care remains largely supportive. This review discusses the lessons learned from recent events and summarizes key advances made toward understanding the basic mechanisms involved in the pathogenesis of typical HUS.
Recent findings
The recent German outbreak of a hybrid organism resulted in an unprecedented number of HUS cases and drastically changed the face of typical (diarrhea-associated) HUS. New findings on the roles of complement and the CXCR4/SDF-1 pathway in HUS pathogenesis are summarized and novel therapeutic strategies are highlighted.
Summary
A better understanding of STEC-mediated HUS underlies improved therapeutic approaches. New studies of the mechanistic basis of the disease, together with patient-based studies, have led to key findings with important clinical implications.
Keywords: complement pathway, CXCR4, hemolytic uremic syndrome, SDF-1, Shiga toxin, Shiga toxin-producing E. coli
INTRODUCTION
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy that is clinically defined by thrombocytopenia, nonimmune hemolytic anemia, and acute renal failure, and is broadly categorized into two classes. Typical or diarrhea-associated (D+) HUS accounts by far for the majority of cases and develops secondary to gastrointestinal infection with Shiga toxin (Stx)-producing Escherichia coli (STEC) [1,2]. Young children are especially at high risk of developing HUS following STEC infection, and the disease is a leading cause of pediatric acute renal failure. On the other hand, atypical HUS accounts for only 10% of cases and has a poor prognosis [1], with a 25% mortality rate and end-stage renal disease (ESRD) in 50% of patients [2]. Familial and sporadic cases of atypical HUS are associated with genetic mutations or acquired deficiencies in complement regulation. Such defects predispose individuals to develop HUS often in the context of an environmental trigger such as pregnancy, nonenteric infection, malignancy, transplantation, and drugs [2].
A recent, high-profile outbreak of STEC in Germany in the middle of 2011 was associated with an unprecedented number of HUS cases and has reengaged the public and medical profession with this global health issue. This review focuses on these latest events in D+ HUS and recent advances in understanding the mechanistic basis of this disease.
OVERVIEW OF TYPICAL HEMOLYTIC UREMIC SYNDROME
HUS is the most serious, life-threatening complication following STEC infection. Fecal contamination of food and drinking water by asymptomatic cattle is often the source, at least for E. coli O157:H7, although secondary infection through person-to-person contact and swimming water may also occur [3]. D+ HUS is most prevalent in infants and young children, and occurs in 5–15% of cases [4,5]. The risk of mortality or ESRD is 12% and long-term renal sequelae are reported in 25% of survivors [6].
Histopathology shows fibrin-rich microvascular thrombi in the renal glomeruli, although thrombosis can also occur in preglomerular arterioles and medium-sized vessels. Endothelial swelling and detachment from the glomerular basement membrane is accompanied by subendothelial deposits and narrowing of the vessel lumen [7]. Thrombocytopenia results from platelet consumption in thrombi, and red blood cell fragmentation occurs as the cells pass through thrombosed vessels. These events occur primarily in the kidneys, but extrarenal complications may develop, particularly in the central nervous system in 10 to up to 50% of patients [4,8]. Clinical and pathological involvement of the gastrointestinal tract, lungs, heart, and pancreas is probably under-recognized.
No specific treatments currently exist for D+ HUS, and patients receive supportive care to manage the gastrointestinal, hematologic, vascular, and renal symptoms. Alternative approaches to antimicrobial treatment are necessary to inhibit disease progression, as antibiotic and antimotility agents may increase the risk of progression to HUS [8]. In fact, antibiotics are now recognized to potentiate the synthesis and release of Stxs from enterohemorrhagic E. coli [9▪]. Curiously, not every patient infected with STEC progresses to HUS and it can be hypothesized that individual genetic predisposition plays a role; however, such factors have yet to be identified. The inability to predict in which patients progression to HUS is imminent is still a significant obstacle facing clinicians. A well designed study to assess affected pedigree members versus incidence in genetically unrelated individuals is required. Sadly, the frequent occurrences of food-associated or water-associated epidemics represent appropriate settings for such studies.
