Abstract
The current state of the AD research field is highly dynamic is some respects, while seemingly stagnant in others. Regarding the former, our current lack of understanding of initiating disease mechanisms, the absence of effective treatment options, and the looming escalation of AD patients is energizing new research directions including a much-needed re-focusing on early pathogenic mechanisms, validating novel targets, and investigating relevant biomarkers, among other exciting new efforts to curb disease progression and foremost, preserve memory function. With regard to the latter, the recent disappointing series of failed Phase III clinical trials targeting Aβ and APP processing, in concert with poor association between brain Aβ levels and cognitive function, have led many to call for a re-evaluation of the primacy of the amyloid cascade hypothesis. In this review, we integrate new insights into one of the earliest described signaling abnormalities in AD pathogenesis, namely intracellular Ca2+ signaling disruptions, and focus on its role in driving synaptic deficits – which is the feature that does correlate with AD-associated memory loss. Excess Ca2+release from intracellular stores such as the endoplasmic reticulum (ER) has been well-described in cellular and animal models of AD, as well as human patients, and here we expand upon recent developments in ER-localized release channels such as the IP3R and RyR, and the recent emphasis on RyR2. Consistent with ER Ca2+ mishandling in AD are recent findings implicating aspects of SOCE, such as STIM2 function, and TRPC3 and TRPC6 levels. Other Ca2+-regulated organelles important in signaling and protein handling are brought into the discussion, with new perspectives on lysosomal regulation. These early signaling abnormalities are discussed in the context of synaptic pathophysiology and disruptions in synaptic plasticity with a particular emphasis on short-term plasticity deficits. Overall, we aim to update and expand the list of early neuronal signaling abnormalities implicated in AD pathogenesis, identify specific channels and organelles involved, and link these to proximal synaptic impairments driving the memory loss in AD. This is all within the broader goal of identifying novel therapeutic targets to preserve cognitive function in AD.
Keywords: Ca2+, endoplasmic reticulum, lysosome, synaptic plasticity, Alzheimer’s Disease, SOCE, RyR, IP3R
Urgency in AD
Among the devastating neurodegenerative diseases, Alzheimer’s disease (AD) alone afflicts over 5 million individuals in the U.S., and is feared to grow to nearly 14 million by 2050. Available FDA-approved therapeutics are limited to three cholinesterase inhibitors, approved in 1996–2000, and a low affinity NMDA-R antagonist, approved in 2003. These are symptomatic treatments, not cures, and are not effective in all patients. While the amyloid hypothesis still largely predominates in the field, decades of research and clinic trials addressing Aβ production and deposition have yet to provide a mechanistic cause of AD or offer new therapeutics. Although expectations and efforts remain high for targeting APP processing as the keystone for AD [136], the amyloid cascade hypothesis is being met with increasing skeptisism and scrutiny [20,21,59]. While ongoing clinical trials take a view more towards preventing than reversing AD, clearly it also is time to increase efforts in earlier or upstream mechanisms that may cause or contribute to AD
As recognized since 1989, it is synapse loss which correlates best with cognitive impairment [36,55,128,150]. This association makes intuitive sense and provides a direct cause for the cognitive impairment in AD, as intact synaptic structure and function are required for the synaptic encoding that forms stable memories [56,96]. By extension, it stands to reason that preserving synapses would be an effective means to prevent loss of cognitive functions in at-risk populations. Until recently, there were few tools to measure synaptic integrity in the human brain prior to autopsy, and studies linking synaptic function to cognitive resilience were largely conducted in mouse models or from post mortem human tissue samples [4,24,41]. However, the recent identification of a PET ligand to measure synaptic density in human patients [44] is an exciting new tool, and stands to provide meaningful diagnostic and predictive information related to synapse loss in disease progression.
Most AD patients, over 95%, have sporadic or late-onset forms of the disease where the etiology is unknown, although ApoE4 is a well-characterized genetic risk factor [34,42,99] and more recently variants in TREM2, which normally serves to trigger phagocytosis, have been identified [102]. In familial AD (FAD), the disease-causing mutations identified to date are in presenilin-1 and 2 (PS1 and PS2) and in amyloid precursor protein (APP) genes. Although APP and PS mutations lead to increased Aβ production or changes in Aβ42:40 ratios [136], Aβ-directed potential therapeutics so far have not met efficacy milestones with regards to memory function in human patients, while multiple lines of evidence connect PS mutations identified in early-onset AD with neuronal dysfunction and apoptosis through Ca2+ dyshomeostasis [37,40]. While Aβ is an obligate diagnostic criterion for AD, it is critically important to expand research in other risk factor mechanisms among cells in the CNS [25,35].
Fundamental and early role of ER Ca2+ dysregulation in AD-related synaptic deficits
Ca2+ is well known as a principal factor in cytotoxicity and apoptosis, and Ca2+ dyshomeostasis is seen in neurons with aging, AD and AD transgenic animal models [17,46,143]. Indeed, PS1 mutations alone, as would occur in FAD, impact Ca2+ signaling at early or asymptomatic disease stages in the absence of Aβ or tau aggregation [25,37,120,122,141,148]. The initiation of this early pathogenic cascade may be due to the γ-secretase independent association of PS with inositol trisphosphate receptors (IP3R) and ryanodine receptors (RyR), the two major Ca2+ release channels in the endoplasmic reticulum. FAD-linked mutant PS can directly increase the gating properties of IP3R and increase the intracellular Ca2+ signaling response to IP3-generating GPCR ligands [100,139]. This is seen in cell models, in brain slice pyramidal neurons from multiple AD mouse models mice, and, importantly, in ectodermal cells (fibroblasts) taken directly from human AD patients [80,139,144]. Both PS1 and PS2 influence RyR2 gating through direct interaction with the PS cytosolic domain, with PS1 increasing channel open probability and single channel currents at physiological Ca2+ concentrations (≤ 1 μM) [131] and PS2 reducing feedback inhibition of RyR2 by Ca2+ at pathological concentrations (≥ 10 μM) [58,115]. In aged mice, PS1 expression is reduced in cerebellum and PS2 levels are increased in cerebellum and forebrain, potentially contributing to age-related increases in cytosolic Ca2+ and cytotoxic elevation of Ca2+ through other mechanisms [69].
RyRs are the other major Ca2+ release channel in the ER. While both RyR and IP3R activities are potentiated by Ca2+, it is RyR that is largely responsible for Ca2+-induced Ca2+ release (CICR) in neurons as well as skeletal muscle, cardiac muscle and other cells [25,37,133]. As such, RyR are poised to amplify other signals elevating cytoplasmic Ca2+. Indeed, Aβ has been found to increase Ca2+ in AD cell and animal models [38,111,153] and elevated Ca2+ can increase Aβ production [37,66,118,125,142] resulting in a pathogenic feed-forward cycle. RyR-mediated Ca2+ release is markedly up-regulated in single AD transgenic mice expressing mutant PS, in AD transgenics expressing a combination of gene mutations, and in APP mutant mice [25,37]. RyR-evoked Ca2+ responses are increased in soma cytoplasm 2–3 fold and up to an order of magnitude in dendrite and spines in the presence or absence of Aβ deposits and from youth throughout life in the transgenic mice [16,50]. Accompanying the elevated responsivity is a 2–5 fold increase in expression of the RyR2 isoform in cell models and transgenic mice at early or asymptomatic disease stages [26,111], and increased RyR3 expression at later stages concomitant with Aβ42 aggregation [149]. Additionally, RyR activity can be further modulated post-translationally through oxidation, nitrosylation, phosphorylation and other mechanisms invoked in AD and other neurodegenerative disorders [37,46,60,120,133,143]. Thus, in AD models, intracellular Ca2+ signaling is enhanced through at least two mechanisms: an increase in RyR expression (both at the message and protein levels), and increased Ca2+ release across individual channels. As a likely result of increased RyR expression and release dynamics, the RyR are now aberrantly coupling to channels and signaling cascades, such as Ca2+-activated K+ channels (normally activated by L-type VGCC) which serve to reduce membrane excitability [23,26,27,144,147], and nitric oxide (NO) synthase (normally activated by NMDAR-mediated Ca2+ influx) which generates the gaseous second messenger, NO, and is linked to synaptic activity, excitotoxicty, and neuroprotection [14,23,33,71,101,164]. This potentiated and aberrant ER Ca2+ release greatly expands the array of signaling and neurophysiological functions modulated by intracellular Ca2+ channels, and in some circumstances, invades other Ca2+ signaling pathways contributing to an altered homeostasis, such as that seen in long-term synaptic plasticity expression [23,27].
Ca2+ is an essential mediator of basal synaptic transmission, short and long forms of synaptic plasticity, and dendritic spine morphology [9,25,57]. The ER extends throughout the neuron, including both postsynaptic dendritic spines and presynaptic nerve terminals, positioning RyR in key loci to influence these processes [8]. One immediate electrophysiological consequence of Ca2+ influx is activation of the hyperpolarizing SK2 Ca2+-dependent K+ channels. In cortical and hippocampal pyramidal neurons, SK2 channels mediate the medium afterhypolarization (mAHP) that reduces neuron excitability and may contribute to the depressed synaptic strength seen in young FAD transgenic mice [26,30,62,67,162]. With aging, expression of the L-type voltage-gated Ca2+ channel (VGCC) is upregulated in normal animals and this has been tied to elevated AHP-mediated deficits in cognitive performance [39,108,151]. RyR-mediated Ca2+ signaling contributes to SK2 channel/AHP activation and recently it was found that the RyR contribution itself is increased with aging due to decreased expression of an FK506-binding protein that associated with and stabilizes RyR2 activity [46,47], implicating RyR stabilization as a target to normalize depressed pyramidal neuron excitability in aging. Indeed, FK506-binding protein expression was found to be reduced in early-AD patients as well as aged rats [46] and, in aged rats, virally mediated over-expression of FK506-binding protein expression reduced RyR mediated Ca2+ signaling and improved spatial memory performance [47]. Thus, in early AD patients, we see evidence for increased RyR expression and destabilization through reduced FK506-binding protein expression, leading to Ca2+ signaling dyshomeostasis that can be magnified with age as Ca2+ influx through VGCC or other sources increases. In FAD, with further enhancements of RyR and IP3R Ca2+ signaling, the disruption would be more severe and be seen at earlier ages.
In addition to the RyR-generated somatic signaling disruptions, these ER channels also are highly expressed in dendrites, along with IP3R, and in dendritic spines from which IP3R appear to be excluded [137]. In dendritic spines, RyR-mediated Ca2+ release can be triggered by Ca2+ influx through NMDA-R and Ca2+-permeable AMPA-R ligand-gated ion channels as well as VGCC [50,129,160] suggesting a complexity of disruptions resulting from aberrant Ca2+ signaling. Homosynaptic NMDA-R dependent long-term potentiation (LTP), a fundamental neuronal mechanism underlying learning and memory, is thought to depend upon depolarization to relieve the Mg2+ block of the NMDA-R channel, permitting Ca2+ influx, coincident with activation of the NMDA-R by glutamate. Simplistically, up-regulation of Ca2+ signaling might be expected to increase LTP. Counterbalancing that, however, is Ca2+ dependent activation of SK channels that can reduce synaptic depolarization, limiting NMDA-R Ca2+ influx and impeding the induction of LTP [1,43,54,104,127]. Further, elevated Ca2+ signaling through RyR and subsequent SK channel activation impairs the post-excitation stabilization of mushroom dendritic spines in cultured neurons and the induction of late-phase LTP in hippocampal brain slices, thus interfering with the morphological and electrophysiological substrates of learning and memory [166].
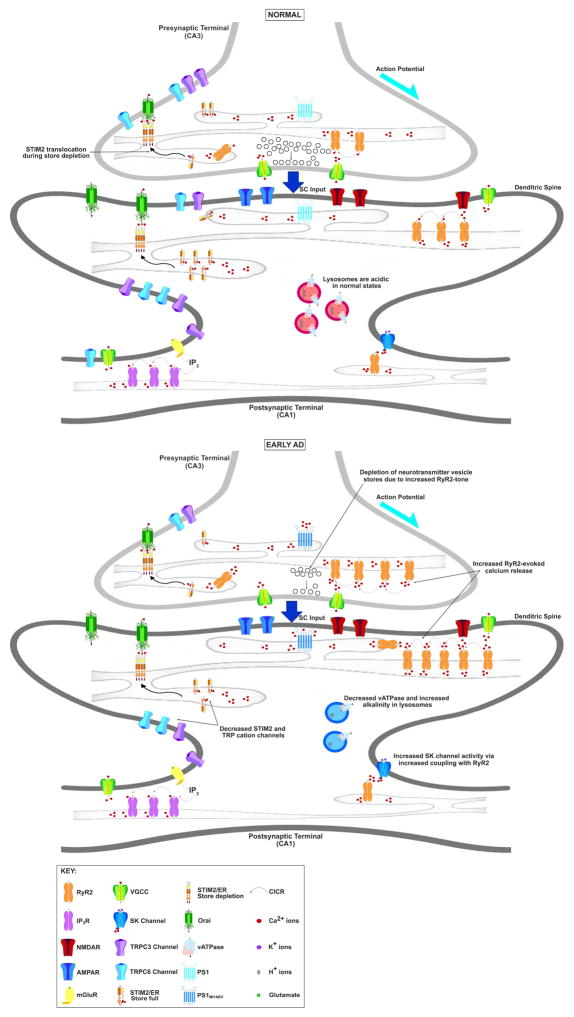
Both presynaptic nerve terminals and postsynaptic dendritic spines are particularly vulnerable to dysregulated RyR Ca2+ signaling as it influences dendritic structure, function [61,74,77] and neurotransmitter release [90], thus impacting neuronal excitability and short and long term plasticity mechanisms essential for learning and memory [10,22,23,121,166] (Figure 1). The ER extends throughout the neuron including into nerve terminals [8] and there is substantial evidence for presynaptic RyR in the hippocampus including localization by immunohistochemistry and electron microscopy, and function by Ca2+ imaging in hippocampal nerve terminals [85,137,154].
Figure 1. Aberrant Ca2+ signaling in early Alzheimer’s disease.
Presynaptically, RyR-mediated CICR can elevate asynchronous release of neurotransmitter vesicles not tied to neuronal action potential. During high frequency activity, VGCC-mediated Ca2+ influx can facilitate RyR-mediated CICR and vesicle release probability. In early AD, CICR may be hyperactive due to increased RyR expression, particularly RyR2 and sensitization of RyR through interaction with mutant PS1, reactive oxygen and nitrogen species. This can lead to increased spontaneous neurotransmitter release resulting in depletion of vesicles from readily-releasable and reserve pools. These maladaptive mechanisms may cause metabolic and oxidative stress resulting in neuronal and synaptic loss. Postsynaptically, in dendritic spines, Ca2+ influx through AMPAR and NMDAR is amplified by RyR-mediated CICR and is essential for induction of long-term synaptic plasticity. Ca2+ also opens Ca2+-dependent potassium (SK) channels which can terminate NMDA-R Ca2+ influx, reduce excitability and modulate postsynaptic action potential firing through repolarization. Additionally, mGluR-phospholipase C signaling generates IP3, activating IP3R to stimulate ER Ca2+ release and magnify CICR. Activation of IP3R supports regenerative Ca2+ waves which can travel to other dendritic regions and soma, influencing gene expression as well as synaptic plasticity. High levels of RyR and IP3R activity can reduce ER Ca2+ content, triggering store-operated Ca2+ entry through STIM2-Orai complexes and TRPC channels. In early AD, increased coupling between RyR2 and SK channels can decrease neuronal excitability and alter the dynamics of synaptic plasticity processes. Altered expression of STIM2 and TRPC3 and TRPC6 can not only disturb store-operated Ca2+ entry but can also destabilize dendritic spine structure and morphology, thus disrupting synaptic plasticity processes. SC input: Schaffer Collateral input; VGCC: voltage-gated Ca2+ channel; STIM: Ca2+-sensing stromal interaction molecule; TRPC channel: transient receptor potential cation channel
Early Transcriptomic and Proteomic Evidence
These findings described above are also consistent with broader proteomic and genomic studies implicating RyR2 in both synaptic functions and AD pathogenesis [19,134,157]. RNA deep sequencing studies show RyR2 transcripts in hippocampal neuropil and dendrites [2,19], and proteomic studies show RyR2 trafficking to synapses [89]. Interestingly, the transcription factor LMO4 (Lim only domain protein 4) is a positive regulator of RyR2 expression, and is one of the genes identified in a GWAS study of aging [157] whose protein expression patterns are altered in the entorhinal cortex and hippocampus, both vulnerable brain regions in AD [82]. Notably, Aβ42 increases expression of LMO4, thus providing a direct mechanism for increased RyR2 in AD brains [6]. Likewise, knockout of LMO4 reduces RyR-mediated Ca2+ signaling and reduces its facilitation of CA3-CA1 glutamatergic synaptic transmission and LTP [124]. Because the molecular and protein machinery are uniquely aligned for synaptic RyR2 expression, these collective findings reveal a previously unappreciated role of RyR2 dysregulation in AD pathophysiology and synaptic degeneration.
Ca2+ and long and short term plasticity defects in AD
Disrupted synaptic plasticity mechanisms concurrent with memory impairments have been widely recognized and reported in FAD transgenic mice at moderate to severe disease stages [68,92,107,136]. Thus, for the purposes of this review, we will not expand upon this well-covered territory, other than to reiterate that overt deficits in hippocampal LTP are usually reported to occur contemporaneously with Aβ aggregation. However, there is strong evidence of more insidious or ‘below the radar’ deficits in asymptomatic AD mice, with overt deficits in certain forms of short term plasticity. It is in these more subtle or emergent stages of synaptic plasticity deficits that interventional strategies may be more effectively employed. For example, in young (3 month old) 3xTg FAD mice, the net magnitude of LTP is remarkably similar to that of age-matched non-transgenic mice, but if Ca2+ homeostasis is tampered with in the AD mice, a profound and underlying shift towards enhanced long-term synaptic depression (LTD) emerges [25,27]. Indeed, in CA3-CA3 pyramidal neuron synaptic transmission, dual patch-clamp electrophysiological studies implicate presynaptic RyR in the induction of homosynaptic LTD [154] and similar mechanisms may underlie the magnified LTD observed with up-regulated RyR Ca2+ signaling in 3xTg FAD mice.
During early stages in 3xTg FAD mice, it is thought that a mixture of NMDA-R dependent and independent mechanisms [30] and enhanced NO signaling boosts postsynaptic RyR-mediated Ca2+ signaling and compensates against enhancement of LTD by other mechanisms [14,23,70,86,154]. In older (8 month) AD Tg mice, LTP is reduced compared to age-matched non-transgenic mice due in part to a reduction in the NMDA-R independent mechanisms [30]. At the same time, however, both younger and older AD Tg mice are impaired in spatial working memory perhaps due to a selective dependence on NMDA-R dependent LTP in this task, or perhaps due to an under-appreciated role for short-term plasticity (paired-pulse facilitation) which is impaired in both young and old 3xTg and reflects aberrations in presynaptic RyR Ca2+ signaling [26,27,30,154]. Notably, the ER Ca2+ signaling abnormalities and plasticity and memory deficits precede detectable amyloid and tau pathology in AD [26,28,144,166].
While seemingly less critical due to its apparent transient nature, short-term plasticity deficits are capable of inflicting long-lasting impairments in memory encoding. While much of the focus has been upon long-lasting changes in synaptic strength, more proximal forms of short-term synaptic plasticity such as synaptic facilitation, post-tetanic potentiation (PTP), paired pulse facilitation (PPF) and early LTP play fundamental roles in stabilizing long term memory encoding. These short forms of synaptic modulation are important in neuronal communication encoding, are impacted by Ca2+ signaling, and participate in the critical synaptic tagging mechanisms which underlie long-term forms of stable synaptic plasticity [45,83,98]. Sustainable long-term memory processing is dependent upon Ca2+-mediated short-term plasticity to transform short-term into long-term memory and establish synapse specificity through synaptic tagging and capture mechanisms [45,126]. The timing and strength of synaptic tagging reflects intracellular Ca2+ signaling patterns, with RyR activation during the plasticity-inducing tetanus serving to increase the duration and durability of the synaptic tag window, thus promoting Hebbbian LTP associativity. In contrast, if RyR channels are active prior to plasticity induction, as demonstrated in the AD models, this critical associative learning window is shortened and plasticity resilience is reduced [83,132]. Thus, in AD pathogenesis, the increased RyR activity at baseline as well as that generated from synaptic activity can blunt the critical window necessary for transforming and stabilizing long term memory formation, resulting in impaired memory performance such as that observed in 3xTgFAD mice at 2 to 3 months of age [30,98,140]. Indeed, working memory deficits are observed in young 3xTg FAD mice well before Aβ deposition [30,140] and underlying this may be a shortening of the associative learning window due to elevated Ca2+ signaling prior to the plasticity induction [83,132].
In AD mouse models at asymptomatic or early disease stages, several synaptic pathophysiological processes mediated by excessive RyR Ca2+ have been observed, such as an increased frequency of spontaneous synaptic potentials, reduced PPF ratios, and increased synaptic depression [16,22,26,27]. Collectively, these would implicate Ca2+-dependent presynaptic mechanisms. PPF, a form of presynaptic Ca2+-dependent short-term plasticity, reflects the probability of neurotransmitter vesicle release, with reduced ratios signifying an increased probability of vesicle release. Existing studies have shown that significant vesicle depletion occurs at synapses with high release probability, which has been demonstrated in several strains of AD mice [50,135]. Thus, presynaptic vesicle release probability is inversely associated with PTP magnitude and the paired pulse ratio [15,53,75,171]. This is consistent with observations in the AD mice, and implicates a Ca2+-mediated increase in release probability, rectified with RyR inhibitors [22,27], in the reduction of presynaptic vesicle stores and blunting of PTP and hippocampal network function. Notably, since synaptic transmission and strength are dependent upon the 3–4th power of Ca2+ concentration within synapses [51], this steep power function is a critical variable influencing synaptic plasticity defects in AD mouse models where synaptically-evoked spine Ca2+ levels are 2–3 times higher than in controls [23,49,117]. Under these extreme conditions, upregulated Ca2+ signaling can create a depleted or burdened synaptic environment.
Emerging Ca2+ sources
Ca2+ is necessary for proper ER function and the concentration of Ca2+ in the ER is one determinant of the magnitude of Ca2+ signaling through IP3R and RyR channels. While it has been proposed that the effect of FAD PS to increase IP3R and RyR Ca2+ signaling is due to elevated ER Ca2+ stores, this mechanism is still under review [12,138]. However, recent modeling studies have demonstrated that knockdown of PS2 can elevate ER Ca2+ stores and support the concept that unprocessed forms of PS act as ER Ca2+ leak channels [5]. ER Ca2+ stores are maintained by store-operated Ca2+ entry (SOCE) and by SERCA pumps which move Ca2+ from cytosol to ER [10,123,156]. As ER Ca2+ levels decrease, Ca2+-sensing stromal interaction molecule (STIM) embedded in the ER membrane associates with the plasma membrane Ca2+ channel Orai to refill ER stores, opening a Ca2+ influx pathway to cytosol which can be measured electrophysiologically as the Ca2+ release activated current ICRAC. Both of the two known STIM homologs (STIM1 and STIM2) are expressed in neurons [97]. In a cultured cortical neuron model, traumatic injury was fount to induce an up regulation of STIM1 and siRNA knockdown of STIM1 reduced the associated increase in Ca2+ signaling and apoptotic cell death [64]. Other recent studies have found a down-regulation of STIM2 in cortex from sporadic AD patients and in hippocampus from aged normal mice (12–16 months old) and in FAD transgenic mice (6–12 but not 3 months old) [146,165]. Further, Ca2+ entry through this pathway was found to be important in the stabilization of memory-associated dendritic mushroom spines of pyramidal neurons through a calmodulin kinase II mechanism. Thus, here Ca2+ influx through ER store-operated channels appears to be favorable for maintaining spine synapses. In striatum from Huntington’s disease (HD) transgenic mice, however, STIM2 and SOCE was up-regulated, disrupting spines in medium spiny neurons [163]. Pharmacologic inhibition of SOCE was beneficial to spine synapse maintenance in the HD model [163], but the same compound may be anticipated to be detrimental to cortical and hippocampal pyramidal neurons. Similarly, while up-regulation of SOCE may be beneficial for AD pyramidal neurons, other CNS synapses could be jeopardized. Clearly, these intriguing new results need further mechanistic dissection that may reveal novel therapeutic targets to address the loss of spine synapses in aging and neurodegenerative disease.
Canonical transient receptor potential (TRPC) channels can also complex with STIM or STIM/Orai to form SOCE pathways [109,123], or they can operate independently to promote synaptic or other neurophysiological functions. There are seven members of the TRPC family with the TRPC1 being the most extensively studied and generally accepted to participate in SOCE. However, TRPC1 was not down- or up-regulated in concert with STIM2 in the aforementioned studies [146,163]. TRPC3, TRPC4 and TRPC5 may also contribute to SOCE alone or in heteromeric complexes with TRPC1, however TRPCs also have the potential to respond more directly to GPCR/diacylglycerol signaling, Ca2+ and redox signals [109] and may be a preferred Ca2+ channel target [29]. TRPC3 channels have been implicated as a Ca2+ source limiting pyramidal neuron firing by activation of the medium and slow AHPs, and the expression of TRPC3 across 23 strains of mice was found to be negatively correlated with learning in the conditioned fear task [103]. Successful learning of the task was associated with down-regulation of TRPC3 and knockdown of TRPC3 improved memory performance. On the other hand, TRPC6 expression improves spatial learning abilities in APP/PS1 mice, and reduces Aβ production and deposition possibly through an interaction between APP and the second transmembrane domain of TRPC6 [158]. These memory-preserving functions of TRPC6 in AD models are likely related to its critical role in forming excitatory synapses and enhancing dendritic spine formation via Ca2+- regulated pathways [170]. Conversely, FAD PS2 mutations have been found to inhibit TRPC6 [81] and thus may contribute to the demise of synapses that are crticial for syanaptic and behavioral plasticity. In other neurodegenerative diseases such as Huntington’s disease, TRPC5 function is closely linked to cell death [63], further implicating the TRPC channel class broadly in neurodegenerative disorders.
Intracellular organelle sources of Ca2+, may include lysosomes in addition to ER and mitochondria. Lysosome dysfunction is implicated along multiple lines of evidence in neurodegenerative disease - AD, Parkinson’s Disease and Huntington’s Disease in particular [13,32,48,65,72,93,119]. Among the numerous molecular pathway links to AD, PS knockout and expression of FAD PS1 have been found to increase Ca2+ release from lysosomes [31,79]. Recent studies suggest that PS1 deficiency leads to reduction in vacuolar ATPase, increasing lysosomal pH which then causes a switch in the main lysosomal Ca2+ efflux pathway from two-pore channels (TPC) to TRPML1 [79]. While the vacuolar ATPase/alkalinization mechanisms remain under investigation is controversial [31,168], there is agreement that PS deficiency increases Ca2+ release from lysosomes and reduces their Ca2+ content [31,79].
Mitochondria are well known as the “powerhouse” of the cell, important in buffering cytosolic Ca2+, and definitive mediator of cytotoxicity when damaged. Numerous reviews have detailed critical processes in mitochondria that may be involved in AD and other neurodegenerative diseases [18,52,91,110,113,145]. While discussion of these mechanisms is beyond the scope of this minreview, of particular note is the expanding interest in mitochondria-associated ER membrane (MAM) [3,76,112,130]. Among the functions attributed to these areas of contact is Ca2+ exchange between ER and mitochondria and the possibility that Ca2+ release from ER may help supply Ca2+ for the ATP powerhouse under normal conditions, or lead to mitochondrial pathology and apoptosis when ER Ca2+ release is aberrant [130].
Opportunities for Therapeutics
RyR are large (>2.2 MDa) allosteric proteins [78,155] in which the intrinsic Ca2+ channel activity is modulated through numerous processes including interaction with other proteins (e.g., FK506-binding protein FKBP12 stabilizes RyRs, L-type VGCC activates RyR1 in skeletal muscle), posttranslational modulation (e.g., phosphorylation, oxidation and nitrosylation), endogenous ligands (e.g., Ca2+ and calmodulin, cyclic ADP-ribose, ATP), and exogenous ligands (e.g., ryanodine, caffeine, dantrolene) [11,60,105,133]. The ligands act as allosteric modulators rather than classic agonist or antagonist. RyR2 is critical for excitation-contraction coupling in cardiac myocytes and it is thought that the RyR2 N- and C-terminal domains, normally coupled or “zipped”, can be destabilized or “unzipped” under conditions of oxidative stress such as in heart failure [106,159]. Dantrolene is able to normalize RyR2 hyperactivity without blocking function [73,95,106,159]. Other studies find that RyR2 destabilization involves “remodeling” by a combination of oxidation/nitrosylation, phosphorylation by protein kinase A, and loss of interaction with FK506-binding protein “calstabin” [94] that can be reversed by the compound S107 [7]. While there is some controversy surrounding the destabilizing mechanisms, e.g. regarding the kinases and phosphorylation sites involved [11], nevertheless it is apparent that small molecules such as dantrolene and S107 can normalize RyR activity without blocking function. Dantrolene is an approved and highly effective drug for use in human to prevent and rescue malignant hyperthermia where it acts by stabilizing RyR1 hyperactivity [161]. Although in vitro binding of dantrolene to RyR2 is low under normal conditions [169], this depends upon the conformational state of the receptor [114]. In cardiac tissue, where RyR2 is expressed specifically and RyR2 Ca2+ release mediates muscle contractility, dantrolene stabilizes aberrant RyR2 function in disease states without blocking normal function [73,152]. Here [106], as in brain [60], oxidative stress may be a key factor in destabilizing RyR2 function in a way that can be normalized by RyR negative allosteric modulators such as dantrolene.
Moreover, treatment of FAD transgenic mice with dantrolene has been found by several laboratories to be effective in reversing a range of AD features, including aberrant ER Ca2+ signaling, disrupted hippocampal synaptic plasticity mechanisms, behavioral deficits, and Aβ plaque deposition [84]. In our laboratory, we found that a 4-week treatment with a brain penetrant nanocrystal formulation of dantrolene normalized the elevated hippocampal RyR2 expression and aberrantly high somatic and dendritic RyR Ca2+ responses in pyramidal neurons from 3xTg and APP/PS1 FAD mice, restored normal short-term and long-term synaptic plasticity mechanisms in 3xTg mice, and reduced the levels of soluble and insoluble Aβ in cortex and hippocampus of APP/PS1 mice [22]. Peng et al. [116] found that memory task performance was improved and Aβ plaque deposition reduced in 3xTg FAD mice following 11 months of i.c.v. infusion of dantrolene. And in Tg2576 FAD mice, which are transgenic for APPswe but not mutant PS, Oules et al. [111] found that 3 months treatment with dantrolene restored levels of the synapse marker PSD-95 and prevented decline of memory performance in hippocampal and cortical-dependent memory tasks while also reducing production of Aβ and C99 fragments. In vitro experiments demonstrated that while expression of FAD APP can increase RyR mediated Ca2+ release, elevated RyR Ca2+ signaling in turn can increase C99 and Aβ production from APP [111], signaling a feed-forward pathogenic mechanism between Aβ and destabilized ER Ca2+ release. Indeed, using a restraint stress model in normal mice, Liu et al. [88] found that the RyR2 allosteric modulator, S107, inhibited RyR biochemical and functional remodeling, and normalized hippocampal synaptic plasticity and behavioral performance in Morris Water Maze and open arm tasks.
These studies favor the potential utility of RyR negative allosteric modulators in AD and other neurodegenerative/cognitive disorders. One study, however, found elevated Aβ plaque load and reduced PSD-95 synaptic marker in APP/PS1 FAD mice fed dantrolene for 6 months [167]. The reasons for this discrepancy are unclear, but may be related to route of administration, low CNS penetration, and low selectivity of dantrolene. Subject age also may be a critical factor, as a knockout study found that RyR3 was protective in young but detrimental in older APP/PS1 FAD mice [87]. Efforts are ongoing to identify superior molecules, as well as further validate the breadth of emerging Ca2+ channels and pathways that likely are contributing to the synaptic pathophysiology and resultant devastating loss of cognitive function in AD.
Supplementary Material
This review highlights recent findings and insights regarding early mechanisms of synaptic pathology in AD, particularly as it involves neuronal calcium signaling abnormalities and manifests as deficits in synaptic plasticity. This discussion is particularly relevant given the strong relationship between synaptic deficits and memory loss is AD, and the pressing need to investigate alternative mechanisms contributing to AD-associated cognitive impairment.
Acknowledgments
The authors would like to thank Alyssa Littlefield and Sarah Mutaly for this assistance is preparing this document.
Abbreviations
- Aβ
amyloid β
- AHP
after-hyperpolarization
- AMPA-R
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid sensitive glutamate receptor
- ApoE
apolipoprotein E
- APP
amyloid precursor protein
- CICR
Ca2+ induced Ca2+ release
- ER
endoplasmic reticulum
- GCPR
G-protein coupled receptor
- ICRAC
Ca2+ release activated Ca2+ current
- IP3
inositol trisphosphate
- IP3R
inositol trisphosphate receptor
- LMO4
Lim only domain protein 4
- LTD
long-term depression
- LTP
long-term potentiation
- MAM
mitochondria-associated membrane
- mGluR
metabotropic glutamate receptor
- NMDA-R
N-methyl-D-aspartate sensitive glutamate receptor
- PPF
paired-pulse facilitation
- PTP
post-tetanic potentiation
- RyR
ryanodine receptor
- SK
small-conductance Ca2+ activated K+ channel
- SERCA
sarco/endoplasmic Ca2+ ATPase
- SOCE
store-operated Ca2+ entry
- TREM
triggering receptor expressed on myeloid cells
- VGCC
voltage-gated Ca2+ channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Allen D, Bond CT, Lujan R, Ballesteros-Merino C, Lin MT, Wang K, Klett N, Watanabe M, Shigemoto R, Stackman RW, Jr, Maylie J, Adelman JP. The SK2-long isoform directs synaptic localization and function of SK2-containing channels. Nat Neurosci. 2011;14:744–749. doi: 10.1038/nn.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonell A, Llado A, Altirriba J, Botta-Orfila T, Balasa M, Fernandez M, Ferrer I, Sanchez-Valle R, Molinuevo JL. A preliminary study of the whole-genome expression profile of sporadic and monogenic early-onset Alzheimer’s disease. Neurobiol Aging. 2013;34:1772–1778. doi: 10.1016/j.neurobiolaging.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Area-Gomez E, Schon EA. Mitochondria-associated ER membranes and Alzheimer disease. Curr Opin Genet Dev. 2016;38:90–96. doi: 10.1016/j.gde.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold SE, Louneva N, Cao K, Wang LS, Han LY, Wolk DA, Negash S, Leurgans SE, Schneider JA, Buchman AS, Wilson RS, Bennett DA. Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiol Aging. 2013;34:157–168. doi: 10.1016/j.neurobiolaging.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandara S, Malmersjo S, Meyer T. Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci Signal. 2013;6:ra56. doi: 10.1126/scisignal.2003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barucker C, Sommer A, Beckmann G, Eravci M, Harmeier A, Schipke CG, Brockschnieder D, Dyrks T, Althoff V, Fraser PE, Hazrati LN, George-Hyslop PS, Breitner JC, Peters O, Multhaup G. Alzheimer amyloid peptide abeta42 regulates gene expression of transcription and growth factors. J Alzheimer’s Dis. 2015;44:613–624. doi: 10.3233/JAD-141902. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, Lacampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci(USA) 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 9.Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pflugers Arch. 2010;459:441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion. 2013;7:2–13. doi: 10.4161/pri.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bers DM. Ryanodine Receptor S2808 Phosphorylation in Heart Failure: Smoking Gun or Red Herring. Circ Res. 2012;110:796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 12.Bezprozvanny I, Supnet C, Sun S, Zhang H, De Strooper B. Response to Shilling et al. J Biol Chem. 2012;287:20469. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanz J, Saftig P. Parkinson’s disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. J Neurochem. 2016 doi: 10.1111/jnc.13517. [DOI] [PubMed] [Google Scholar]
- 14.Bradley SA, Steinert JR. Nitric oxide-mediated posttranslational modifications: impacts at the synapse. Oxid Med Cell Longev. 2016;2016:5681036. doi: 10.1155/2016/5681036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003;6:551–552. doi: 10.1038/nn1067. [DOI] [PubMed] [Google Scholar]
- 16.Briggs CA, Schneider C, Richardson JC, Stutzmann GE. Beta amyloid peptide plaques fail to alter evoked neuronal calcium signals in APP/PS1 Alzheimer’s disease mice. Neurobiol Aging. 2013;34:1632–1643. doi: 10.1016/j.neurobiolaging.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brini M, Cali T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Q, Tammineni P. Alterations in mitochondrial quality control in Alzheimer’s disease. Front Cell Neurosci. 2016;10:24. doi: 10.3389/fncel.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cajigas IJ, Tushev G, Will TJ, Stom D, Fuerst N, Schuman EM. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron. 2012;74:453–466. doi: 10.1016/j.neuron.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellani RJ, Smith MA. Compounding artefacts with uncertainty, and an amyloid cascade hypothesis that is ‘too big to fail’. J Pathol. 2011;224:147–152. doi: 10.1002/path.2885. [DOI] [PubMed] [Google Scholar]
- 21.Castello MA, Jeppson JD, Soriano S. Moving beyond anti-amyloid therapy for the prevention and treatment of Alzheimer’s disease. BMC Neurol. 2014;14:169. doi: 10.1186/s12883-014-0169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, Stutzmann GE. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer’s disease. PLoS One. 2012;7:e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer’s disease mice. J Neurosci. 2015;35:6893–6902. doi: 10.1523/JNEUROSCI.4002-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakroborty S, Stutzmann GE. Early calcium dysregulation in Alzheimer’s disease: setting the stage for synaptic dysfunction. Sci China Life Sci. 2011;54:752–762. doi: 10.1007/s11427-011-4205-7. [DOI] [PubMed] [Google Scholar]
- 25.Chakroborty S, Stutzmann GE. Calcium channelopathies and Alzheimer’s disease: insight into therapeutic success and failures. Eur J Pharmacol. 2014;739:83–95. doi: 10.1016/j.ejphar.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakroborty S, Kim J, Schneider C, Jacobson C, Molgó J, Stutzmann GE. Early pre- and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J Neurosci. 2012;32:8341–8353. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi S, Maleth J, Jha A, Lee KP, Kim MS, So I, Ahuja M, Muallem S. The TRPCs-STIM1-Orai interaction. Handb Exp Pharmacol. 2014;223:1035–1054. doi: 10.1007/978-3-319-05161-1_13. [DOI] [PubMed] [Google Scholar]
- 30.Clark JK, Furgerson M, Crystal JD, Fechheimer M, Furukawa R, Wagner JJ. Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol Learn Mem. 2015;125:152–162. doi: 10.1016/j.nlm.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coen K, Flannagan RS, Baron S, Carraro-Lacroix LR, Wang D, Vermeire W, Michiels C, Munck S, Baert V, Sugita S, Wuytack F, Hiesinger PR, Grinstein S, Annaert W. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J Cell Biol. 2012;198:23–35. doi: 10.1083/jcb.201201076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, Van Nostrand WE, Weinberg JB, Dawson H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci(USA) 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Sci. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 35.De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 36.Dekosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 37.del Prete D, Checler F, Chami M. Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener. 2014;9:21–29. doi: 10.1186/1750-1326-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demuro A, Parker I. Cytotoxicity of intracellular abeta42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate. J Neurosci. 2013;33:3824–3833. doi: 10.1523/JNEUROSCI.4367-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Disterhoft JF, Oh MM. Learning, aging and intrinsic neuronal plasticity. Trends Neurosci. 2006;29:587–599. doi: 10.1016/j.tins.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Duggan SP, McCarthy JV. Beyond gamma-secretase activity: The multifunctional nature of presenilins in cell signalling pathways. Cell Signal. 2016;28:1–11. doi: 10.1016/j.cellsig.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. J Alzheimer’s Dis. 2015;47:231–242. doi: 10.3233/JAD-150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Haj M, Antoine P, Amouyel P, Lambert JC, Pasquier F, Kapogiannis D. Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer’s disease: a review. Ageing Res Rev. 2016;27:15–22. doi: 10.1016/j.arr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- 44.Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin S, Chen MK, Dhaher R, Matuskey D, Baum E, Holden D, Spencer DD, Mercier J, Hannestad J, Huang Y, Carson RE. Imaging synaptic density in the living human brain. Science Translational Medicine. 2016;8:348ra96. doi: 10.1126/scitranslmed.aaf6667. [DOI] [PubMed] [Google Scholar]
- 45.Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 46.Gant JC, Blalock EM, Chen KC, Kadish I, Porter NM, Norris CM, Thibault O, Landfield PW. FK506-binding protein 1b/12.6: a key to aging-related hippocampal Ca2+ dysregulation? Eur J Pharmacol. 2014;739:74–82. doi: 10.1016/j.ejphar.2013.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gant JC, Chen KC, Kadish I, Blalock EM, Thibault O, Porter NM, Landfield PW. Reversal of aging-related neuronal Ca2+ dysregulation and cognitive impairment by delivery of a transgene encoding FK506-binding protein 12.6/1b to the hippocampus. J Neurosci. 2015;35:10878–10887. doi: 10.1523/JNEUROSCI.1248-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez-Sintes R, Ledesma MD, Boya P. Lysosomal cell death mechanisms in aging. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Goussakov I, Chakroborty S, Stutzmann GE. Generation of dendritic Ca2+ oscillations as a consequence of altered ryanodine receptor function in AD neurons. Channels (Austin) 2011;5:9–13. doi: 10.4161/chan.5.1.14124. [DOI] [PubMed] [Google Scholar]
- 50.Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca2+ influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grigoryan G, Segal M. Ryanodine-mediated conversion of STP to LTP is lacking in synaptopodin-deficient mice. Brain Struct Funct. 2016;221:2393–2397. doi: 10.1007/s00429-015-1026-7. [DOI] [PubMed] [Google Scholar]
- 52.Grimm A, Friedland K, Eckert A. Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer’s disease. Biogerontology. 2016;17:281–296. doi: 10.1007/s10522-015-9618-4. [DOI] [PubMed] [Google Scholar]
- 53.Habets RL, Borst JG. Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol. 2005;564:173–187. doi: 10.1113/jphysiol.2004.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamos JE, DeGennaro LJ, Drachman DA. Synaptic loss in Alzheimer’s disease and other dementias. Neurology. 1989;39:355–361. doi: 10.1212/wnl.39.3.355. [DOI] [PubMed] [Google Scholar]
- 56.Harris KM, Weinberg RJ. Ultrastructure of synapses in the mammalian brain. Cold Spring Harb Perspect Biol. 2012;4:a005587. doi: 10.1101/cshperspect.a005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi Y, Majewska AK. Dendritic Spine Geometry: Functional Implication and Regulation. Neuron. 2005;46:529–532. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44:507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 60.Hidalgo C, Arias-Cavieres A. Calcium, reactive oxygen species, and synaptic plasticity. Physiology (Bethesda) 2016;31:201–215. doi: 10.1152/physiol.00038.2015. [DOI] [PubMed] [Google Scholar]
- 61.Higley MJ, Sabatini BL. Calcium signaling in dendritic spines. Cold Spring Harb Perspect Biol. 2012;4:a005686. doi: 10.1101/cshperspect.a005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hinrich AJ, Jodelka FM, Chang JL, Brutman D, Bruno AM, Briggs CA, James BD, Stutzmann GE, Bennett DA, Miller SA, Rigo F, Marr RA, Hastings ML. Therapeutic correction of ApoER2 splicing in Alzheimer’s disease mice using antisense oligonucleotides. EMBO Mol Med. 2016;8:328–345. doi: 10.15252/emmm.201505846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong C, Seo H, Kwak M, Jeon J, Jang J, Jeong EM, Myeong J, Hwang YJ, Ha K, Kang MJ, Lee KP, Yi EC, Kim IG, Jeon JH, Ryu H, So I. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington’s disease. Brain. 2015;138:3030–3047. doi: 10.1093/brain/awv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou PF, Liu ZH, Li N, Cheng WJ, Guo SW. Knockdown of STIM1 improves neuronal survival after traumatic neuronal injury through regulating mGluR1-dependent Ca(2+) signaling in mouse cortical neurons. Cell Mol Neurobiol. 2015;35:283–292. doi: 10.1007/s10571-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener. 2015;4:18. doi: 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Isaacs AM, Senn DB, Yuan M, Shine JP, Yankner BA. Acceleration of amyloid beta-peptide aggregation by physiological concentrations of calcium. J Biol Chem. 2006;281:27916–27923. doi: 10.1074/jbc.M602061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci(USA) 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang SS, Chung HJ. Emerging link between Alzheimer’s disease and homeostatic synaptic plasticity. Neural Plast. 2016;2016:7969272. doi: 10.1155/2016/7969272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaja S, Sumien N, Shah VV, Puthawala I, Maynard AN, Khullar N, Payne AJ, Forster MJ, Koulen P. Loss of spatial memory, learning, and motor function during normal aging Is accompanied by changes in brain presenilin 1 and 2 expression levels. Mol Neurobiol. 2015;52:545–554. doi: 10.1007/s12035-014-8877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kakizawa S, Yamazawa T, Iino M. Nitric oxide-induced calcium release: activation of type 1 ryanodine receptor by endogenous nitric oxide. Channels (Austin) 2013;7:1–5. doi: 10.4161/chan.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakizawa S, Takeshima H, Iino M. Nitric oxide-induced calcium release: a novel calcium-mobilizing mechanism mediated by S-nitrosylation-dependent modulation of ryanodine receptor. Messenger. 2012;1:133–140. [Google Scholar]
- 72.Kilpatrick BS, Magalhaes J, Beavan MS, McNeill A, Gegg ME, Cleeter MW, Bloor-Young D, Churchill GC, Duchen MR, Schapira AH, Patel S. Endoplasmic reticulum and lysosomal Ca(2+) stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium. 2016;59:12–20. doi: 10.1016/j.ceca.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing inter-domain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci(USA) 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Korogod N, Lou X, Schneggenburger R. Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held. J Neurosci. 2005;25:5127–5137. doi: 10.1523/JNEUROSCI.1295-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krols M, van IG, Asselbergh B, Kremer A, Lippens S, Timmerman V, Janssens S. Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol. 2016;131:505–523. doi: 10.1007/s00401-015-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lessard CB, Lussier MP, Cayouette S, Bourque G, Boulay G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell Signal. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Leuba G, Vernay A, Vu D, Walzer C, Belloir B, Kraftsik R, Bouras C, Savioz A. Differential expression of LMO4 protein in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2004;30:57–69. doi: 10.1046/j.0305-1846.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 83.Li Q, Rothkegel M, Xiao ZC, Abraham WC, Korte M, Sajikumar S. Making synapses strong: metaplasticity prolongs associativity of long-term memory by switching synaptic tag mechanisms. Cereb Cortex. 2014;24:353–363. doi: 10.1093/cercor/bhs315. [DOI] [PubMed] [Google Scholar]
- 84.Liang L, Wei H. Dantrolene, a treatment for Alzheimer disease? Alzheimer Dis. Assoc Disord. 2015;29:1–5. doi: 10.1097/WAD.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang Y, Yuan LL, Johnston D, Gray R. Calcium signaling at single mossy fiber presynaptic terminals in the rat hippocampus. J Neurophysiol. 2002;87:1132–1137. doi: 10.1152/jn.00661.2001. [DOI] [PubMed] [Google Scholar]
- 86.Lima B, Forrester MT, Hess DT, Stamler JS. S-Nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Supnet C, Sun S, Zhang H, Good L, Popugaeva E, Bezprozvanny I. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels (Austin) 2014;8:230–242. doi: 10.4161/chan.27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, Arancio O, Marks AR. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150:1055–1067. doi: 10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu XA, Kadakkuzha B, Pascal B, Steckler C, Akhmedov K, Yan L, Chalmers M, Puthanveettil SV. New approach to capture and characterize synaptic proteome. Proc Natl Acad Sci(USA) 2014;111:16154–16159. doi: 10.1073/pnas.1401483111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 91.Magi S, Castaldo P, Macri ML, Maiolino M, Matteucci A, Bastioli G, Gratteri S, Amoroso S, Lariccia V. Intracellular Calcium Dysregulation: Implications for Alzheimer’s Disease. Biomed Res Int. 2016;2016:6701324. doi: 10.1155/2016/6701324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marchetti C, Marie H. Hippocampal synaptic plasticity in Alzheimer’s disease: what have we learned so far from transgenic models? Rev. Neurosci. 2011;22:373–402. doi: 10.1515/RNS.2011.035. [DOI] [PubMed] [Google Scholar]
- 93.Martini-Stoica H, Xu Y, Ballabio A, Zheng H. The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci. 2016;39:221–234. doi: 10.1016/j.tins.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marx SO, Marks AR. Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. J Mol Cell Cardiol. 2013;58:225–231. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol- Heart Circ Physiol. 2012;302:H953–H963. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4:a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L, Lodola F, D’Angelo E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front Cell Neurosci. 2015;9:153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moncada D, Ballarini F, Viola H. Behavioral tagging: a translation of the synaptic tagging and capture hypothesis. Neural Plast. 2015;2015:650780. doi: 10.1155/2015/650780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mufson EJ, Ikonomovic MD, Counts SE, Perez SE, Malek-Ahmadi M, Scheff SW, Ginsberg SD. Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav Brain Res. 2016;311:54–69. doi: 10.1016/j.bbr.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller M, Cheung KH, Foskett JK. Enhanced ROS generation mediated by Alzheimer’s Disease presenilin regulation of InsP3R Ca2+ signaling. Antioxid Redox Signal. 2011;14:1225–1235. doi: 10.1089/ars.2010.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura T, Lipton SA. Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ. 2011;18:1478–1486. doi: 10.1038/cdd.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neumann H, Daly MJ. Variant TREM2 as risk factor for Alzheimer’s disease. New England J Med. 2013;368:182–184. doi: 10.1056/NEJMe1213157. [DOI] [PubMed] [Google Scholar]
- 103.Neuner SM, Wilmott LA, Hoffmann BR, Mozhui K, Kaczorowski CC. Hippocampal proteomics defines pathways associated with memory decline and resilience in normal aging and Alzheimer’s disease mouse models. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 105.O’Brien F, Venturi E, Sitsapesan R. The ryanodine receptor provides high throughput Ca2+-release but is precisely regulated by networks of associated proteins: a focus on proteins relevant to phosphorylation. Biochem Soc Trans. 2015;43:426–433. doi: 10.1042/BST20140297. [DOI] [PubMed] [Google Scholar]
- 106.Oda T, Yang Y, Uchinoumi H, Thomas DD, Chen-Izu Y, Kato T, Yamamoto T, Yano M, Cornea RL, Bers DM. Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca(2+) release and pathologically alter, RyR structure and calmodulin affinity. J Mol Cell Cardiol. 2015;85:240–248. doi: 10.1016/j.yjmcc.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 108.Oh MM, Simkin D, Disterhoft JF. Intrinsic hippocampal excitability changes of opposite signs and different origins in CA1 and CA3 pyramidal neurons underlie aging-related cognitive deficits. Front Syst Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ong HL, de Souza LB, Ambudkar IS. Role of TRPC channels in store-operated calcium entry. Adv Exp Med Biol. 2016;898:87–109. doi: 10.1007/978-3-319-26974-0_5. [DOI] [PubMed] [Google Scholar]
- 110.Onyango IG, Dennis J, Khan SM. Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7:201–214. doi: 10.14336/AD.2015.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oules B, del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Brechot P, Trebak M, Checler F, Benfenati F, Chami M. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci. 2012;32:11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paillusson S, Stoica R, Gomez-Suaga P, Lau DH, Mueller S, Miller T, