Summary
Endothelial injury is a characteristic finding in chronic kidney disease and is associated with both markedly increased cardiovascular risk and chronic kidney disease progression. The past decade has seen a remarkable surge of interest in the role of bone marrow– derived cells for the protection, repair, and regeneration of injured endothelium. In particular, despite controversies regarding their mechanisms of action, endothelial progenitor cells have garnered considerable attention, with multiple reports suggesting that these cells exhibit remarkable pro-angiogenic effects. Recent advances in our understanding of how the bone marrow responds to endothelial injury now suggest that multiple bone marrow cell populations, including both endothelial progenitor cells and a novel group of cells called early outgrowth cells, promote endothelial repair and regeneration through different, yet complementary, mechanisms. Moreover, certain subsets of bone marrow– derived cells also appear to have novel, potent, angiogenesis-independent tissue-protective properties. The bone marrow should thus now be viewed not only as a hematopoiesis organ, but also as a rich reservoir of cells capable of protecting and even regenerating nonhematopoietic tissues such as the kidney. To harness the prognostic and therapeutic potential of the bone marrow, the renal community must be aware of recent advances in our understanding of the nature and therapeutic potential of these cells.
Keywords: Endothelial progenitor cell, early outgrowth cell, chronic kidney disease, angiogenesis, fibrosis, oxidative stress
Chronic kidney disease (CKD) is a major cause of hospitalization, premature death, and impaired quality of life.1 Despite its increasing prevalence and associated disease burden, the mechanisms underlying CKD progression and its complications are still being worked out. It increasingly has become recognized that the endothelium plays an important role in maintaining homeostasis of not only the cardiovascular system but also the normal functioning kidney. Indeed, damage and dysfunction of the endothelium has been associated with not only increased cardiovascular risk, but also with acute renal injury2 and progression of chronic kidney disease.3 The shared effects of endothelial dysfunction on cardiovascular and renal health suggest a central role for endothelial disease as a mediator of both kidney damage and cardiovascular injury.
In 1997, Asahara et al4 described a population of bone marrow– derived angiogenic cells that had the ability to regenerate new blood vessels in ischemic tissue. They called these cells “endothelial progenitor cells” (EPCs), sparking a wave of interest in bone marrow progenitor cell populations that had the ability to replace injured or lost endothelial cells. This seminal report was followed by reports of benefit using both endogenous and exogenously administered bone marrow– derived cells (BMDCs) in various ischemic disease models, such as myocardial infarction,5–8 peripheral arterial disease,9,10 pulmonary hypertension,11 and ischemia-reperfusion renal injury.12 In all these reports, BMDC therapy appeared to enhance neovascularization of ischemic tissue, reduce tissue damage, and preserve organ function. Investigators focused on the ability of BMDCs to engraft and trans-differentiate into resident endothelial cells in ischemic organs, a process termed vasculogenesis and reminiscent of embryonic vascular development. Given the importance of microvascular damage in mediating the progression of renal injury and the pathogenesis of the vascular injury associated with CKD, these reports suggested a possible new tool in the treatment of CKD and its vascular complications.
Because many different BMDC populations with tissue-protective properties have been described to date, a comprehensive discussion of all such cells is beyond the scope of this review. This article therefore aims to critically re-examine the EPC literature from a renal perspective, highlighting more recent advances that have shed light on bone marrow– derived cells with angiogenic properties that are relevant for the renal research community.
ENDOTHELIAL PROGENITOR CELLS AS A NOVEL CELL POPULATION THAT DIRECTLY REGENERATE THE INJURED OR LOST ENDOTHELIUM: THE ENGRAFTMENT HYPOTHESIS
The initial report by Asahara et al4 described the existence of adult bone marrow–derived cells that had the ability to contribute to new vessel formation in ischemic tissue. In support of their claim that these cells were endothelial progenitor cells, they found that these cells expressed genes that are expressed in mature endothelium, and at least a portion of the cells appeared to incorporate into vessels along the inner lining as one would expect of mature endothelial cells. Importantly, these cells improved blood flow to the ischemic leg of mice with surgically induced limb ischemia following intracardiac cell infusion.9 Hints also came from the work of others, as a host of studies showed that bone marrow–derived cells appeared to contribute to endothelialization of left ventricular assist devices13 and to neovascularization of other ischemic tissues, such as the infarcted heart,5–8 the ischemic retina,14 and the ischemic brain.15 In the kidney, bone marrow–derived cells were shown to accelerate endothelial cell recovery in a model of Thy1 nephritis,16 and to maintain capillary density in a model of unilateral renal artery stenosis.17 We and other groups also have shown that bone marrow– derived cells could be used to attenuate the glomerular and peritubular capillary rarefaction that occurs in experimental chronic kidney disease.18–20
Mechanistic studies also began to tease out the manner in which these cells are mobilized from the bone marrow and subsequently home to areas of ischemia. Angiogenic chemokines such as vascular endothelial growth factor-A (VEGF-A) and stromal cell–derived factor-1α were shown to activate bone marrow cell mobilization through matrix metalloproteinase–dependent mechanisms.21–23 Moreover, these same chemokines, the expression of which is regulated by ischemia through stabilization of hypoxia-inducible factor 1α,24,25 also were shown to play critical roles in attracting mobilized bone marrow-derived cells to sites of ischemia and vascular injury.26 Accordingly, exogenous delivery of stromal cell– derived factor-1, either via plasmid-mediated gene transfer27 or via direct protein injection,28 enhanced bone marrow cell–mediated vasculogenesis in models of ischemic peripheral vascular disease. Similarly, ectopic up-regulation of VEGF-A in either the liver or the heart efficiently recruited bone marrow–derived cells that promote neo-vascularization.26
These reports lead to the theory that a bone marrow–based population of EPCs responds to ischemic stimuli by migrating from the bone marrow to areas of ischemia, after which they transdifferentiate into mature endothelial cells, either replacing injured cells, or even forming new vessels through vasculogenesis.4 The resulting progeny endothelial cells were thought to help maintain perfusion to the ischemic tissue, leading to improvements in structure and function.
PROBLEMS WITH THE ENGRAFTMENT HYPOTHESIS
Despite the excitement generated by these reports, lingering concerns have persisted regarding the mechanisms by which these so-called EPCs mediated their benefits. Careful review of the literature revealed a growing discrepancy between the consistent and often dramatic structural and functional benefits seen with bone marrow cell–based therapies, and the highly variable retention rates of these cells in the injured organs being targeted, with reports ranging from 0% to 80% retention.29,30 Moreover, careful tracking studies of either exogenously infused cells or endogenous bone marrow– derived cells questioned whether retained cells actually could be found in significant numbers lining the inner wall of blood vessels.31–34 Indeed, these studies showed that recruited bone marrow– derived cells were located more commonly in perivascular areas rather than in an intraluminal location.31–35 Along similar lines, it was recognized that expression of proteins characteristic of endothelial cells such as VEGF receptor-2 or CD31 (platelet and endothelial cell adhesion molecule-1) does not necessarily infer transdifferentiation into mature functional endothelial cells.31,36
NOT ALL EPCs ARE EPCs
The earlier-described studies raised important questions regarding how precisely to define what an endothelial progenitor cell is. In the strictest sense, an EPC must have the ability to self-renew, and also must give rise to progeny endothelial cells that can form a functional endothelium in vivo.36 Although multiple bone marrow–derived cell populations can exert potent pro-angiogenic effects both in vitro and in vivo,36,37 how these cells promote new endothelial formation is a critical issue with important mechanistic and therapeutic implications. Cells that are recruited to areas of ischemia and that promote local neovascularization, but do not engraft as endothelial cells themselves, are not true EPCs because they do not give rise to progeny endothelium. Likewise, by this definition, cells that are not found in significant numbers in ischemic tissue despite a profound neovascularization response also should not be termed EPCs. To add to the complexity of this issue, the cell populations being studied often are heterogeneous groups of cells, the cellular constituents of which may play different, yet complementary, roles in promoting neovascularization.
