Abstract
Although testosterone (T) has been characterized as universally immunosuppressive across species and sexes, recent ecoimmunology research suggests that T’s immunomodulatory effects (enhancing/suppressing) depend on the organism’s reproductive context. Very little is known about the immune effects of T in healthy females, and even less about how reproductive effort modulates the immune effects of T in humans. We investigated how the interaction between endogenous T and sexual activity predicted menstrual cycle-related changes in several measures of immunity: inflammation (indexed by interleukin-6, IL-6), adaptive immunity (indexed by immunoglobulin A, IgA), and functional immunity (indexed by bactericidal assay). Thirty-two healthy women (sexually abstinent, N=17; sexually active with one male partner, N= 15) provided saliva samples at four points in the menstrual cycle: menses, follicular, ovulation, and luteal phases. Among sexually abstinent women, T was positively associated with IL-6 across the cycle; for sexually active women, however, T was positively associated with IL-6 in the luteal phase only, and negatively associated with IL-6 at ovulation. High T predicted higher IgA among women who reported infrequent intercourse, but lower IgA among women who reported very frequent intercourse. Finally, across groups, T was positively associated with greater bacterial killing at menses, but negatively associated in the luteal phase. Overall, rather than being universally immunosuppressive, T appeared to signal immunomodulation relevant to reproduction (e.g., lowering inflammation at ovulation, potentially preventing immune interference with conception). Our findings support the hypothesis that the immunomodulatory effects of endogenous T in healthy females depend on sexual and reproductive context.
Keywords: Testosterone, female, immune, IL-6, inflammation, IgA, menstrual cycle, ovulation, sexual activity, immunosuppression
Introduction
Early models of sex differences in immunity proposed that testosterone (T) is immunosuppressive, leading to generally lower immune response in males across species (Klein, 2000; Schuurs and Verheul, 1990). Investment in T-dependent structures could be a signal of a robust immune system that could afford suppression, a phenomenon central to the immunocompetence handicap hypothesis (Folstad and Karter, 1992). Indeed, numerous studies in animal models have shown that T can suppress different aspects of immune response, measured either by level (e.g., antibody counts) or by functional outcome (e.g., parasite load); see (Roberts et al., 2004) for a critical meta-analysis. However, other work has shown that T is not universally immunosuppressive across contexts: for example, T has been shown to increase some aspects of immune defense in males living in the wild, in birds (Peters, 2000) and mammals (Ezenwa et al., 2012) including humans (Rantala et al., 2012).
That T has been found inconsistently immunosuppressive highlights the need to consider the ecological context of the effects of T on immunity. T does appear to be involved in tradeoffs between immunity and reproduction (broadly construed, potentially including fertility/fecundity, reproductive development, frequency of sexual behavior, and other variables pertaining to investing in the creation of offspring). As such, how T will influence immune response may depend on the organism’s physical state (including reproductive development), available resources, and mating system. It may be more fruitful to conceptualize T as an immune signal relevant to coordinating immune resources (Braude et al., 1999), rather than a substance that has a fixed immune effect.
Of note, most of the research on the immunomodulatory effects of T in humans have been conducted in men, with considerably less known about the role of T in women’s immunity. Women generally have greater immune reactivity and higher rates of autoimmune disorders than men (Klein, 2000). This fact is often cited as evidence of the immunosuppressive effects of T, despite the existence of many other potential sex or gender differences such as behavioral patterns leading to different rates of pathogen exposure (Fish, 2008; Klein, 2000). The ambiguous link between T and sex differences in immunity is further highlighted by the high variability in findings on the effect of T on women’s immune response.
Studies examining the effect of exogenous T added to immune cells taken from women show inconsistent effects, with some studies showing increased immune activity and/or levels of immune markers (Holdstock et al., 1982; Konecna et al., 2000; Posma et al., 2004), some showing decreased activity (Kanda et al., 1996; Sthoeger et al., 1988), and some showing no effect (Giron-Gonzalez et al., 2000). Notably, to date there has been no research on the effects of in vivo exogenous T administration on healthy women’s immune response. However, administration of androgens in clinical populations (e.g., women with autoimmune arthritis) is associated with improvement of symptoms (Van Vollenhoven et al., 1998). These symptom changes are sometimes accompanied by decreases in markers of inflammation and other immune responses (Booji et al., 1996; Petri et al., 2004), but not always (Huang et al., 2014).
Studies of the immune effects of endogenous T are no more consistent: some show a negative association between women’s endogenous T and markers of adaptive immunity such as antibody production (Furman et al., 2014), while others show a positive association (Ding et al., 2007) or no significant association (van Anders, 2010). Studies in healthy premenopausal women have generally found that no association between endogenous T and markers of inflammation (Benson et al., 2008; Guzelmeric et al., 2007; Kelly et al., 2001; Tarkun et al., 2004); in postmenopausal women, some studies have found a positive association (Maggio et al., 2011; Maturana et al., 2008) and others, a negative association (Joffe et al., 2006). Even when controlling for group-wise differences in age, high endogenous T is associated with slower wound healing in premenopausal women, but faster wound healing in postmenopausal women (Engeland et al., 2009). Part of this confusion may arise from lack of consideration of hormonal medications that influence women’s fertility (such as hormonal contraceptives (HC) or hormone replacement therapies), which are not always reported – let alone included in models of T’s immune effects. In short, T has a complex immunomodulatory role in females: the direction of the effect of T on women’s immune response is dependent on the source of T (endogenous or exogenous), the aspect of immunity measured, and the fertility of the woman.
It has been argued that inconsistent findings in studies of immune markers in premenopausal women may be due to natural variation in sex steroid hormones – that is, due to the menstrual cycle (Schisterman et al., 2014). Menstrual cycle-related variations in immunity may support fertility by downregulating immune responses that may interfere with conception (e.g., inflammation, mucosal antibodies) near ovulation (Lorenz et al., 2015a; Lorenz et al., under review; Lorenz et al., 2015d), and upregulating immune responses that promote implantation (e.g., T-helper 2 cell activity) in the luteal phase (Lorenz et al., 2015c). As T appears to be relevant to tradeoffs between fertility and immunity, among premenopausal women the effect of T may vary across cycle phases (e.g., from the follicular to luteal phase). In one study, exogenous T was added to blood cells taken from women several times across the menstrual cycle. T increased production of IL-6 (an inflammation marker) during the follicular phase, but decreased IL-6 production during the luteal phase (Konecna et al., 2000). Other studies, however, have not found phase-specific immunomodulatory effects. Exogenous T added to blood cells taken from women at several cycle phases (e.g., follicular and luteal phases) yielded similar effects on B-cell suppression (Sthoeger et al., 1988) and T-cell differentiation (Giron-Gonzalez et al., 2000). Similarly, high endogenous T appears to delay wound healing across both follicular and luteal phases (Engeland et al., 2009). Such cycle-specific effects could be mediated through variations in ovarian hormones, either acutely (e.g., the acute surge in P prior to ovulation) or through continued exposure (e.g., the steady rise in P during the 10 – 18 days of the luteal phase). They may also relate to discrete events in the ovarian cycle such as ovulation. While different women may require different absolute hormone levels to trigger ovulation, once the mature follicle is present, it appears to have consistent immune parameters such as inflammation (Lorenz et al., 2015d).
There are clearly critical factors missing from the research on the effect of T on women’s immune function. One such factor may be sexual activity (van Anders, 2014): the effect of T on women’s immune function would likely depend on her capacity for conception, which is a function of phase of the menstrual cycle (Aronoff et al., 2014) and degree of sexual activity (Fortenberry et al., 2014). Among young women not currently caring for young children, sexual activity level may correspond to degree of reproductive effort: arguably, sexually active and abstinent women are not under the same constraints in balancing investment in immunity vs. reproduction. As few studies have measured – let alone accounted for – differences in women’s sexual activity levels, we should expect inconsistencies in findings regarding T’s immunomodulatory effects.
We examined changes in T as a predictor of immune response across the menstrual cycle in healthy women who were or were not sexually active. To examine the effects of T across different measures of immune response, we collected a marker of inflammation (IL-6), a marker of adaptive immunity (salivary Ig A, or SIgA) and an index of immune function in response to an ex vivo challenge (percent bacterial killing). IL-6 is a cytokine, or immune signaling molecule, that is commonly used by immune actors such as macrophages and T cells to stimulate or target inflammation processes. In the central nervous system, IL-6 also acts as a neurotransmitter that triggers the suite of behaviors and subjective symptoms associated with illness such as lethargy/fatigue, loss of appetite, and social withdrawal. In men, T is inversely associated with IL-6 production (Furman et al., 2014; Li et al., 1992; Maggio et al., 2006); however, as noted above, the effect of T on women’s IL-6 appears to depend on the menstrual cycle phase (Konecna et al., 2000).