SHIGA TOXIN-MEDIATED PATHOGENESIS
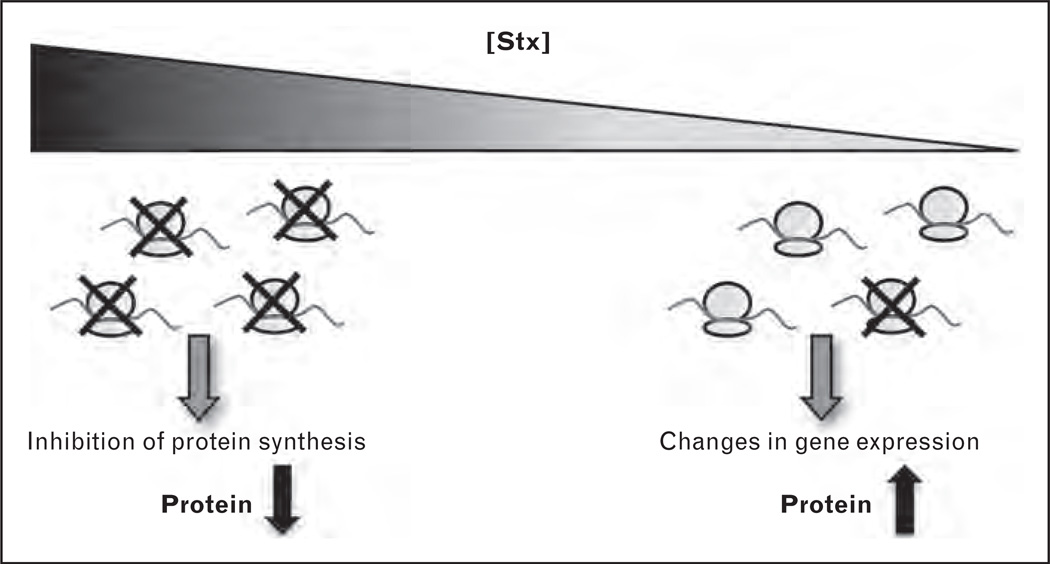
Stx is quintessential to HUS pathogenesis following STEC infection. Stx-mediated damage to the endothelium is thought to be the inciting event leading to thrombotic microangiopathy [6,10]. The toxins are released by STEC in the gastrointestinal tract and translocate across the intestinal epithelium into the systemic circulation, where they bind to globotriaosylceramide (Gb3) expressed on the surface of susceptible host cells, including the glomerular endothelium [11]. The Stx family comprises Stx1 and Stx2 together with their variants, Stx1c, Stx2c, Stx2d, Stx2e, Stx2f, and Stx2g. These AB5 protein toxins consist of a homopentameric ring of receptor-binding B subunits and a single enzymatic A subunit that depurinates host 28S rRNA and inhibits protein synthesis [12]. Although classically viewed as ribosome inactivators causing cell injury and death through inhibition of protein translation, it is becoming increasingly apparent that at concentrations that have negligible effects on protein synthesis, Stxs have dramatic effects on gene expression and RNA metabolism, essentially altering the endothelial phenotype (Fig. 1) [10,13,14▪]. Basally, the endothelium exhibits a vasodilatory profile, is thrombo-resistant, antiadhesive, and anti-inflammatory. However, in the presence of Stx, increased endothelial expression of hallmark proadhesive, prothrombotic, and inflammatory molecules is observed [10]. The important effect of Stx on enhancing endothelial monolayer permeability is increasingly recognized. However, the factors that mediate these effects are poorly understood.
FIGURE 1.
Molecular mechanisms of Shiga toxin (Stx) pathobiology. Stx inactivates host ribosomes by removing a specific adenine residue from the 28S rRNA, thus inhibiting protein synthesis. At concentrations that have only minor effects on global protein synthesis, Stx causes changes in gene expression and endothelial cell phenotype.
EMERGENCE OF HYBRID ORGANISMS GIVE A NEW FACE TO HEMOLYTIC UREMIC SYNDROME
Karmali et al.’s [15] seminal reports over 25 years ago made the association between STEC infection and sporadic cases of HUS. Since then, E. coli O157:H7 has become recognized as the leading cause of HUS globally [4,16]. Beginning in May 2011, an unprecedented STEC outbreak struck Germany, and affected 15 other European and North American countries. Contaminated sprouts were incriminated as the source of the outbreak [17▪]. The Robert Koch Institute officially declared the outbreak over on 26 July 2011, but not before 3816 people in Germany became ill [18▪▪]. Among these, 845 individuals developed HUS and 54 deaths were reported (HUS and gastroenteritis combined) [18▪▪]. Elsewhere, 51 cases of HUS and 2 deaths were linked to the outbreak [18▪▪]. Several key and remarkably unusual clinical features of this outbreak are highlighted below.
Unlike typical STEC outbreaks in which young children are most often affected, 88% of HUS cases in the German outbreak occurred in adults (i.e., >17 years; median age, 42) [18▪▪], and only 2% of HUS patients were under the age of 5 years [19]. Curiously, women were disproportionately affected (68% of cases in women vs. 56% between 2001 and 2010 in Germany) [19]. The median incubation period to the onset of diarrhea was longer than usual at 8 days [19]. Another unusual aspect of this outbreak was the higher proportion of individuals who developed HUS. A total of 20–22% of infected patients progressed to HUS, highlighting the increased virulence of the causative agent [18▪▪]. Early reports indicated that an alarmingly high proportion of HUS patients developed neurological complications, while HUS seemed to be resolving [7,20] (Table 1).
Table 1.
Characteristics of E. coli O157:H7, the most common serotype associated with HUS globally, compared with those of E. coli O104:H4, the highly virulent causative agent in the German outbreak of 2011
| STEC serovar | ||
|---|---|---|
| O157:H7 | O104:H4 (German isolate) | |
| Incidence of HUS | 5–15% [4,5] | 20–22% [18▪▪] |
| Patient demographics | Children | 88% adults (median age, 42) [18▪▪] 68% of HUS cases occurred in women [18▪▪] |
| Clinical features | Neurologic involvement uncommon Bloody diarrhea frequent |
Neurologic involvement common Bloody diarrhea infrequent |
| Incubation period | 3–5 days [4, 18▪▪] | 8 days [19] |
| Reservoir | Zoonosis – cattle and other ruminants | Not a zoonosis – unknown, possibly humans |
| Pathogroup | EHEC | EAEC |
| stx2 | + | + [21▪▪, 22▪▪] |
| eae | + | − [23▪] |
| ehx | + | − [23▪] |
| aggR, aap, aatA, aggA | − | + [23▪] |
| Sorbitol fermentation | − | + |
aap, dispersin; aatA, outer membrane TolC; aggA, pilin subunit of aggregative adherence fimbriae I; aggR, transcriptional regulator; eae, intimin; ehx, enterohemolysin; EAEC, enteroaggregative E. coli; EHEC, enterohemorrhagic E. coli; HUS, hemolytic uremic syndrome; STEC, Shiga toxin-producing E. coli; stx2, Shiga toxin 2.
What exactly was responsible for this unusual outbreak? The exceptionally virulent pathogen responsible for the German outbreak was identified as E. coli O104:H4, an enteroaggregative E. coli (EAEC) that colonizes the bowel in a characteristic ‘stacked brick’ pattern and normally causes watery diarrhea in children, travelers’ diarrhea, and persistent diarrhea in HIV patients [24,25]. The organism could not be isolated from cattle in northern Germany, and it appears it may not have a zoonotic origin [26]. Unlike O157:H7, O104:H4 had not previously been associated with large HUS outbreaks, and until May 2011 E. coli O104:H4 had only been associated with a handful of sporadic HUS cases [25,27]. Additionally, the German outbreak prompted retrospective analysis of an isolate obtained in 2009 from a pediatric HUS patient in Italy, which was also shown to be O104:H4 [28]. Genomic sequencing of the German isolate indicated it was, surprisingly, a hybrid organism, possessing features common to both EAEC and STEC [21▪▪,22▪▪,23▪] (Table 1). Comparison to historic O104:H4 isolates indicated the current strain had acquired the stx2 gene (Stx2a variant) and extended-spectrum β-lactamase production via horizontal gene transfer [9▪].
That an organism not typically associated with HUS could cause such a significant outbreak upon acquiring the unusual capacity to produce Stx underscores the seminal role that the toxin plays in the pathophysiology of HUS. Why adults were primarily affected in this outbreak is intriguing. Whether this reflects variations in the consumption of contaminated sprouts by adults and children, and the ability of the EAEC to colonize the bowel of adults compared with children remain to be determined. Interestingly, the fact that more adults experienced hemorrhagic colitis, while vomiting was more common in children, could possibly be related to differences in susceptibility to intestinal colonization [18▪▪]. The sad lesson learned from this outbreak is that exchange of genetic material among organisms can lead to the emergence of dangerously hypervirulent pathogens, targeting unexpected populations. The long incubation period also confounded epidemiological assessments of common source contaminants. This event underscores the importance of testing for the presence of Stx and not relying solely on E. coli serotype. Furthermore, an emphasis should be placed on food and water safety in order to control the spread of STEC and related HUS cases.
Several newer approaches to therapy were assessed in this epidemic. The use of anti-C5 (eculizumab) and immunoadsorption therapy are discussed below. In addition, Colic et al. [29] treated five adult patients with plasma exchange and suggest that early plasma exchange correlated with reduced lactate dehydrogenase. However, the use of plasma exchange as the first-line therapy for D+ HUS remains controversial.
A ROLE FOR COMPLEMENT IN TYPICAL HEMOLYTIC UREMIC SYNDROME
Mutations in complement regulatory genes are found in 50% of patients with atypical HUS [30]. Until recently, studies on the involvement of complement in D+ HUS were lacking. However, there is now growing evidence for a role for activation of complement in this disease. Elevated levels of the terminal complement complex (TCC) were reported in a small group of children with STEC-mediated HUS [31]. Purified Stx was found to induce formation of TCC when incubated with normal serum in vitro [32]. In addition, Stx was shown to bind factor H and this binding delayed cell surface factor H activity [32]. These in-vitro studies used relatively high concentrations of toxin, which is not detectable in patient blood, and whether these events have a role in HUS patients remains to be determined.
More recently, Morigi et al. [33▪] demonstrated that Stx-mediated upregulation of P-selectin on microvascular endothelial cells increased C3 deposits and favored platelet thrombus formation under flow conditions in vitro. This resulted in increased C3a levels that, in turn, further enhanced P-selectin expression [33▪]. These results were confirmed in vivo in a mouse model. In an independent study, increased levels of C3 were observed on platelet–leukocyte complexes in a patient with D+ HUS compared with levels at recovery and with normal controls [34]. This study also demonstrated increased levels of TCC in the acute phase of disease in HUS patients, further supporting the work by Orth et al. [32].
Eculizumab (Soliris, Alexion Pharmaceuticals, Cheshire CT, USA) was approved by the U.S. Food and Drug Administration (FDA) in September 2011 for the treatment of atypical HUS. It is currently the most expensive drug on the market at a cost exceeding 400 000 USD per patient per year for patients being treated for paroxysmal nocturnal hemoglobinuria [35]. The humanized monoclonal antibody directed against C5 prevents cleavage to C5a and C5b and inhibits TCC formation. During the German outbreak, Lapeyraque et al. [36▪] reported the use of eculizumab with promising results in three 3-year-old patients suffering from severe D+ HUS with neurologic involvement. In an effort to determine the utility of eculizumab in STEC-mediated HUS, the drug was administered free of charge to over 200 patients affected by the German outbreak [1]. Patient outcomes have yet to be reported. The sum of these recent data suggests that uncontrolled activation of complement is an important facet not only of atypical HUS but also of typical HUS, and they highlight the need for well designed studies. A complicating factor is that controlled trials are difficult to implement during an infectious outbreak, especially one that crosses international boundaries.
PATHOPHYSIOLOGY OF LATE-ONSET SYMPTOMS IN TYPICAL HEMOLYTIC UREMIC SYNDROME
The 2011 outbreak has provided important insight into the pathogenesis of neurological complications accompanying D+ HUS. Greinacher et al. [37▪] conducted a noncontrolled, prospective trial of 12 adult patients with severe neurological complications and recent E. coli O104:H4 infection who did not respond to eculizumab therapy or plasma exchange. Delayed neurological symptoms in these patients suggested to them the possibility that an immune-mediated pathogenesis was operative. Therefore, immunoadsorption therapy was used to deplete IgG. Over 80% of patients experienced complete recovery of neurological and renal function in this study. A competing model is the interaction between the host and the Stx-producing bacterium. Just as the time from contaminated food source to diarrhea is prolonged in this adult-enriched 2011 cohort, it is possible that the systemic access of the toxin is also prolonged in this new disease. Complicating our understanding of these clinical features is the prior realization that even for water-borne E. coli O157:H7-associated HUS epidemics, mortality is evident predominantly in older patients and is associated with neurological involvement [38]. The unfortunate epidemic underscores that myriad organs are affected by Stx: blood, bone marrow, and kidney, but also brain and heart, among others. The role of anti-Stx and anticomplement factor H responses in organ injury merits closer scrutiny. It has also been suggested that differences in the effectiveness of eculizumab between adults infected with O104:H4 and children infected with O157:H7 may be because of the differences in E. coli serotype or age-related differences in complement activation [39].
RECENT BASIC ADVANCES IN UNDERSTANDING SHIGA TOXIN PATHOGENESIS
A better understanding of Stx biology is crucial to improved patient therapies. A recent report describes a novel in-vitro model for the study of Stx effects on the microvasculature [40▪]. This 3-dimensional, ‘endothelialized’ microfluidic chamber allows the study of Stx in a physiologically relevant setting that recapitulates in-vivo size scale and hemodynamics. This model will be useful in studying complex biological processes of Stxmediated thrombosis and vessel occlusion in vitro.
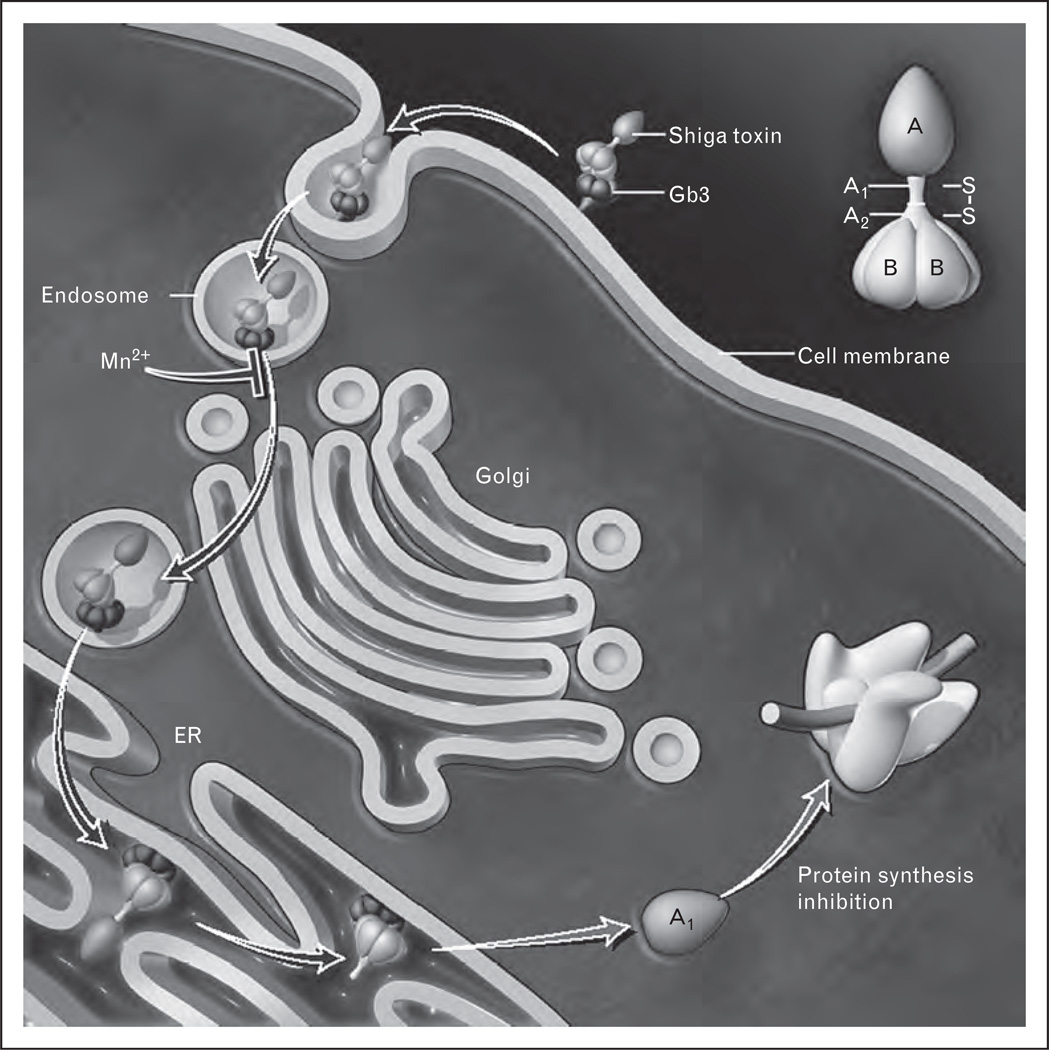
A second recent study shed light on the intracellular trafficking of Stx and how it may be targeted therapeutically [41▪]. Mn2+ was shown to block retrograde transport of Stx to the Golgi, a requisite step in Stx cellular intoxication, and resulted in lysosomal degradation (Fig. 2). When Mn2+ was given daily to mice beginning 5 days before the injection of Stx1, and then daily after the toxin, survival was enhanced. As it is not clear when in the course of disease Stx is released and reaches target organs, it is important to also consider additional therapeutic approaches aimed at inhibiting pathogenesis after it has already started and the patient has presented with symptoms. Therefore, studies on the effectiveness of Mn2+ on animal survival beginning after Stx challenge are warranted.
FIGURE 2.
Manganese blocks intracellular transport of Shiga toxin (Stx). Receptor-bound Stx is internalized by endocytosis and is transported retrogradely to the Golgi and endoplasmic reticulum (ER). The A subunit becomes activated upon cleavage and is translocated to the cytosol where it exerts its enzymatic activities. Manganese blocks retrograde transport of the toxin from the endosome to the Golgi leading to lysosomal degradation, and thereby disrupts Stx pathogenesis. Gb3, globotriaosylceramide. Adapted with permission [42].