With these issues in mind, despite the fact that many bone marrow– derived cell populations induce potent angiogenic and tissue-protective responses, very few of them truly qualify as EPCs. Further complicating matters, various groups studying EPCs have assigned them different names in the literature, identifying them variously as “late outgrowth EPCs,”38 “blood outgrowth endothelial cells,”39 or “endothelial colony forming cells.”40,41 Importantly, because no unique identifying cell surface markers have yet been described to identify these cell populations, these cells have been defined jointly by the culture techniques used for their generation, and their ability to incorporate into endothelial networks both in vitro and in vivo.36 In short, late outgrowth EPCs, blood outgrowth endothelial cells, and endothelial colony forming cells are different names for the same EPC cell type generated by culturing blood mononuclear cells on an extracellular matrix protein such as collagen or fibronectin in an endothelial differentiation medium. Adherent cell colonies emerge after either 5 to 7 days of culture (if using umbilical cord blood–derived mononuclear cells), or 10 to 24 days of culture (if using adult peripheral blood–derived mononuclear cells).42 It is not clear whether culturing bone marrow cells in a similar manner produces the same EPC phenotype. True EPCs have remarkable clonal proliferative potential, express endothelial-like proteins, and give rise to functional blood vessels in vivo in preclinical animal models.41,43–46 The major function of these cells, at least in preclinical studies, appears to be their ability to incorporate into new vascular networks in areas of ischemia. Whether these cells exert other tissue-protective effects beyond their ability to integrate into vascular networks as endothelial cells is not clear.
EARLY OUTGROWTH CELLS
More recent studies have clearly shown that some bone marrow–derived cells previously labeled as EPCs, although not meeting the earlier-mentioned criteria of an endothelial progenitor cell, show significant pro-angiogenic properties. Commonly used techniques to generate these non-EPC pro-angiogenic cells involve the culture of peripheral blood mononuclear cells or unfractionated bone marrow on collagen or fibronectin for shorter culture times (4–10 days) than those used to generate EPCs.47,48 In contrast to EPCs, these cells appear to be a heterogeneous mix of hematopoietic cells including monocytes and lymphocytes that show angiogenic properties.41,49,50 Because unfractionated, uncultured bone marrow cells also have been shown to have pro-angiogenic effects,51 it is possible that this short ex vivo culture period enriches the resulting cell population with hematopoietic cells that have a pro-angiogenic phenotype. More recently renamed “circulating angiogenic cells”36 or “early outgrowth cells” (EOCs),38 this heterogeneous group of cells expresses some endothelial-like proteins,36 but importantly does not incorporate into blood vessels in significant numbers either in vitro or in vivo.52 Although it is difficult to compare between cell populations given their heterogeneous composition and because different investigators have prepared their cells in different ways, the common emerging theme is that early outgrowth cells release soluble factors that promote angiogenic and/or vasculogenic processes such as EPC and endothelial cell survival,53 migration,54 proliferation,44 and tube formation.52,55,56 Many such factors have been identified, and include VEGF-A, stromal cell–derived factor-1α, and basic fibroblast growth factor, among others.54,57
In various preclinical models of ischemic injury, including both ischemic acute16 and chronic17–20 kidney injury models, EOC infusion has been associated with a robust preservation of microvascular density and associated improvements in tissue function, despite being found only rarely in a classic intraluminal endothelial location.26,31,32,35 More commonly, early outgrowth cells appear to be found at least transiently in perivascular locations in ischemic tissue,31–35 leading some investigators to suggest that these cells may act in a paracrine manner to promote angiogenesis.54,58 Interestingly, other studies have reported much higher early outgrowth cell retention in reticulo-endothelial organs such as the liver and spleen, suggesting that these cells may be inducing their pro-angiogenic effects from remote locations.18,59 Taken together, these results suggest that early outgrowth cells create an environment that promotes other cell types to participate in endothelial network formation.
THE CLINICAL RELEVANCE OF EPCS AND EARLY OUTGROWTH CELLS
Despite the great interest in both EPCs and early outgrowth cells in the preclinical world, relatively little is known about their utility in the clinical setting. Indeed, despite the theoretical importance of EPCs in mediating endothelial repair, to our knowledge, there have been no clinical reports showing the utility of these cells as a therapeutic agent to date, although this may reflect the fact that this cell population only recently was precisely defined.