IgA is an antibody predominantly expressed in mucosal sites such as the mouth, gastrointestinal tract, and vagina. As the mucosal linings are frequently shed and replenished, IgA must be produced continuously (Reinholdt and Husby, 2013). Thus, the changes in immune system resource allocation to antibody production – that is, ongoing immunomodulation – can be readily observed in changes in IgA. One study of healthy participants found no association between endogenous T and SIgA in either men or women (van Anders, 2010); however, this study did not report the menstrual phase of the female participants. IgA has been found to be significantly different across cycle phases (Gomez et al., 1993; Lorenz et al., 2015a), although other studies have found no significant cycle-related variation (Brown et al., 2008; Garde et al., 2000; Gillum et al., 2014).
Finally, bacterial killing is a functional measure of how well the immune system can respond to a realistic challenge (Demas et al., 2011). To date, only one study has examined the effects of T on bacterial killing in healthy humans. This study found a positive association between endogenous T and degree of killing in both men and women; however, women’s cycle phase was not reported (Prall et al., 2011). In sum, while there is reason to believe that T may influence these three indices of immunity in women, the direction of the effect and possible interaction with either cycle phase or sexual activity status was unknown.
We predicted that in our sample of healthy women, high T would be immunosuppressive in contexts in which selection pressures corresponding to reproductive-immune tradeoffs are salient, but neutral (or even immunoenhancing) in contexts where such selection pressures are low. Specifically, we predicted that at mid-cycle, T would be immunosuppressive, temporarily down-regulating immune responses that could interfere with conception (e.g., inflammation). At other points of the cycle, however, T would not be immunosuppressive, and could potentially be immunoenhancing. Importantly, we predicted the immunosuppressive effects of T would be strongest in women who sexually active, as selection pressures would be greater for women who could potentially conceive that cycle than for those who could not. Specifically, we predicted that frequency of sexual activity would amplify T’s immunomodulatory effects.
Materials and Methods
The present analysis was drawn from a larger study on the effects of sexual activity on women’s health; other papers from this study include (Lorenz et al., 2015a; Lorenz et al., 2015b).
Participants
All participants were healthy, premenopausal women who reported regular menstrual cycles (cycle every 26 – 34 days, with no more than one missing period in the last six months). The sexually active group included women who reported regular (≥ 1x/week) penile-vaginal intercourse with one and only one male partner. The sexually abstinent group included women who reported no partnered genital sexual contact in the previous four months; however, women who reported lifetime history of sexual contact (or current masturbation) could be included in this group. We excluded women taking hormonal medications such as HCs; as such, all sexually active participants had to report either a non-hormonal IUD or condoms as their primary contraceptive method. Other exclusion criteria were: use of any medication on a regular basis (infrequent use of over-the-counter pain or allergy medications was allowed), drinking >15 units of alcohol per week, medical conditions known to impact immune response (e.g., cancer), pregnancy or lactation in the past year, or diagnosis of sexually transmitted infection within the past year (even if currently asymptomatic).
Women were recruited from the community via flyers and online advertisements; all participants were screened over the phone to ensure fit with study criteria. Thirty-five healthy women enrolled in the study; of these, three dropped out, creating a total N=32. All participants provided informed consent, and all procedures were approved by the Indiana University Institutional Review Board.
A total of 17 sexually abstinent and 15 sexually active women participated. The majority of participants were Caucasian (n = 23, 72% of sample) with 5 women reporting Asian race and 6 women reporting mixed race or other. The average age was 23.32 (SD = 5.62). On average, sexually active women were older (Mage = 24.65) than sexually abstinent women (Mage = 22.16); this difference was not significant (F(1, 31) = 1.55, p = 0.22). Average body fat percentage was 27.31% (SD = 7.77). Among the abstinent women, 82% were single and 18% in a dating relationship (without sexual contact), while among the sexually active women, 40% were married or cohabiting and 60% were in a dating relationship. The average length of relationship was 3.97 years (SD = 6.77).
Salivary T was higher in the sexually abstinent group (M = 74.25 pg/mL, SD = 47.14) than the sexually active group (M = 64.00 pg/mL, SD = 36.07); however, this difference was not statistically significant (F(1, 118) = 1.75, p = 0.19). In the present sample, there was no significant association with salivary T and relationship status (F(1, 41.58) = 1.54, p = 0.201), even accounting for potential differences over time (F(11, 70.64) = 0.95, p = 0.504). Means and standard deviations of all measures are reported in Supplementary Appendix A.
Laboratory session procedures
Participants attended laboratory sessions during menses (within the first two days of onset of bleeding) and at ovulation (within two days of ovulation). Timing of ovulation was confirmed with a commercially available urine test for luteinizing hormone (LH), a marker of ovulation (OneStep Urine Ovulation Test, BlueCross Biomedical, Beijing, China). Participants received a packet of five ovulation test strips at their first laboratory visit, and were instructed to complete tests in the days prior to a likely ovulation date estimated via backwards counting (van Anders et al., 2014). All participants had a positive LH test strip within 48 hours of their second laboratory session. Additionally, all participants (including the sexually abstinent women) completed commercially available urine tests for human chorionic gonadotropin, a marker of pregnancy, at both visits. No participant was found pregnant during the study. All sessions were scheduled in the afternoon to minimize the effects of circadian variations on immune and endocrine measures; 83% of in-lab samples were collected between 1:30 – 6:30pm.
In the lab, participants were measured for height and body fat with a Tanita floor scale (FitScale 585F, Tanita Corporation, Illinois USA). They provided unstimulated saliva samples into polypropylene tubes, which were frozen immediately after collection. Saliva samples were timed as flow rate significantly influences measurement of IgA (Miletic et al., 1996); IgA concentrations were divided by flow rate to create a measure of µg/min. Participants also provided demographic information as well as self-reported illness and stress; no participant reported significant illness or traumatic stress during the study.
At-home procedures
In addition to the two saliva samples collected during laboratory sessions, participants self-collected saliva samples during the follicular phase (7–10 days following the onset of menses) and during the luteal phase (7–10 days following ovulation). Participants were asked to time their samples, and to complete samples in the afternoon; 69% of at-home samples were taken between 1:30 – 6:30pm. Immediately upon completion, participants put the saliva samples in their home freezer. Samples were transported to the lab in Styrofoam containers lined with deep-freeze packs. Accordingly, each participant provided four samples (and thus, four measures of T, P4, IL-6, IgA and bacterial killing) timed to points of the menstrual cycle: menses, follicular phase, ovulation, and luteal phase.
Sexually active participants were also asked to complete an online diary measure each time they engaged in partnered sexual activity. We coded number of intercourse events from these diary measures as “sexual frequency”. The range of intercourse events reported by sexually active women was 1 – 18 (average = 6.67, SD = 4.81 events). Abstinent participants were coded as reporting zero intercourse events in the sexual frequency measure; accordingly, all analyses reflect sexual activity level as both state (sexually active vs. abstinent) and trait (frequency of sexual activity). We did not assess if abstinent participants had partnered sexual activity during the study; however, no participant reported a significant change in relationship status (e.g., from “single, not having sex with anyone” to “single, having sex”).
Assay procedures
Saliva samples were stored at −80C until analysis, and no sample was subjected to more than 2 freeze-thaw cycles. We measured T, progesterone (P4), estradiol (E2), SIgA and unstimulated IL-6 with commercially available enzyme-linked immunosorbent assay (ELISA) kits, using procedures recommended by kit manufacturers (Salimetrics LLC, Pennsylvania, USA). Assay coefficients of variance (CVs) were as follows (intra-/inter-): SIgA: 5.09%/2.14%; IL-6: 14.27%/15.71%; T: 6.73%/10.55%; P 3.06%/13.47%; (E2 5.25%/12.04%). Functional immune response was measured with an ex-vivo bacterial killing assay (Demas and Carlton, 2015; Demas et al., 2011), adapted for saliva (Muehlenbein et al., 2011). In the bacterial killing assay, a common pathogen (in this case, Escherichia coli ATCC #8739) was incubated with the saliva sample on an agar plate. The number of colonies formed on the sample plates were compared to plates with unimpeded growth; the degree of immune function is thus termed “percent killing”. High percent killing indicates the immune responses of the saliva were successful in impeding bacterial growth (and thus, higher immune function, reflecting lower chance of infection) while low percent killing indicates bacterial growth was unchecked (and thus, lower immune function).
Analytic plan
Missing data
A total of 13 saliva samples were not turned in or could not be used due to pragmatic reasons (e.g., at-home sample was returned to the lab unfrozen), and thus 10% of data were missing. The loss of data from these samples was unrelated to the associations of interest and thus can be considered missing at random (Heitjan and Basu, 1996). We confirmed this assumption statistically with Dixon’s test (Little, 1988): there were no significant differences between women with and without missing samples in any of the variables of interest (T, IL-6, SIgA, percent killing) at non-missing timepoints. Missing values were thus addressed using statistical techniques robust to missing data (see below).
Covariates and alternative models
In all tests below, we controlled for age and body fat percentage, as these have strong associations to variability in immune factors (Gardner and Murasko, 2002; Nguyen et al., 2004; Pacifico et al., 2006) and, in the present sample, there were (non-significant) group-wise differences in both age and body composition. Additionally, sexually active and abstinent women differ in patterns of E2 and P4 across the menstrual cycle (Prasad et al., 2014). Although we confirmed all women in the present sample had an LH surge (a marker of ovulation), the sexually active women in this sample did have significantly higher luteal-phase P4 than did the sexually abstinent women (see (Lorenz et al., 2015b) for more details). T can be converted into E2, and thus changes in T may be indirectly an index of changes in E2. As such, we tested all of the models described below with E2 and P4 as covariates.
Previous studies have found lower T in partnered women relative to unpartnered/ abstinent women (van Anders and Goldey, 2010; van Anders and Watson, 2007). Even though we did not find a significant effect of partnership type on salivary T, it is reasonable to suspect there may be relevant differences between partnered and unpartnered women that would contribute to both T and immune response. Unfortunately, in the present design, all of the sexually active women were partnered and thus it would not be statistically sound to include both partnership and sexual activity status in the same model. Thus, we conducted a separate set of analyses examining relationship status (without sexual activity); these analyses are presented in Supplementary Appendix B.
IL-6
The rate of non-detection of IL-6 was relatively high (N = 36, ∼26% of values), as can be expected for unstimulated cytokine levels in a population of young, healthy women (Riis et al., 2014). The distribution was heavily right-skewed (beyond the point at which transformation was feasible) and the loss of data from these samples was potentially related to the outcome (i.e., not missing at random). To counter these issues, we used the quantile regression approach recommended by Eilers et al. (2012) for the treatment of immunologic data with many non-detects. We first characterized IL-6 values according to quantiles (below limit of detection: IL-6 < 0.78 pg/mL; low: 0.78 pg/mL – 1.80 pg/mL; moderate: 1.80 pg/mL – 4.95 pg/mL; high: IL-6 > 4.95). We then conducted a repeated measures generalized linear mixed model with IL-6 quantile group (non-detect, lowest detected, moderate, high) as an ordinal outcome variable with a multinomial logit distribution. We included time (menses, follicular, ovulation, luteal) as a repeated measures variable, as well as sexual frequency, T and their interactions, and covariates (progesterone, age, and body fat percentage) as fixed effects, as well as a subject-level random intercept to account for individual differences at baseline.
SIgA and percent killing
As the distribution of SIgA was right-skewed, we used a square root Box-Cox transformation and back-transformed results for presentation. Two of the percent killing values were >3 SD from the mean; following removal of these outliers, distribution of percent killing was normal without further transformation (Kolmogorov-Smirnov test, p = 0.20). We examined changes in SIgA and percent killing across the menstrual cycle with repeated measures mixed general linear models, controlling for progesterone, age and body fat percentage. As in the model of IL-6, these models included sexual frequency, time as a repeated measure, T, and their interactions as fixed effects, and specified subject-level random intercepts.
All analyses were performed with IBM SPSS Statistics version 22.0; we set the significance criterion of α =0.05, with marginal significance considered up to p < 0.06. In keeping with guidelines for interpretation of non-additive relationships, we did not interpret lower-order (constituent) interaction effects when a higher-order interaction was significant, (Braumoeller, 2004).
Results
Changes in IL-6 across the menstrual cycle
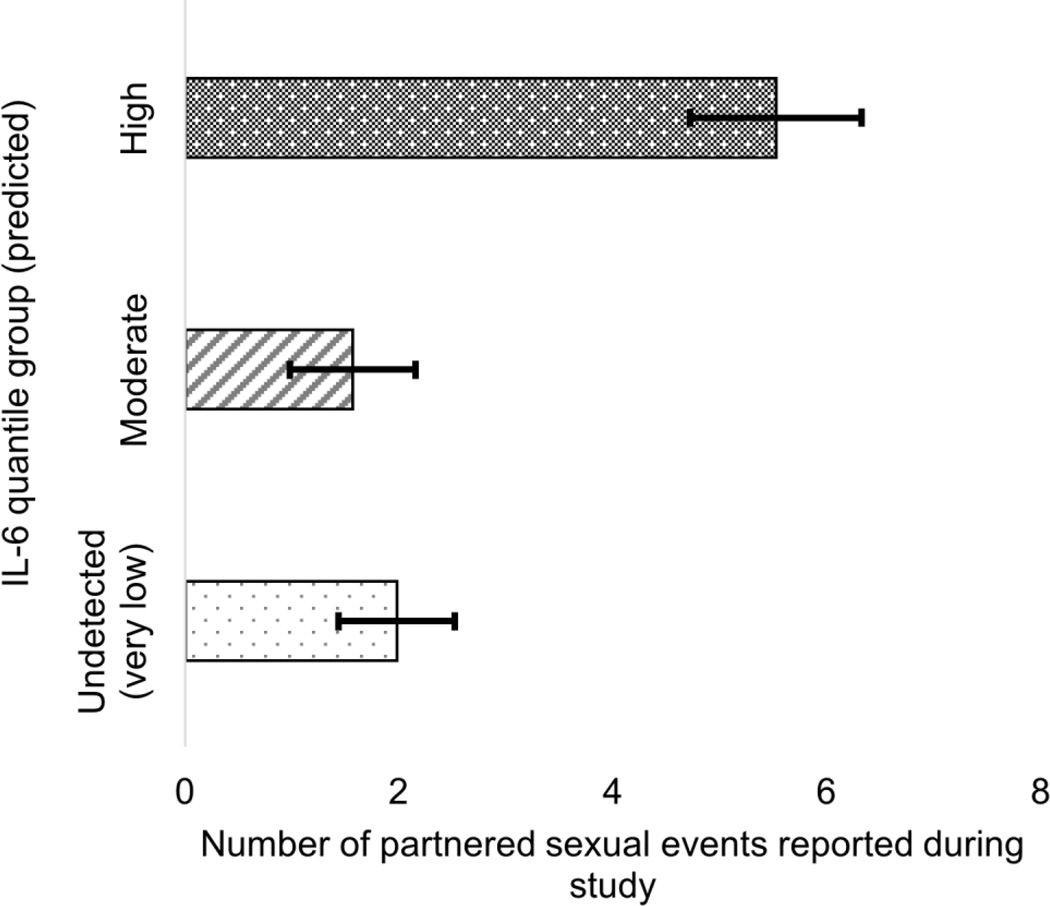
There was a marginally significant effect of sexual frequency on IL-6 quantile group (Wald χ2 = 3.79, p = 0.051, Cramer’s V = 0.181), such that women in the highest quantile of IL-6 had higher rates of sexual intercourse than women in the lower quantiles (Fig 1).
Figure 1.
Association between sexual frequency and predicted IL-6 quantile group, collapsed across time. Women who reported sexual activity more than 1x/week were more likely to be in the highest IL-6 group.
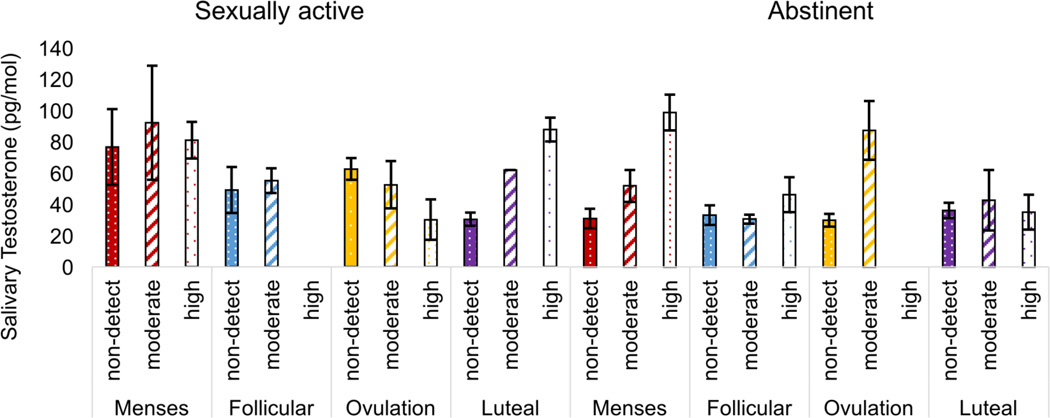
The interaction between time and sexual frequency was significant (Wald χ2 = 12.09, p = 0.007, Cramer’s V = 0.186), as was the interaction between T and sexual frequency (Wald χ2 = 7.87, p = 0.008, Cramer’s V = 0.245). There was also a significant interaction between time, T, and sexual frequency (Wald χ2 = 12.23, p = 0.007, Cramer’s V = 0.187). For sexually abstinent women, higher levels of T were associated with higher IL-6 (more specifically, higher likelihood of being in the moderate or high quantile of IL-6). This was particularly true at the menses and ovulation time points (Figure 2). For sexually active women, there was no association between T and IL-6 quantile at menses and follicular time points. At ovulation, higher T was associated with lower IL-6 (that is, lower likelihood of being in the moderate or high quantiles). And finally, in the luteal phase of sexually active women, higher T was associated with higher IL-6 (higher likelihood of being in the highest quantile).