THE CXCR4/SDF-1 CHEMOKINE PATHWAY IN HEMOLYTIC UREMIC SYNDROME PATHOGENESIS
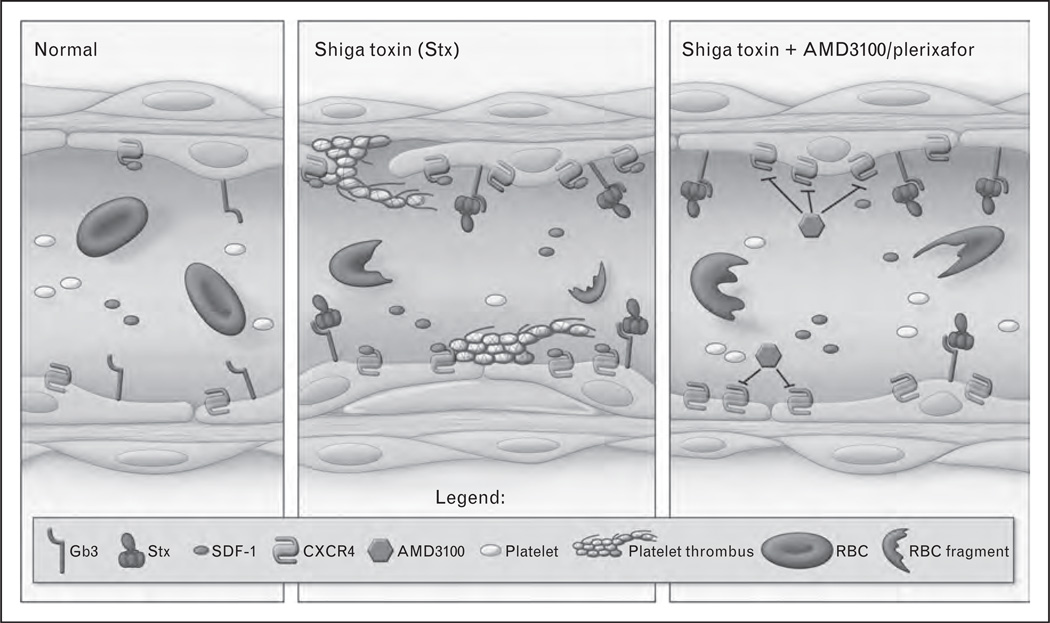
Recent studies in our laboratory have shown that Stx-mediated activation of the CXCR4/stromal cellderived factor 1 (SDF-1) pathway contributes to Stx-associated disease [14▪]. CXCR4 is a chemokine receptor whose endogenous ligand is SDF-1. The pathway is crucial in the development of the vascular beds affected in HUS, namely the renal glomerulus and gastrointestinal vessels [43,44]. Postnatally, it plays roles in vascular repair [45,46], but may also be pathogenic when overactivated [47]. Using an unbiased gene-expression profiling approach, we observed upregulation of both CXCR4 and SDF-1 mRNA by Stx in microvascular endothelial cells. SDF-1 levels also increased in the blood of Stx-treated animals [14▪]. Inhibition of CXCR4/SDF-1 interaction with the drug plerixafor (also known as AMD3100 and Mozobil, Genzyme Corporation, Cambridge, MA, USA) inhibited platelet adhesion to endothelial cells in vitro (Fig. 3). In vivo, the drug improved animal survival when administered in a therapeutic regimen (i.e., after toxin challenge) and was found to reduce the markers of kidney dysfunction and restore platelet levels (Fig. 3). To determine the relevance of these experimental findings in HUS patients, SDF-1 blood levels were measured in a prospective cohort of pediatric HUS patients with documented O157:H7 infection. The samples were obtained from children with uncomplicated infection and children in whom HUS subsequently developed. Hematocrit, platelet counts, and serum creatinine levels were normal in both groups. Despite the absence of clinically evident microangiopathic changes and renal insufficiency, the children in whom HUS subsequently developed had a four-fold increase in median plasma levels of SDF-1 preceding onset of HUS [median, 94.2 pg/ml; interquartile range (IQR), 79.3–81.6 pg/ml vs. median, 23.0 pg/ml, IQR, 7.3–69.46 pg/ml in individuals who were later diagnosed with the disease compared with children with uncomplicated infection, respectively] [14▪]. This finding supports the survival data in that the CXCR4/SDF-1 pathway contributes to HUS pathogenesis, but also suggests the intriguing possibility that SDF-1 could potentially serve as a biomarker to help predict patient outcome after STEC infection. Currently, clinicians cannot predict who will develop complications leading to HUS and who will recover spontaneously, as it is impossible to differentiate between the two clinically identical populations at presentation. The use of a prognostic marker from a simple blood test at presentation could allow for more aggressive treatment in the population at high risk for HUS. If further studies confirm these findings in a broader cohort, regardless of STEC serotype or patient age, there would be a strong argument for trials with plerixafor, which is already FDA approved for use in autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma and multiple myeloma.
FIGURE 3.
The contribution of the CXCR4/SDF-1 pathway to Shiga toxin (Stx) pathophysiology. Stx induces changes in endothelial gene expression and phenotype. Among the changes observed is detachment of the endothelium to expose the underlying basement membrane, subendothelial edema, and increased platelet adhesion. In vivo, this is accompanied by thrombocytopenia and red blood cell (RBC) fragmentation. CXCR4 and its ligand, SDF-1, are upregulated by Stx both in vitro and in vivo. Inhibition of CXCR4/SDF-1 interaction in vitro using plerixafor prevents Stx-mediated platelet adhesion to the endothelium under flow conditions. In vivo, plerixafor restores platelets to basal levels in an animal model of Stx challenge, and significantly attenuates Stx-mediated mortality. Individuals infected with E. coli O157:H7 exhibit elevated SDF-1 levels prior to onset of clinical features of hemolytic uremic syndrome. Taken together, these findings implicate an important role for the CXCR4/SDF-1 pathway in Stx-mediated pathogenesis. Gb3, globotriaosylceramide.