In contrast, preliminary findings in clinical studies have suggested important roles for various EOC populations in the setting of vascular injury. An early report, for example, showed an inverse correlation between circulating EOC number and cardiovascular risk in patients with coronary artery disease,47 suggesting a possible role for these cells in mediating vascular repair, although the exact reason for this association has not yet been elucidated. Furthermore, several clinical trials, as described later in this review, have examined the therapeutic efficacy of various early outgrowth cell populations in the treatment of human disease, showing encouraging but somewhat conflicting results.
Although no published clinical trials have tested early outgrowth cells as a treatment for acute or chronic kidney injury, a number of studies have tested EOC infusion for the treatment of ischemic vascular disease, a common complication of CKD. For the most part, these clinical trials have used freshly isolated, uncultured bone marrow cells that were not enriched by ex vivo culture, or fractionated into homogenous cell populations, meaning that an unselected, heterogeneous cell population was used. Tateishi-Yuyama et al,60 for example, showed that injection of such freshly isolated cells into the gastrocnemius muscle of patients with refractory ischemic peripheral vascular disease of the leg was associated with significant improvements in ischemic leg vascular density, perfusion, and function when compared with either saline or freshly isolated peripheral blood mononuclear cells, a remarkable finding given the relative paucity of effective medical therapies for this devastating disease.
Similar studies of such freshly isolated bone marrow cells have been performed in the setting of ischemic heart disease. Early post-myocardial infarction, intracoronary EOC infusion has yielded conflicting results, with several studies showing modest improvements,61,62 whereas others reported no significant change63,64 in left ventricular infarct remodeling and systolic function several months after cell therapy. In the chronic ischemic heart disease setting, studies testing infusion of either freshly isolated bone marrow cells or GCSF-mobilized CD34+ cells have demonstrated modest yet significant benefits in left ventricular function65 and anginal control66 when compared with standard-of-care therapy.
This variation in reported EOC efficacy may be related in part to differences in cell preparation and/or differences in patient selection, suggesting that our understanding of what cell types are most effective, and which patients are most responsive to therapy, needs further refinement. Taken together, however, these preliminary results suggest the exciting possibility that at least certain human early outgrowth cell populations may harbor the ability to promote vascular repair or even regeneration in human disease. Many important questions, of course, still remain. The exact cell population responsible for these benefits, their mode(s) of action, the disease settings in which these cells are most effective, the optimum route of administration, the dose of cells required, and the duration of effect are merely some of the questions that remain unanswered in this exciting, yet developing, field, and are actively being investigated.
INFLUENCE OF KIDNEY DISEASE ON EARLY OUTGROWTH CELL FUNCTION
In line with the fact that CKD commonly is associated with diffuse endothelial injury and dysfunction, recent data suggest that chronic renal dysfunction, even with the institution of conventional in-center hemodialysis, is associated with significant reductions in circulating EOC number and impairments in EOC function.67–70 Although it is unclear what exactly is responsible for this EOC defect, it appears that even short-term exposure to uremic serum significantly inhibits the function of EOCs derived from healthy donors in vitro.67 Consistent with the importance of adequate uremic clearance in maintaining EOC health, strategies that achieve extensive uremic toxin removal, such as home nocturnal hemodialysis and kidney transplantation, both are associated with significant improvements in EOC function in vitro.71,72 We recently extended these findings to the in vivo setting, showing that although EOCs cultured from conventional hemodialysis patients fail to promote neovascularization and restoration of blood flow in a rodent model of peripheral vascular disease, EOCs cultured from home nocturnal hemodialysis patients show potent neovascularization capacity.73 Taken together, these results point to the intriguing possibility that uremia-induced EOC dysfunction may be responsible, in part, for the endothelial injury observed in CKD. Because EOC-based therapies typically are autologous given the risk of immune rejection with allogeneic cell infusion, these results also suggest that the effectiveness of EOC-based therapies in the setting of uremia may be limited unless uremic toxin clearance also is augmented.