Figure 2.
Interaction between menstrual cycle phase, testosterone, and sexual frequency on predicted IL-6 group. Among sexually abstinent women, higher testosterone was associated with higher likelihood of being in the high IL-6 group, particularly at menses and ovulation. Among sexually active women, higher testosterone was associated with significantly lower likelihood of being in the high IL-6 group at ovulation, but higher likelihood during the luteal phase.
Changes in SIgA across the menstrual cycle
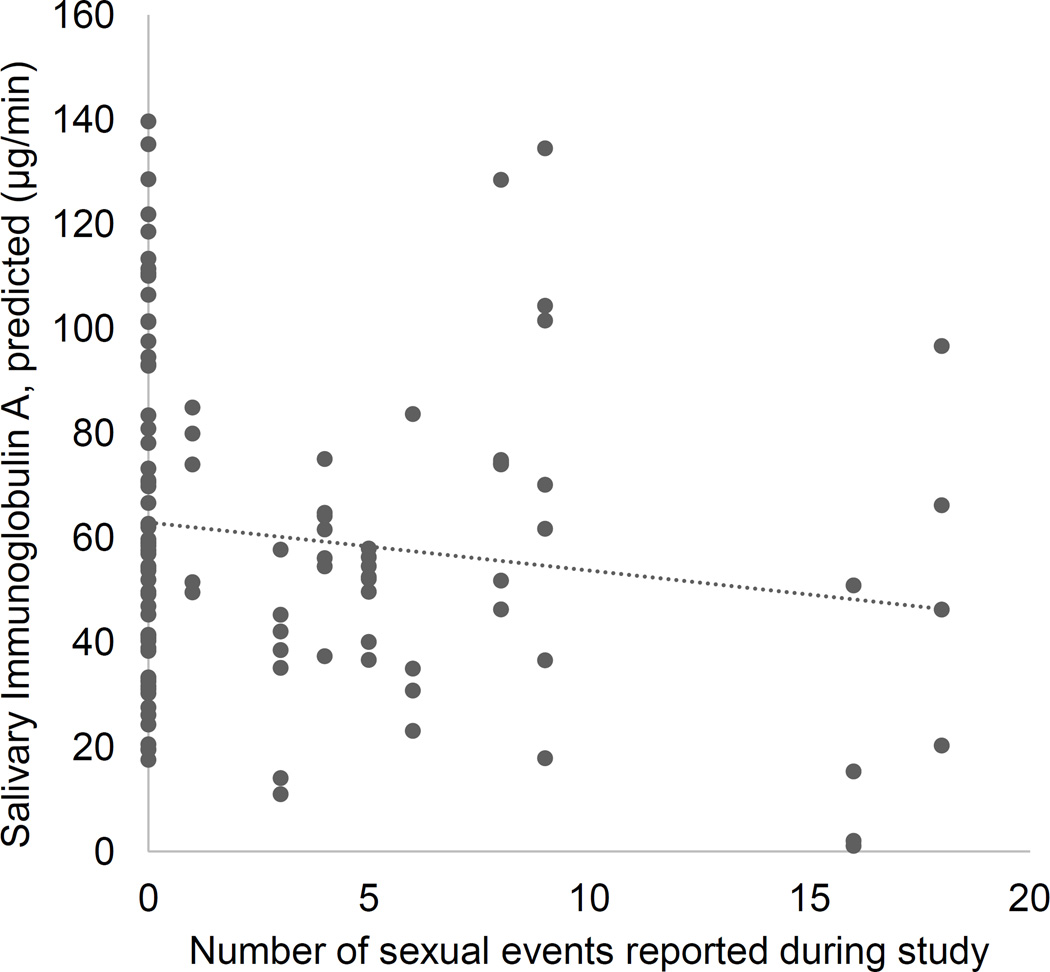
There was a significant positive association between T and SIgA (F(1, 111.72) = 5.92, p = 0.017, Cohen’s local f2= 0.057). There was also a significant negative association between SIgA and sexual frequency (F(1, 73.41) = 4.75, p = 0.033, Cohen’s local f2= 0.051, Figure 3). The main effect of time on SIgA was also significant, F(1, 88.23) = 3.79, p = 0.013, Cohen’s local f2= 0.056. The effect of time was quadratic (specific contrast estimate t(86.05) = −3.34, p = 0.001), with significantly higher SIgA at mid-cycle (follicular phase and ovulation) than at early or late cycle.
Figure 3.
Association between sexual frequency and salivary immunoglobulin A, collapsed across time.
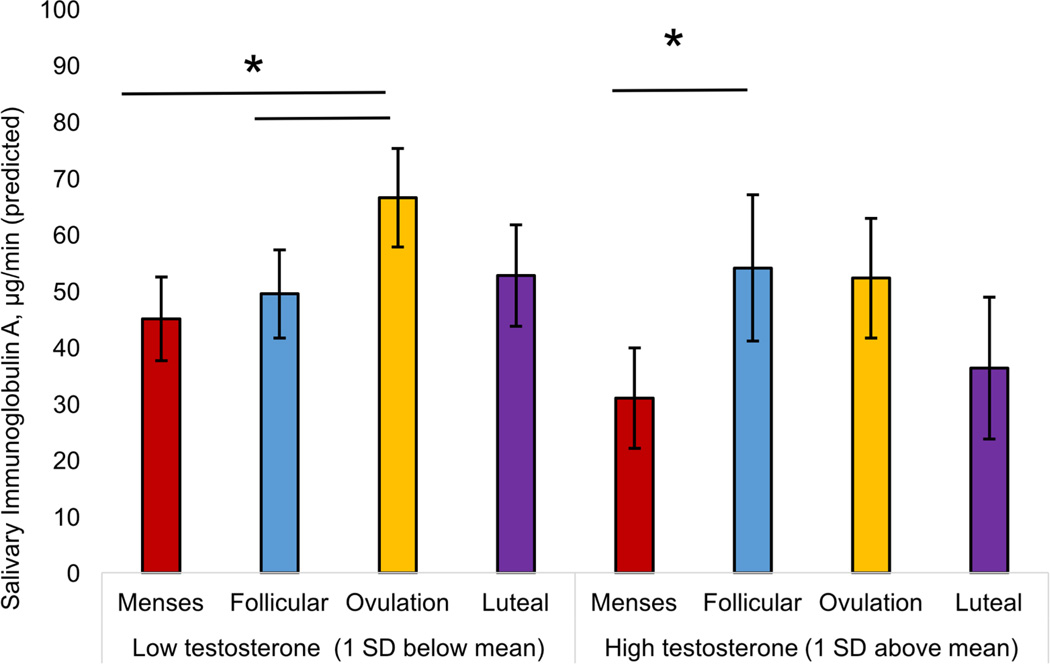
The interaction between time and T was marginally significant (F(3, 91.73) = 2.21, p = 0.092, Cohen’s local f2= 0.050). The mid-cycle increase in SIgA described above was significant at low levels of T (1 SD below the mean). At high levels of T (1 SD above the mean), the direction of effects was similar but non-significant.
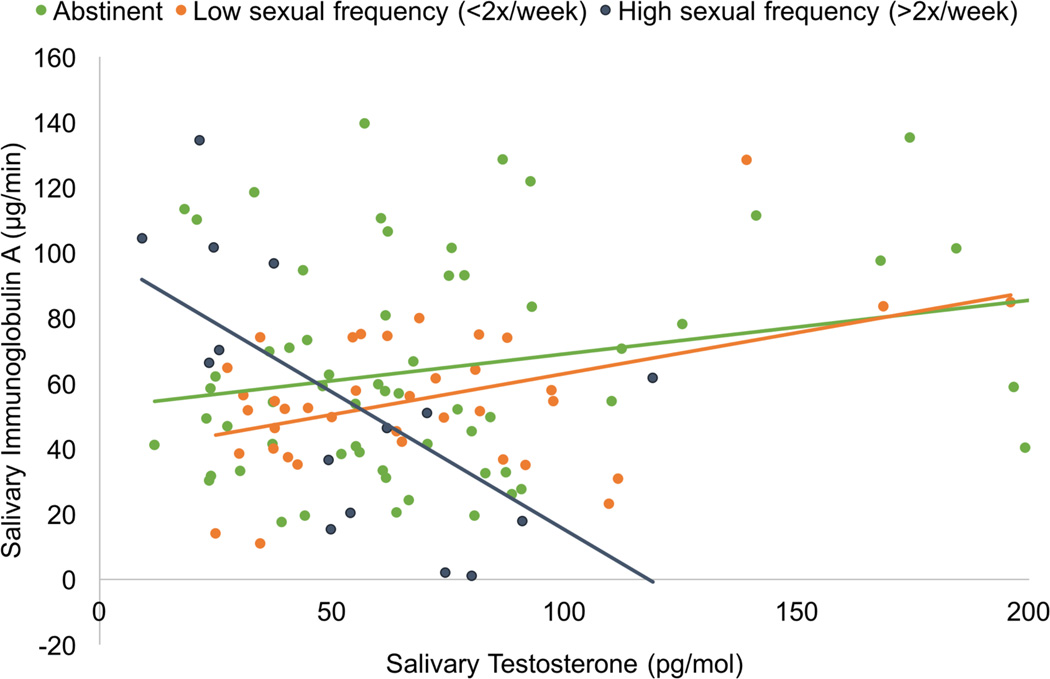
Finally, the interaction between T and sexual frequency was significant, F(1, 99.02) = 7.19, p = 0.009, Cohen’s local f2= 0.113 (Figure 5). For women who engaged in no sexual activity (abstinent), there was a marginally significant positive association between T and SIgA (r(61) = 0.22, p = 0.091). Among women who engaged in moderate levels of sexual activity (< 2x/week), there was a significant positive association between T and SIgA (r(38) = 0.44, p = 0.006). However, for the women who engaged in high levels of sexual activity (>2x/week), there was a significant negative association (r(15) = −0.63, p = 0.012).
Figure 5.
Interaction between sexual activity and testosterone in predicting salivary immunoglobulin A levels. There was a positive association between testosterone and immunoglobulin A levels in sexually abstinent women (green line) and moderately sexually active women (orange line), but a negative association in highly sexually active women (blue-grey line).
Changes in percent bacterial killing across the menstrual cycle
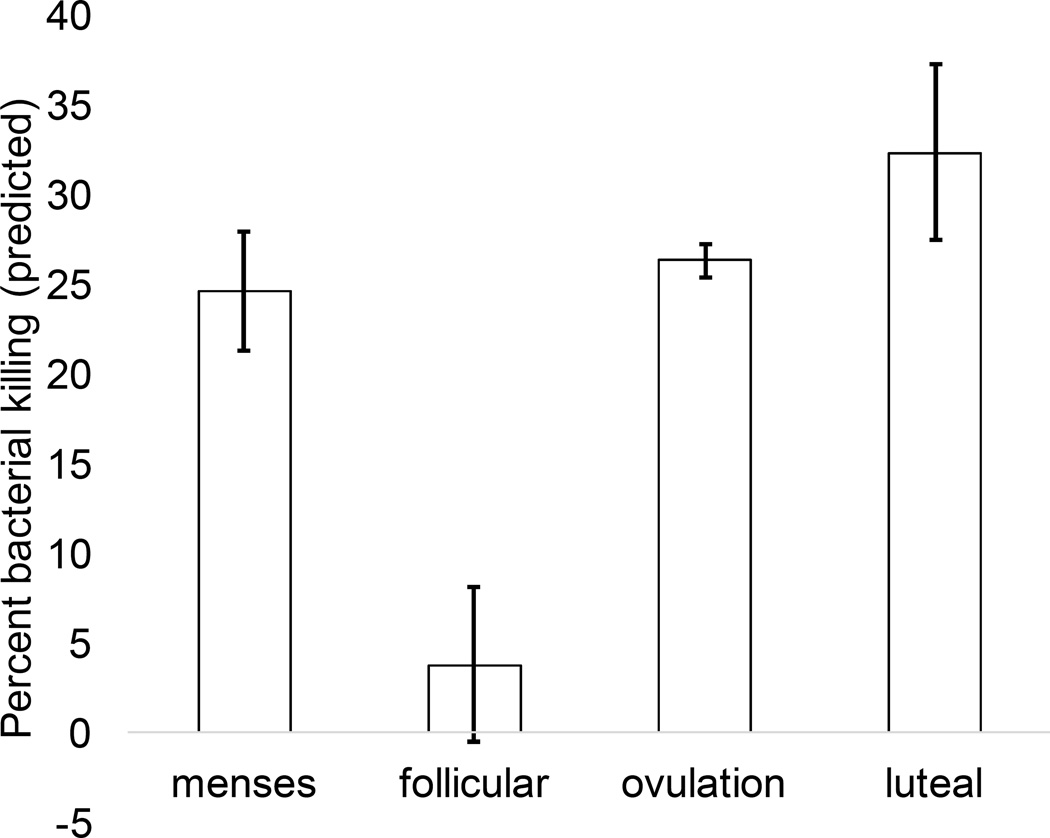
There was a significant main effect of time on percent bacterial killing (F(3, 48.97) = 4.78, p = 0.005, Cohen’s local f2= 0.310). As with SIgA, the effect of time was quadratic (specific contrast estimate t(80.26) = 2.10, p = 0.039); however, in this case, the non-linearity of the effect was driven primarily by very low percent killing in the follicular phase, relative to all other phases (see Figure 6).
Figure 6.
Effect of menstrual cycle phase on functional immunity (percent bacterial killing), collapsed across sexual frequency groups. There was a significant main effect of time, with functional immunity significantly lower during the late follicular phase relative to all other phases.
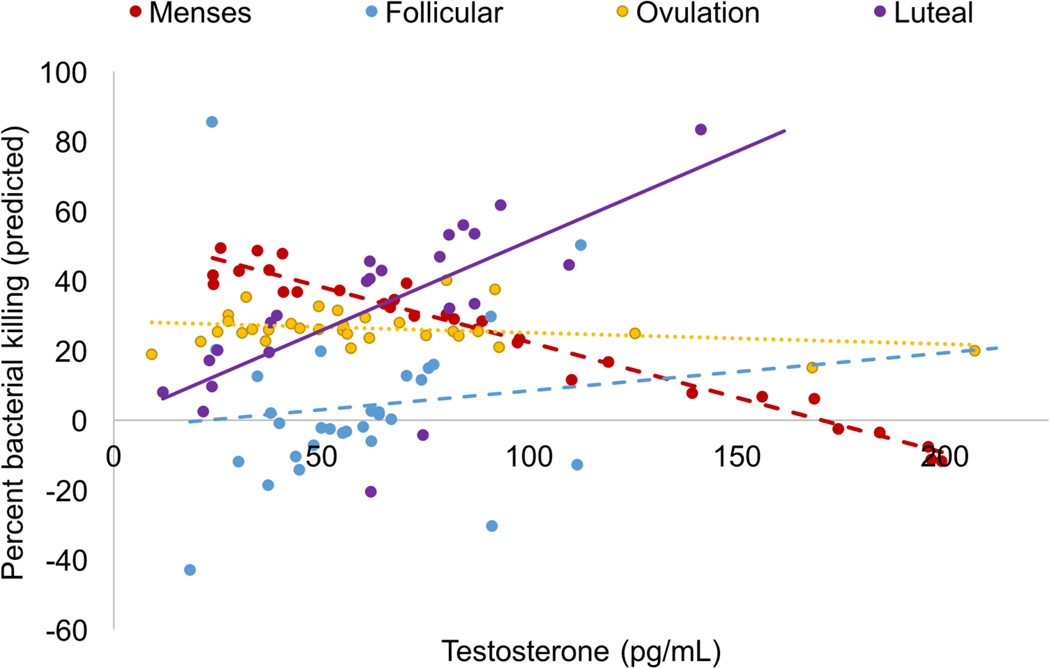
The interaction between time and T was significant (F(3, 56.74) = 4.33, p = 0.008, Cohen’s local f2= 0.115, Figure 7). At menses, there was a significant negative association between T and percent bacterial killing. At follicular and ovulation phases, there was a nonsignificant association between T and percent bacterial killing. Finally, during the luteal phase, there was a significant positive association between T and percent killing.
Figure 7.
Interaction between testosterone and menstrual cycle phase in predicting functional immunity (percent bacterial killing). There was a significant negative association between testosterone and bacterial killing at menses (dashed red line), no association at follicular phase (dashed blue) or ovulation (dotted gold line), and a positive association at luteal phase (solid purple line).
Discussion
Testosterone has been traditionally considered as universally immunosuppressive (Schuurs and Verheul, 1990). However, in the present study, the immunomodulatory effects of testosterone were dependent on temporal and behavioral context. While higher testosterone was associated with significantly lower levels of IgA (a mucosal antibody) in highly sexually active women, there was no effect in sexually abstinent women. In general, higher testosterone was predictive of higher levels of the pro-inflammatory cytokine IL-6; however, among sexually active women at ovulation, higher testosterone was associated with lower levels of IL-6. And finally, while higher testosterone was associated with lower functional immunity (as measured by ex vivo bacterial killing) at menses, the opposite was true during the luteal phase. In sum, while the effect of testosterone was sometimes immunosuppressive in these healthy premenopausal women, there were important exceptions to this trend, exceptions that appear to be relevant to fertility. These findings support the hypothesis that testosterone is a signal of reproductive conditions that may require immunomodulation (either suppression or enhancement), rather than a universal immunosuppressant.
Replicating the findings of several other studies (Brown et al., 2008; Charnetski and Brennan, 2004; Lorenz and van Anders, 2014), we found that higher levels of sexual activity was associated with lower SIgA in healthy women. Novel to this study, we found that T was associated with lower IgA among highly sexually active women. Sexual activity is associated with acute, transitory elevations in T in women (Morris et al., 1987; Tuiten et al., 2000) although the direction of causality is unclear (Goldey and van Anders, 2011; van Anders, 2012). T may be either a mediator of the association between sexual activity and IgA, or the two variables may be co-related to a third factor (e.g., sexual desire, frequency of non-sexual intimate touch (van Anders et al., 2007). In the present sample, T levels were not significantly higher in women who were frequently active: in fact, it was lower. Other studies have similarly found lower T in women who are highly vs. infrequently sexually active (Prasad et al., 2014). Taken together, these findings suggest that the effect of T on the immune system is as a relative signal: when baseline T is low (e.g., as in highly sexually active women), the T response to sexual activity would be relatively high, triggering an immunosuppressive response. When baseline T is higher (e.g., as in less frequently sexually active women), the T response to sexual activity would be relatively lower, and the corresponding immunosuppressive signal would be attenuated. Indeed, in the present study, there was no significant association between T and IgA among sexually abstinent women. This may also explain why sexual frequency in men is not consistently associated with changes in IgA (Lorenz and van Anders, 2014), as the increase in T associated with sexual activity would likely not be sufficient to trigger suppression of mucosal immunity.
Among sexually active women, higher T was generally associated with higher levels of IL-6, a marker of inflammation. Of note, however, at ovulation this association was reversed: higher T was associated with lower levels of IL-6. Systemic inflammation may interfere with conception by creating an inhospitable environment for sperm and follicle (Suarez and Pacey, 2006) or interfering with local inflammation-dependent mechanisms underlying implantation (Mor et al., 2011). As noted above, sexual activity is associated with elevations in T and there is some evidence that sexual activity near ovulation is associated with greater elevations in T than at other phases (Caruso et al., 2014). Here the female body faces a dilemma: sexual activity must occur around ovulation for conception to take place, and sexual activity may elevate T. If T is interpreted as a pro-inflammatory signal in the time close to ovulation, it could interfere with conception. If the female body interpreted T as an anti-inflammatory signal around ovulation, it would circumvent this dilemma. While speculative, the present data are consistent with this idea.
More surprising, though, was the finding that T was associated with higher IL-6 at non-ovulatory time points in sexually active women, and at all time points in sexually abstinent women. T may stimulate IL-6 production via interactions with the hypothalamic-pituitary-adrenal (HPA) axis. Except in cases of extreme or chronic stress, cortisol and other hormones of the HPA axis suppress inflammation, including production of IL-6 (Franchimont, 2004), while IL-6 stimulates cortisol production (Steensberg et al., 2003). T decreases HPA responsiveness to IL-6, permitting higher levels of IL-6 before cortisol is triggered to suppress inflammation (Papadopoulos and Wardlaw, 2000); in males, this is counteracted by mutual inhibition between T and activity of the HPA axis (Handa et al., 1994). Relative to men, women’s HPA sensitivity to IL-6 is even lower (Rohleder et al., 2001), and the combined inhibition of the HPA and T significantly weaker (Liening et al., 2010; McCormick et al., 2002). Thus, in women, T may reduce HPA suppression of IL-6 (and thus be associated with higher IL-6), but unlike in men, this mechanism would not be as restrained by negative feedback. This possibility does not exclude other potential mechanisms such as interactions with the sympathetic nervous system (Fernandez-Real et al., 2001; Hermans et al., 2007), or other endocrine inputs such as oxytocin. It also is possible that some behavioral factor, such as physical activity, co-varies with both T and IL-6 (Cumming et al., 1986; Haahr et al., 1991).
Finally, T at menses was associated with significantly higher bacterial killing (our index of functional immune response) while luteal T was associated with significantly lower bacterial killing. These findings underscore the importance of functional indices of immunity as a counterpoint to enumerative measures, as they are not entirely parallel with changes in IgA or IL-6 as outlined above. Functional immune assays take into account both the direct actions and interactions of all immune cells as well as endocrine factors present in the sample. Given this, a possible mechanism for these effects is an interaction between T and P and/or E2 (which are low at menses, but high in the luteal phase); although we controlled for the main effects of P and E2, this would not account for dynamic interactions between hormones. Speculatively, it is possible that these luteal-phase effects are a byproduct of an association that would be adaptive during pregnancy, when P and E2 are high. High levels of P and/or E2 may decrease immune cell receptivity for T (McMurray et al., 2001), reducing T’s immunosuppressive effect. This would improve the survival of mothers of male offspring, as they would be protected from an immunosuppressive effect of fetal-derived T. Of course, even this speculative hypothesis does not explain why T would have an immunoenhancing effect: clearly, much more work is needed.
Limitations
The findings from the present study are preliminary, and some considerations must be made in future research. We investigated a limited number of immune factors in a small sample of healthy, predominantly White, premenopausal women. Replication and extension to other aspects of immunity, as well as to other ethnic and age groups, is needed to confirm our results. Of particular importance is replication in markers of vaginal immunity, which may differ from that of saliva. Both aspects of immune response are relevant to women’s health, but vaginal responses are likely more directly critical for fertility and sexually transmitted disease risk. While many of the factors that drive changes in immune response apply equally across mucosal membranes, there may be important effects in the local vaginal immune responses that are either not present or differentially active in saliva. For example, one study showed significant effect of intercourse on the vagina’s production of antimicrobial peptides (AMPs) (Aronoff et al., 2014; Fortenberry et al., 2014); however, there is often a low correlation between vaginal and salivary measures of AMPs (Nittayananta et al., 2016). The vagina and mouth also differ significantly in their microbiome (Peterson et al., 2009), which may contribute to immune responses to sexual activity.
It is also worth noting that in the present study, all of the sexually active women but only a few sexually abstinent women were in relationships, making it difficult to tease apart the effects of sexual activity and partnership (however, see Supplementary Appendix B for analyses examining the effect of partnership on associations between T and immune markers). It is also worth noting that we coded all sexually abstinent women as having a sexual frequency of zero, and thus our findings reflect two non-exclusive levels of analysis: zero vs. non-zero sexual frequency (that is, a trait-like variable) and the continuous count (that is, a state-like variable). We did observe some differential effects at low vs. high frequency of sexual activity; however, it is difficult to know if these differences were due to passing a threshold (reflecting changes at the state level) or a non-linear but continuous effect (reflecting changes at the trait level). Further work examining a wider range of sexual frequencies, as well as transitions from abstinent to sexually active state, may clarify this distinction.
The immune effects of sexual activity with more than one partner, or with non-male partners, cannot be determined based on this study. Similarly, sexual activity comes in many forms. In humans, much (perhaps most) sexual activity is non-procreative in intent or form (Meston and Buss, 2007) yet may still serve an important role in reproductive fitness (e.g., by strengthening pair bonds). It is critical, then, that future research address the possibility that non-intercourse sexual activity may similarly impact women’s endocrine and immune function. Given that we do not yet know the mechanisms by which sexuality, T and immune function interact in healthy women, it is reasonable to examine if these effects replicate across a broad range of sexual activities.
None of the women in the present study were taking HCs; as about 17% of reproductive-aged US women use HCs (Jones et al., 2012), our findings are limited in generalizability. Given the impact of endocrine responses in the effect of sexual activity on immune response, there will likely be differences in these effects among women who do and do not use HCs. Moreover, exclusion of women using HCs means that all of the sexually active women in this study reported using condoms or non-hormonal IUDs. While much work has documented how condom use reduces the risk of sexually-transmitted infections, is unknown what effect condoms (or IUDs) may have on mucosal immunity in healthy (pre-infection) women. Finally, T can be converted into estrogens in both men and women (Longcope et al., 1969), and thus it is possible that the T we observed in saliva does not correlate perfectly with the degree to which T is active on a cellular level.