CONCLUSION
HUS following STEC infection is an important global health concern. The emergence of new, more virulent pathogens and changing demographics in 2011 remind us of the importance of an improved understanding of the pathogenic mechanisms driving the disease. Considerable advances have been made in the last year toward elucidating these mechanisms and these novel concepts warrant further study toward the development of new therapeutic options.
KEY POINTS
The emergence of new, hypervirulent Shiga toxin-producing microorganisms via horizontal gene transfer highlights the continuing threat to public health and the need to develop specific therapeutic options.
Recent studies suggest a role for complement in typical HUS. Shiga toxin mediates complement activation and subsequent thrombus formation.
Activation of the CXCR4/SDF-1 chemokine pathway by Shiga toxin contributes to HUS pathogenesis, and blockade of this pathway represents an attractive therapeutic option.
Patients destined to develop HUS following STEC infection exhibit increased SDF-1 chemokine levels in their blood prior to the clinical features of HUS.
Acknowledgements
This work was supported by the grants from the Canadian Institutes of Health, especially MOP 79475, and grant PO1 HL076540-06A1 from NHLBI/NIH to P.A. Marsden. P.A. Marsden holds the Keenan Chair in Medical Research at St. Michael’s Hospital and the University of Toronto.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 457–461).
- 1.Johnson S, Waters A. Is complement a culprit in infection-induced forms of haemolytic uraemic syndrome? Immunobiology. 2012;217:235–243. doi: 10.1016/j.imbio.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 3.Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC) Vet Microbiol. 2010;140:360–370. doi: 10.1016/j.vetmic.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 5.Obrig TG. Escherichia coli Shiga toxin mechanisms of action in renal disease. Toxins (Basel) 2010;2:2769–2794. doi: 10.3390/toxins2122769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoja C, Buelli S, Morigi M. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr Nephrol. 2010;25:2231–2240. doi: 10.1007/s00467-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 7.Jansen A, Kielstein JT. The new face of enterohaemorrhagic Escherichia coli infections. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 8.Boyer O, Niaudet P. Hemolytic uremic syndrome: new developments in pathogenesis and treatment. Int J Nephrol. 2011;2011 doi: 10.4061/2011/908407. 908407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med. 2011;365:709–717. doi: 10.1056/NEJMoa1106920. The authors propose that horizontal gene transfer was responsible for the acquisition of the stx2 gene and extended-spectrum β-lactamase production in the O10:H4 strain responsible for the outbreak in Germany.
- 10.Petruzziello TN, Mawji IA, Khan M, Marsden PA. Verotoxin biology: molecular events in vascular endothelial injury. Kidney Int Suppl. 2009;75:S17–S19. doi: 10.1038/ki.2008.612. [DOI] [PubMed] [Google Scholar]
- 11.Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function – where membrane structure and pathology intersect. FEBS Lett. 2010;584:1879–1886. doi: 10.1016/j.febslet.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 12.Endo Y, Tsurugi K, Yutsudo T, et al. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 13.Bitzan MM, Wang Y, Lin J, Marsden PA. Verotoxin and ricin have novel effects on preproendothelin-1 expression but fail to modify nitric oxide synthase (ecNOS) expression and NO production in vascular endothelium. J Clin Invest. 1998;101:372–382. doi: 10.1172/JCI522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petruzziello-Pellegrini TN, Yuen DA, Page AV, et al. The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice. J Clin Invest. 2012;122:759–776. doi: 10.1172/JCI57313. This study demonstrates that the inhibition of the CXCR4/SDF-1 pathway improves survival in animals treated with Stx, that SDF-1 blood levels increase in patients in the pre-HUS phase.
- 15.Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolyticuraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;1:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 16.Johannes L, Romer W. Shiga toxins – from cell biology to biomedical applications. Nat Rev Microbiol. 2010;8:105–116. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 17. Buchholz U, Bernard H, Werber D, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365:1763–1770. doi: 10.1056/NEJMoa1106482. This study identified sprouts as the source of transmission of E. coli O104:H4 during the German outbreak.
- 18. Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. This study provides epidemiological data on the O104:H4 outbreak. It highlights key unusual features of this outbreak that will be important for surveillance in future outbreaks.
- 19.Report: Final presentation and evaluation of epidemiological findings in the EHEC O104:H4 outbreak. Berlin: Robert Koch Institute; 2011. [Google Scholar]
- 20.The German EHEC-HUS Registry. The German 2011 epidemic of Shiga toxin-producing E. Coli – the nephrological view. Nephrol Dial Transplant. 2011;26:2723–2726. doi: 10.1093/ndt/gfr462. [DOI] [PubMed] [Google Scholar]
- 21. Rohde H, Qin J, Cui Y, et al. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med. 2011;365:718–724. doi: 10.1056/NEJMoa1107643. The authors sequenced the genome of the German O104:H4 strain and showed that the organism possessed genes from both EAEC and EHEC, including the stx2 gene.
- 22. Mellmann A, Harmsen D, Cummings CA, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022751. e22751. The authors sequenced the genome of the German O104:H4 strain and showed that the organism possessed genes from both EAEC and EHEC, including the stx2 gene.
- 23. Bielaszewska M, Mellmann A, Zhang W, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11:671–676. doi: 10.1016/S1473-3099(11)70165-7. In this study, virulence characteristics of 80 isolates from the German outbreak were analyzed and compared with other E. coli pathogroups.
- 24.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 25.Scheutz F, Nielsen EM, Frimodt-Moller J, et al. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill. 2011;16 doi: 10.2807/ese.16.24.19889-en. pii – 19889. [DOI] [PubMed] [Google Scholar]
- 26.Wieler LH, Semmler T, Eichhorn I, et al. No evidence of the Shiga toxin-producing E. coli O104:H4 outbreak strain or enteroaggregative E. coli (EAEC) found in cattle faeces in northern Germany, the hotspot of the 2011 HUS outbreak area. Gut Pathog. 2011;3:17. doi: 10.1186/1757-4749-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control and European Food Safety Authority. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EEA, with special reference to the German outbreak strain STEC O104. Stockholm: ECDC; 2011. [Google Scholar]
- 28.Scavia G, Morabito S, Tozzoli R, et al. Similarity of Shiga toxin-producing Escherichia coli O104:H4 strains from Italy and Germany. Emerg Infect Dis. 2011;17:1957–1958. doi: 10.3201/eid1710.111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colic E, Dieperink H, Titlestad K, Tepel M. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet. 2011;378:1089–1093. doi: 10.1016/S0140-6736(11)61145-8. [DOI] [PubMed] [Google Scholar]
- 30.Benz K, Amann K. Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens. 2010;19:242–247. doi: 10.1097/MNH.0b013e3283378f25. [DOI] [PubMed] [Google Scholar]
- 31.Thurman JM, Marians R, Emlen W, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1920–1924. doi: 10.2215/CJN.02730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orth D, Khan AB, Naim A, et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J Immunol. 2009;182:6394–6400. doi: 10.4049/jimmunol.0900151. [DOI] [PubMed] [Google Scholar]
- 33. Morigi M, Galbusera M, Gastoldi S, et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol. 2011;187:172–180. doi: 10.4049/jimmunol.1100491. This study shows evidence of alternative complement activation in children with D+ HUS. These results encouraged the study of complement in Stx-mediated disease and therapeutic inhibition of complement in patients.
- 34.Stahl AL, Sartz L, Karpman D. Complement activation on platelet–leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117:5503–5513. doi: 10.1182/blood-2010-09-309161. [DOI] [PubMed] [Google Scholar]
- 35.Herper M. The world’s most expensive drugs. Forbes. 2010 [Google Scholar]
- 36. Lapeyraque AL, Malina M, Fremeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–2563. doi: 10.1056/NEJMc1100859. Eculizumab treatment was reported to dramatically improve symptoms in three children with severe D+ HUS. This observation has important implications for future treatment of patients with STEC-mediated HUS. Well designed studies addressing the therapeutic efficacy and safety of this drug are warranted.
- 37. Greinacher A, Friesecke S, Abel P, et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: a prospective trial. Lancet. 2011;378:1166–1173. doi: 10.1016/S0140-6736(11)61253-1. This study describes immunoadsorption therapy for HUS patients with severe neurological complications.
- 38.Garg AX, Clark WF, Salvadori M, et al. Microalbuminuria three years after recovery from Escherichia coli O157 hemolytic uremic syndrome due to municipal water contamination. Kidney Int. 2005;67:1476–1482. doi: 10.1111/j.1523-1755.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim JJ. Immunoadsorption for haemolytic uraemic syndrome. Lancet. 2011;378:1120–1122. doi: 10.1016/S0140-6736(11)61342-1. [DOI] [PubMed] [Google Scholar]
- 40. Tsai M, Kita A, Leach J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–418. doi: 10.1172/JCI58753. A novel in-vitro system is described consisting of a 3-dimensional endothelialized structure under flow conditions that more closely resembles the in-vivo microvasculature and provides a model to study Stx biology.
- 41. Mukhopadhyay S, Linstedt AD. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science. 2012;335:332–335. doi: 10.1126/science.1215930. This study demonstrates that Mn2+ can block Shiga toxin intracellular transport and improve animal survival when administered before toxin challenge.
- 42.Matouk CC, Marsden PA. Molecular insights into the thrombotic microangiopathies. In: Mount DB, Pollack MR, editors. Molecular and genetic basis of renal disease: a companion to Brenner & Rector’s the kidney. Philadelphia: Saunders; 2008. pp. 453–480. [Google Scholar]
- 43.Takabatake Y, Sugiyama T, Kohara H, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 45.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 46.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding M, Cui S, Li C, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]