NOVEL ANTIFIBROTIC PROPERTIES OF EARLY OUTGROWTH CELLS
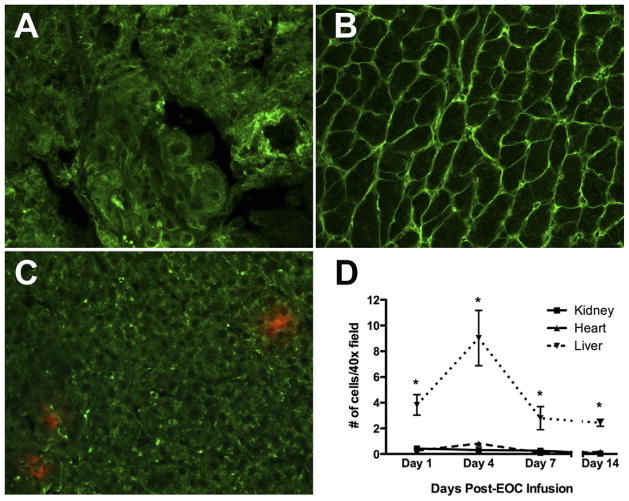
Aside from their well-described pro-angiogenic activity, recent reports have suggested other novel angiogenesis-independent mechanisms of action for EOCs. For example, EOCs were shown recently to exert potent antifibrotic effects when intravenously infused into rat models of experimental cirrhosis, with EOC-treated livers showing increased matrix metalloproteinase activity and correspondingly reduced type I collagen levels.74 It is unlikely that this resulted from angiogenic effects in the liver because this organ, through its dual blood supply, largely is ischemia-resistant. Interestingly, endogenous bone marrow cells and EOCs both have been shown to express matrix metalloproteinase-2, -9, and -13 in vitro and in vivo,74,75 providing a possible explanation for the antifibrotic activity of these cells. We recently showed that EOCs release soluble factors that inhibit transforming growth factor–β–induced fibroblast collagen production in vitro, possibly through inhibition of phosphorylation of Smad2, a key intracellular signaling molecule in the profibrotic transforming growth factor–β signaling pathway.18 Furthermore, intravenous EOC infusion was associated with marked attenuation of renal and cardiac fibrosis in an experimental chronic kidney disease rat model characterized by up-regulation of transforming growth factor–β expression in the kidney76 and heart,77 and subsequent fibrosis-associated organ dysfunction (Fig. 1).18 Importantly, EOC therapy was associated with improvement in both kidney and heart function, demonstrating the potential of these cells to target not only kidney injury, but also fibrosis-associated diastolic dysfunction, an important cardiac complication of CKD. These antifibrotic effects were noted despite minimal retention of EOCs within either organ, both at early and later time points after infusion, whereas infused cells were found in significant numbers within reticulo-endothelial organs such as the liver and spleen (Fig. 2). Such data suggest that the primary mechanism by which EOCs prevent extracellular matrix accumulation may be through the release of systemically circulating soluble factors that modulate fibrotic pathways via an endocrine mechanism of action.18 In line with this hypothesis, Bi et al78 recently suggested that mesenchymal stem cells, another bone marrow– derived cell population, protect against cisplatin-induced acute kidney injury through the release of systemically circulating factors.
Figure 1.
Both renal and cardiac fibrosis were improved markedly after systemic EOC infusion into rats with experimentally induced chronic kidney disease (5/6 subtotal nephrectomy [SNX]). (A–C) Representative type IV collagen immunostained kidney sections. Original magnification, ×160. (A) Sham-operated animal. (B) SNX animal. (C) SNX–EOC animal. (D–F) Representative picrosirius red staining of interstitial cardiac fibrosis. Original magnification, ×160. (D) Sham-operated animal. (E) SNX animal. (F) SNX–EOC animal.
Adapted from Yuen DA et al.18
Figure 2.
After infusion of 1 × 106 fluorophore-labeled EOCs, very few cells could be found in the kidney despite dramatic structural and functional benefits, whereas cells were found in the liver. (A–C) Representative confocal microscopy images of kidney, heart, and liver, respectively, at 4 days after EOC infusion. Original magnification, ×20. (A) Kidney cortex. (B) Heart. (C) Liver. (D) Time-course of EOC retention in kidney, heart, and liver. n = 3 animals per time point. *P< .05 versus kidney.