Conclusions
The present study investigated the effects of naturally varying T on several measures of immunity in healthy, premenopausal women who were sexually active or abstinent. We found evidence that the effects of T on women’s immune response depended on their cycle phase and degree of sexual activity. There were significant changes in the associations between T and specific immune measures at different points of the menstrual cycle, particularly among sexually active women. T was not universally immunosuppressive, but rather, appeared to coordinate immune changes relevant to supporting fertility: for example, among sexually active women, higher T was associated with higher inflammation markers at most cycle phases, but lower inflammation markers at ovulation (which may potentially promote conception). Alongside the other studies in this special issue, the findings from this study underscore the importance of considering ecological context in humans as in non-humans when evaluating the immunomodulatory effects of reproductively-relevant hormones such as testosterone.
Supplementary Material
Figure 4.
Interaction between testosterone and menstrual cycle phase on salivary immunoglobulin A. Women with low levels of testosterone showed significant increase in SIgA from menses to the follicular phase, which was maintained through ovulation. Women with high levels of testosterone showed a significant decrease in SIgA from menses to the follicular phase, followed by a significant increase at ovulation.
Highlights.
We examined associations between women’s testosterone (T) and immune markers.
In sexually abstinent women, higher T predicted higher interleukin-6 (IL-6).
In sexually active women, higher T at ovulation predicted lower IL-6.
In women who reported frequent sex, higher T predicted lower immunoglobulin A.
Higher T predicted greater bacterial killing at menses, but lower in luteal phase.
In this sample of healthy young women, T was not universally immunosuppressive.
Acknowledgments
Dr. Lorenz would like to recognize Dr. Sari van Anders’ support of this work; conversations with Dr. van Anders during a visiting research fellowship were instrumental in developing an early version of the hypotheses presented here. This work was partially funded by the Office of the Vice Provost of Research at Indiana University-Bloomington through the Collaborative Research and Creative Activity Funding Award, and partially by the American Psychological Foundation’s Henry P. David Award for Research in Human Reproductive Behavior and Population Studies. Dr. Lorenz was supported by grant T32HD049336-09 from the National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- Aronoff DM, Fordyce K, Salzman E, Fortenberry JD, Rogers M, King S, van Anders SM. Antiviral Activity of Vaginal Secretions Is Menstrual Cycle Phase-Dependent. Florence, Italy: Society for Gynecologic Investigation; 2014. [Google Scholar]
- Benson S, Janssen O, Hahn S, Tan S, Dietz T, Mann K, Pleger K, Schedlowski M, Arck P, Elsenbruch S. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain, Behav., Immun. 2008;22:177–184. doi: 10.1016/j.bbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Booji A, Biewenga-Booji CM, Huber-Bruning O, Cornelis C, Jacobs JW, Bijlsma JW. Androgens as adjuvant treatment in postmenopausal female patients with rheumatoid arthritis. Ann. Rheum. Dis. 1996;55:811–815. doi: 10.1136/ard.55.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude S, Tang-Martinez Z, Taylor GT. Stress, testosterone, and the immunoredistribution hypothesis. Behav. Ecol. 1999;10:345–350. [Google Scholar]
- Braumoeller BF. Hypothesis testing and multiplicative interaction terms. International organization. 2004;58:807–820. [Google Scholar]
- Brown SG, Morrison L, Calibuso MJ, Christiansen BA. The menstrual cycle and sexual behavior: Relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48:429–444. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S, Agnello C, Malandrino C, Lo Presti L, Cicero C, Cianci S. Do hormones influence women’s sex? Sexual activity over the menstrual cycle. The journal of sexual medicine. 2014;11:211–221. doi: 10.1111/jsm.12348. [DOI] [PubMed] [Google Scholar]
- Charnetski CJ, Brennan FX. Sexual frequency and Immunoglobulin A (IgA) Psychol. Rep. 2004;94:839–844. doi: 10.2466/pr0.94.3.839-844. [DOI] [PubMed] [Google Scholar]
- Cumming DC, Brunsting L, 3rd, Strich G, Ries AL, Rebar RW. Reproductive hormone increases in response to acute exercise in men. Med. Sci. Sports Exerc. 1986;18:369–373. [PubMed] [Google Scholar]
- Demas GE, Carlton ED. Ecoimmunology for psychoneuroimmunologists: Considering context in neuroendocrine-immune-behavior interactions. Brain, Behav., Immun. 2015;44:9–16. doi: 10.1016/j.bbi.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Zysling DA, Beechler BR, Muehlenbein MP, French SS. Beyond phytohaemagglutinin: Assessing vertebrate immune function across ecological contexts. J. Anim. Ecol. 2011;80:710–730. doi: 10.1111/j.1365-2656.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- Eilers PH, Röder E, Savelkoul HF, van Wijk RG. Quantile regression for the statistical analysis of immunological data with many non-detects. BMC Immunol. 2012;13:37. doi: 10.1186/1471-2172-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland CG, Sabzehei B, Marucha PT. Sex hormones and mucosal wound healing. Brain, Behav., Immun. 2009;23:629–635. doi: 10.1016/j.bbi.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa VO, Stefan Ekernas L, Creel S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012;26:123–133. [Google Scholar]
- Fernandez-Real J-M, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. The Journal of Clinical Endocrinology & Metabolism. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992:603–622. [Google Scholar]
- Fortenberry JD, Rogers M, Fordyce K, King S, Aronoff DM, Van Anders SM. HIV Inhibition and Variation in Anti-Microbial Peptides Associated With Intercourse; Boston, MA. Conference on Retroviruses and Opportunistic Infections.2014. [Google Scholar]
- Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N. Y. Acad. Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garde AH, Hansen ÅM, Skovgaard LT, Christensen JM. Seasonal and Biological Variation of Blood Concentrations of Total Cholesterol, Dehydroepiandrosterone Sulfate, Hemoglobin A1c, IgA, Prolactin, and Free Testosterone in Healthy Women. Clin. Chem. 2000;46:551–559. [PubMed] [Google Scholar]
- Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–290. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- Gillum T, Kuennen M, Miller T, Riley L. The effects of exercise, sex, and menstrual phase on salivary antimicrobial proteins. Exerc. Immunol. Rev. 2014;20:23–38. [PubMed] [Google Scholar]
- Giron-Gonzalez J, Moral FJ, Elvira J, Garcia-Gil D, Guerrero F, Gavilan I, Escobar L. Consistent production of a higher TH1: TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- Goldey KL, van Anders SM. Sexy thoughts: Effects of sexual cognitions on testosterone, cortisol, and arousal in women. Horm. Behav. 2011;59:754–764. doi: 10.1016/j.yhbeh.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gomez E, Ortiz V, SAINT-MARTIN B, Boeck L, DÍAZ-SÁNCHEZ V, Bourges H. Hormonal Regulation of the Secretory IgA (sIgA) System: Estradiol-and Progesterone-induced Changes in sIgA in Parotid Saliva Along the Menstrual Cycle. Am. J. Reprod. Immunol. 1993;29:219–223. doi: 10.1111/j.1600-0897.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C. Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2007;23:505–510. doi: 10.1080/09513590701554306. [DOI] [PubMed] [Google Scholar]
- Haahr P, Pedersen B, Fomsgaard A, Tvede N, Diamant M, Klarlund K, Halkjaer-Kristensen J, Bendtzen K. Effect of physical exercise on in vitro production of interleukin 1, interleukin 6, tumour necrosis factor-alpha, interleukin 2 and interferon-gamma. Int. J. Sports Med. 1991;12:223–227. doi: 10.1055/s-2007-1024672. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal Steroid Hormone Receptors and Sex Differences in the Hypothalamo-Pituitary-Adrenal Axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Heitjan DF, Basu S. Distinguishing “missing at random” and “missing completely at random”. The American Statistician. 1996;50:207–213. [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Holdstock G, Chastenay B, Krawitt E. Effects of testosterone, oestradiol and progesterone on immune regulation. Clin. Exp. Immunol. 1982;47:449. [PMC free article] [PubMed] [Google Scholar]
- Huang G, Tang E, Aakil A, Anderson S, Jara H, Davda M, Stroh H, Travison TG, Bhasin S, Basaria S. Testosterone dose-response relationships with cardiovascular risk markers in androgen-deficient women: a randomized, placebo-controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2014;99:E1287–E1293. doi: 10.1210/jc.2013-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe HV, Ridker PM, Manson JE, Cook NR, Buring JE, Rexrode KM. Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann. Epidemiol. 2006;16:105–112. doi: 10.1016/j.annepidem.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. National health statistics reports. 2012;60:1–25. [PubMed] [Google Scholar]
- Kanda N, Tsuchida T, Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin. Exp. Immunol. 1996;106:410–415. doi: 10.1046/j.1365-2249.1996.d01-842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. The Journal of Clinical Endocrinology & Metabolism. 2001;86:2453–2455. doi: 10.1210/jcem.86.6.7580. [DOI] [PubMed] [Google Scholar]
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Konecna L, Yan MS, Miller LE, Schölmerich J, Falk W, Straub RH. Modulation of IL-6 production during the menstrual cycle in vivo and in vitro. Brain, Behav., Immun. 2000;14:49–61. doi: 10.1006/brbi.1999.0570. [DOI] [PubMed] [Google Scholar]
- Li Z, Danis V, Brooks P. Effect of gonadal steroids on the production of IL-1 and IL-6 by blood mononuclear cells in vitro. Clin. Exp. Rheumatol. 1992;11:157–162. [PubMed] [Google Scholar]
- Liening SH, Stanton SJ, Saini EK, Schultheiss OC. Salivary testosterone, cortisol, and progesterone: Two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol. Behav. 2010;99:8–16. doi: 10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J. Clin. Invest. 1969;48:2191. doi: 10.1172/JCI106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Demas GE, Heiman JR. Interaction of menstrual phase and sexual activity predicts mucosal and systemic humoral immunity in healthy women. Physiol. Behav. 2015a;152:92–98. doi: 10.1016/j.physbeh.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Demas GE, Heiman JR. Partnered sexual activity moderates menstrual cycle-related changes in inflammation in healthy women. doi: 10.1016/j.fertnstert.2016.11.010. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Heiman JR, Demas GE. Sexual activity modulates shifts in Th1/Th2 cytokine profile across the menstrual cycle: An observational study. Fertil. Steril. 2015b;104:1513–1521. doi: 10.1016/j.fertnstert.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Heiman JR, Demas GE. Sexual activity modulates shifts in Th1/Th2 cytokine profile across the ovarian cycle. Fertil. Steril. 2015c;104:1513–1521. doi: 10.1016/j.fertnstert.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, van Anders SM. Interactions of sexual activity, gender, and depression with immunity. J. Sex. Med. 2014;11:966–979. doi: 10.1111/jsm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T, Worthman C, Vitzthum VJ. Links among inflammation, sexual activity and ovulation Evolutionary trade-offs and clinical implications. Evolution, Medicine, and Public Health. 2015d;2015:304–324. doi: 10.1093/emph/eov029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. The Journal of Clinical Endocrinology & Metabolism. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- Maggio M, Ceda GP, Lauretani F, Bandinelli S, Corsi AM, Giallauria F, Guralnik JM, Zuliani G, Cattabiani C, Parrino S. SHBG, sex hormones, and inflammatory markers in older women. The Journal of Clinical Endocrinology & Metabolism. 2011;96:1053–1059. doi: 10.1210/jc.2010-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961–965. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and Central Sex Steroids Have Differential Effects on the HPA Axis of Male and Female Rats. Stress. 2002;5:235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- McMurray RW, Suwannaroj S, Ndebele K, Jenkins JK. Differential effects of sex steroids on T and B cells: modulation of cell cycle phase distribution, apoptosis and bcl-2 protein levels. Pathobiology. 2001;69:44–58. doi: 10.1159/000048757. [DOI] [PubMed] [Google Scholar]
- Meston CM, Buss DM. Why humans have sex. Arch. Sex. Behav. 2007;36:477–507. doi: 10.1007/s10508-007-9175-2. [DOI] [PubMed] [Google Scholar]
- Miletic I, Schiffman S, Miletic V, Sattely-Miller E. Salivary IgA secretion rate in young and elderly persons. Physiol. Behav. 1996;60:243–248. doi: 10.1016/0031-9384(95)02161-2. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry JR, Khan-Dawood F, Dawood MY. Marital sex frequency and midcycle female testosterone. Arch. Sex. Behav. 1987;16:27–37. doi: 10.1007/BF01541839. [DOI] [PubMed] [Google Scholar]
- Muehlenbein M, Prall S, Chester E. Development of a noninvasive salivary measure of functional immunity in humans. Am. J. Hum. Biol. 2011:267–268. [Google Scholar]
- Nguyen DP, Genc M, Vardhana S, Babula O, Onderdonk A, Witkin SS. Ethnic differences of polymorphisms in cytokine and innate immune system genes in pregnant women. Obstet. Gynecol. 2004;104:293–300. doi: 10.1097/01.AOG.0000133486.85400.5e. [DOI] [PubMed] [Google Scholar]
- Nittayananta W, Weinberg A, Malamud D, Moyes D, Webster-Cyriaque J, Ghosh S. Innate immunity in HIV-1 infection: epithelial and non-specific host factors of mucosal immunity- a workshop report. Oral Dis. 2016;22:171–180. doi: 10.1111/odi.12451. [DOI] [PubMed] [Google Scholar]
- Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-γ-secreting cells in obese children. Eur. J. Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- Papadopoulos AD, Wardlaw SL. Testosterone Suppresses the Response of the Hypothalamic-Pituitary-Adrenal Axis to Interleukin-6. Neuroimmunomodulation. 2000;8:39–44. doi: 10.1159/000026451. [DOI] [PubMed] [Google Scholar]
- Peters A. Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free- living males with high testosterone are more immunocompetent. Proceedings of the Royal Society of London B: Biological Sciences. 2000;267:883–889. doi: 10.1098/rspb.2000.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C. The NIH human microbiome project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri MA, Mease PJ, Merrill JT, Lahita RG, Iannini MJ, Yocum DE, Ginzler EM, Katz RS, Gluck OS, Genovese MC. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004;50:2858–2868. doi: 10.1002/art.20427. [DOI] [PubMed] [Google Scholar]
- Posma E, Moes H, Heineman MJ, Faas MM. The Effect of Testosterone on Cytokine Production in the Specific and Non-specific Immune Response. Am. J. Reprod. Immunol. 2004;52:237–243. doi: 10.1111/j.1600-0897.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- Prall S, Blanchard S, Hurst D, Ireland E, Lewis C, Martinez L, Rich A, Singh E, Taboas C, Muehlenbein M. Salivary measures of testosterone and functional innate immunity are directly associated in a sample of healthy young adults. American Journal of Physical Anthropology. 2011;52:243. [Google Scholar]
- Prasad A, Mumford SL, Louis GMB, Ahrens KA, Sjaarda LA, Schliep KC, Perkins NJ, Kissell KA, Wactawski-Wende J, Schisterman EF. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Horm. Behav. 2014;66:330–338. doi: 10.1016/j.yhbeh.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nat. Commun. 2012;3:694. doi: 10.1038/ncomms1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt J, Husby S. IgA and Mucosal Homeostasis, Madame Curie Bioscience Database. Austin, TX: Landes Bioscience; 2013. [Google Scholar]
- Riis JL, Out D, Dorn LD, Beal SJ, Denson LA, Pabst S, Jaedicke K, Granger DA. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Dev. Psychobiol. 2014;56:797–811. doi: 10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom. Med. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol. Rev. 2014;36:71–82. doi: 10.1093/epirev/mxt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurs A, Verheul H. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. American Journal of Physiology-Endocrinology And Metabolism. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Sthoeger Z, Chiorazzi N, Lahita R. Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. The Journal of immunology. 1988;141:91–98. [PubMed] [Google Scholar]
- Suarez S, Pacey A. Sperm transport in the female reproductive tract. Hum. Reprod. Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Tarkun I, Arslan BnÇ, Canturk Z, Turemen E, Şahn T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. The Journal of Clinical Endocrinology & Metabolism. 2004;89:5592–5596. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- Tuiten A, Van Honk J, Koppeschaar H, Bernaards C, Thijssen J, Verbaten R. Time course of effects of testosterone administration on sexual arousal in women. Arch. Gen. Psychiatry. 2000;57:149–153. doi: 10.1001/archpsyc.57.2.149. [DOI] [PubMed] [Google Scholar]
- van Anders SM. Gonadal steroids and salivary IgA in healthy young women and men. Am. J. Hum. Biol. 2010;22:348–352. doi: 10.1002/ajhb.20997. [DOI] [PubMed] [Google Scholar]
- van Anders SM. Testosterone and Sexual Desire in Healthy Women and Men. Arch. Sex. Behav. 2012;41:1471–1484. doi: 10.1007/s10508-012-9946-2. [DOI] [PubMed] [Google Scholar]
- van Anders SM. Nomenclature and knowledge-culture, or, we don’t call semen ‘penile mucous’. Psychology & Sexuality. 2014;5:349–356. [Google Scholar]
- van Anders SM, Goldey KL. Testosterone and partnering are linked via relationship status for women and ‘relationship orientation’for men. Horm. Behav. 2010;58:820–826. doi: 10.1016/j.yhbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL, Bell SN. Measurement of testosterone in human sexuality research: Methodological considerations. Arch. Sex. Behav. 2014;43:231–250. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Horm. Behav. 2007;51:477–482. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships. Horm. Behav. 2007;51:286–291. doi: 10.1016/j.yhbeh.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Van Vollenhoven R, Morabito L, Engleman E, McGuire J. Treatment of systemic lupus erythematosus with dehydroepiandrosterone: 50 patients treated up to 12 months. The Journal of rheumatology. 1998;25:285–289. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.