Adapted from Yuen DA et al.18
Because endothelial injury, ischemia, and fibrosis often are tightly interlinked processes,79 it is perhaps not surprising that cells with an angiogenic phenotype also possess the ability to suppress profibrotic pathways. Given the importance of fibrosis in mediating progressive injury in almost all forms of chronic kidney disease,80 and the importance of cardiac fibrosis and the resulting diastolic dysfunction that serves as a poor prognostic factor for CKD patients,81 the finding that EOC-derived factors can exert potent antifibrotic effects within the injured kidney and heart strengthens the possibility that such factors could be used to attenuate progression of human CKD and its cardiac complications.
NOVEL ANTI-OXIDANT PROPERTIES OF EARLY OUTGROWTH CELLS
Reactive oxygen species play a critical role in mediating injury in many forms of kidney disease.82 Interestingly, certain human EOC populations have been shown to express high levels of anti-oxidant enzymes such as manganese superoxide dismutase, catalase, and glutathione peroxidase, rendering them more resistant to oxidative stress stimuli.83 More recently, Yang et al53 extended this concept, showing that peripheral blood mononuclear cell–derived EOCs released factor(s) that could inhibit hydrogen peroxide–induced oxidant stress in cultured endothelial cells, reducing subsequent apoptosis rates. These EOC-derived soluble factor(s) up-regulated the expression of anti-oxidant enzymes within endothelial cells, suggesting that EOCs can activate cytoprotective mechanisms in target cells through the release of such soluble factors.53 Taken together, these results suggest that in addition to their antifibrotic activity, EOCs may exert important anti-oxidant effects through the release of soluble factors that could contribute, at least in some settings, to the tissue-protective properties of these cells.
THE BONE MARROW AS A RESERVOIR FOR TISSUE-PROTECTIVE AND TISSUE-REGENERATIVE CELL POPULATIONS
Advances in our understanding of the tissue-protective and tissue-regenerative properties of bone marrow–derived cell populations have transformed our understanding of the bone marrow. The bone marrow no longer can be considered solely as a hematopoietic reticulo-endothelial organ, but rather as a rich reservoir of cells, some of which have the potential to protect and regenerate injured nonhematopoietic tissues such as the kidney.
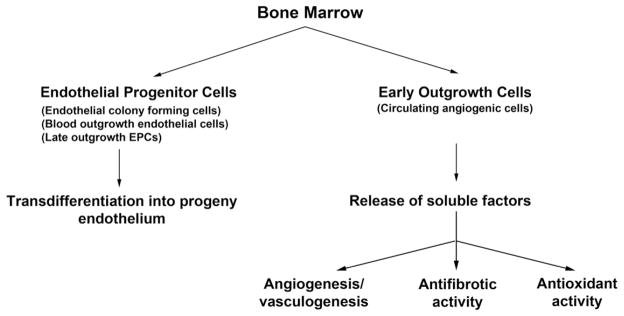
Endothelial injury, in particular, is a common complication of many disease states, including acute and chronic kidney injury, and itself can lead to important downstream ischemic consequences that often are tightly linked with other injurious processes such as fibrosis and oxidative stress. Importantly, the bone marrow harbors different populations of cells with protective and regenerative effects that target these pathologic processes, which broadly can be divided into two groups of cells: EPCs and EOCs (Fig. 3). Whereas EPCs can integrate directly into new or existing vascular networks to maintain perfusion, EOCs play important supportive roles for this process, promoting both EPC and local endothelial cell survival,53 migration,54 proliferation,44 and tube formation (Fig. 3).52,55,56 In addition, EOCs also show novel angiogenesis-independent tissue-protective activity, being able to suppress pathologic fibrosis18,74 and reactive oxygen species generation53 in vitro and in certain in vivo models (Fig. 3). Whether specific EOC subtypes in isolation can reciprocate one or more of these functions, or if the entire heterogeneous cell population is required for these functions, has yet to be determined.
Figure 3.
New proposed classification scheme for bone marrow–derived cell populations that participate in endothelial repair/regeneration.
Recognizing the different but potentially complementary roles played by these cell populations, Yoon et al44 tested whether a combined EPC/EOC strategy would have additive effects on top of either cell population alone. Interestingly, the addition of EOC-released factors enhanced EPC proliferation and integration into vascular networks in vitro.44 This synergism also was seen in vivo because a mixed EPC/EOC infusion strategy induced greater neovascularization in ischemic mouse hindlimbs compared with either cell population alone.44 Whether such a combined cell strategy would have similar additive effects in the treatment of kidney injury, in which endothelial injury, fibrosis, and oxidative stress all play major roles, is not known.
CONCLUSIONS
In the past 20 years, our concept of the bone marrow has evolved from a hematopoietic cell factory to a diverse reservoir of cells capable of protecting and regenerating many different tissues in the body. Recent advances in our understanding of the bone marrow’s response to endothelial damage, and tissue injury in general, has lead to the theory that multiple bone marrow–derived cell populations have the potential to respond, in different yet complementary ways, to tissue injury. This new paradigm, however, brings with it important new questions. By using the endothelium as an example, what the relative contribution of each of these cell populations to injury and normal endothelial turnover, for instance, is not well understood, although preliminary evidence would suggest that in certain settings, the combined use of EPCs and EOCs may augment tissue protection.44 Whether recruitment of EOCs to the site of endothelial injury is required also is not clear, although recent studies have suggested that, at least in certain settings, EOCs can mediate their beneficial effects by the release of systemically circulating factors.78 Furthermore, how disease states such as CKD might adversely affect the function of EPCs or EOCs also largely is unknown.
Better defining these cell populations will be critical to understand the specific roles that these cells play in both health and disease. In particular, further characterizing the specific EOC cell types that are responsible for the different tissue-protective properties ascribed to these cells will be instrumental in understanding their biology and harnessing their therapeutic potential. Together, these next steps hopefully will lay the foundation for the successful translation of bone marrow cell– based therapies into the clinic for the treatment of injured tissue.
Acknowledgments
Financial support: Supported in part by grants from the Canadian Institutes of Health Research; Darren Yuen is supported by a Canadian Society of Transplantation fellowship, and was the previous recipient of a KRESCENT postdoctoral fellowship; Richard Gilbert is the Canada Research Chair in Diabetes Complications and as such this work was supported in part by the Canadian Diabetes Association and the Canada Research Chair Program; and Philip Marsden is a Heart and Stroke Foundation of Canada Career Investigator, the Keenan Chair in Medical Research, and is supported by a grant from the National Institutes of Health (PPG HL076540-06A1).
Footnotes
Conflict of interest statement: none.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007;72:151–6. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 3.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–47. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 6.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–30. doi: 10.1111/j.1749-6632.2001.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and enos gene therapy in established disease. Circ Res. 2005;96:442–50. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 12.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–35. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 13.Rafii S, Oz MC, Seldomridge JA, Ferris B, Asch AS, Nachman RL, et al. Characterization of hematopoietic cells arising on the textured surface of left ventricular assist devices. Ann Thorac Surg. 1995;60:1627–32. doi: 10.1016/0003-4975(95)00807-1. [DOI] [PubMed] [Google Scholar]
- 14.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–8. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 16.Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, et al. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol. 2005;16:997–1004. doi: 10.1681/ASN.2004050367. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–57. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen DA, Connelly KA, Advani A, Liao C, Kuliszewski MA, Trogadis J, et al. Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLoS One. 2010;5:e9543. doi: 10.1371/journal.pone.0009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangidorj O, Yang SH, Jang HR, Lee JP, Cha RH, Kim SM, et al. Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am J Physiol Renal Physiol. 2010;299:F325–35. doi: 10.1152/ajprenal.00019.2010. [DOI] [PubMed] [Google Scholar]
- 20.Alexandre CS, Volpini RA, Shimizu MH, Sanches TR, Semedo P, di Jura VL, et al. Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells. 2009;27:682–92. doi: 10.1634/stemcells.2008-0496. [DOI] [PubMed] [Google Scholar]
- 21.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with amd3100, a cxcr4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–14. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through hif-1 induction of sdf-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL. Targeting hif-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 26.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. Vegf-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 29.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 30.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–52. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 31.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–5. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. Pdgfrbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 36.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 38.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, et al. Human cd34+ac133+vegfr-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 43.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–5. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 45.Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Vandenwijngaert S, et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010;55:2232–43. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 46.Medina RJ, O’Neill CL, Humphreys MW, Gardiner TA, Stitt AW. Outgrowth endothelial cells: characterization and their potential for reversing ischemic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:5906–13. doi: 10.1167/iovs.09-4951. [DOI] [PubMed] [Google Scholar]
- 47.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 48.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 49.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–67. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 50.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–52. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 51.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 52.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell sub-populations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–8. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–9. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 56.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 57.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 58.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mrna. Blood. 2007;110:2440–8. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 59.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 60.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 61.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 62.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with st-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 63.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 64.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled boost (bone marrow transfer to enhance st-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 65.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 66.Boyle AJ, Whitbourn R, Schlicht S, Krum H, Kocher A, Nandurkar H, et al. Intra-coronary high-dose cd34+ stem cells in patients with chronic ischemic heart disease: a 12-month follow-up. Int J Cardiol. 2006;109:21–7. doi: 10.1016/j.ijcard.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 67.de Groot K, Bahlmann FH, Sowa J, Koenig J, Menne J, Haller H, et al. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66:641–6. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 68.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246–52. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 69.Westerweel PE, Hoefer IE, Blankestijn PJ, de Bree P, Groeneveld D, van Oostrom O, et al. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am J Physiol Renal Physiol. 2007;292:F1132–40. doi: 10.1152/ajprenal.00163.2006. [DOI] [PubMed] [Google Scholar]
- 70.Herbrig K, Pistrosch F, Oelschlaegel U, Wichmann G, Wagner A, Foerster S, et al. Increased total number but impaired migratory activity and adhesion of endothelial progenitor cells in patients on long-term hemodialysis. Am J Kidney Dis. 2004;44:840–9. [PubMed] [Google Scholar]
- 71.de Groot K, Bahlmann FH, Bahlmann E, Menne J, Haller H, Fliser D. Kidney graft function determines endothelial progenitor cell number in renal transplant recipients. Transplantation. 2005;79:941–5. doi: 10.1097/00007890-200504270-00012. [DOI] [PubMed] [Google Scholar]
- 72.Chan CT, Li SH, Verma S. Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am J Physiol Renal Physiol. 2005;289:F679–84. doi: 10.1152/ajprenal.00127.2005. [DOI] [PubMed] [Google Scholar]
- 73.Yuen DA, Kuliszewski MA, Liao C, Rudenko D, Leong-Poi H, Chan CT. Nocturnal hemodialysis is associated with restoration of early-outgrowth endothelial progenitor-like cell function. Clin J Am Soc Nephrol. 2011;6:1345–53. doi: 10.2215/CJN.10911210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura T, Torimura T, Sakamoto M, Hashimoto O, Taniguchi E, Inoue K, et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007;133:91–107. e101. doi: 10.1053/j.gastro.2007.03.110. [DOI] [PubMed] [Google Scholar]
- 75.Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, et al. Bone marrow-derived cells express matrix met-alloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–22. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 76.Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE. Transforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat: role of the renin-angiotensin system. Kidney Int. 1997;51:1553–67. doi: 10.1038/ki.1997.214. [DOI] [PubMed] [Google Scholar]
- 77.Koleganova N, Piecha G, Ritz E, Gross ML. Calcitriol ameliorates capillary deficit and fibrosis of the heart in subtotally nephrectomized rats. Nephrol Dial Transplant. 2009;24:778–87. doi: 10.1093/ndt/gfn549. [DOI] [PubMed] [Google Scholar]
- 78.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–96. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 79.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–72. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 80.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 81.Parfrey PS, Harnett JD, Barre PE. The natural history of myocardial disease in dialysis patients. J Am Soc Nephrol. 1991;2:2–12. doi: 10.1681/ASN.V212. [DOI] [PubMed] [Google Scholar]
- 82.Okamura DM, Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol. 2009;24:2309–19. doi: 10.1007/s00467-009-1199-5. [DOI] [PubMed] [Google Scholar]
- 83.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–7. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]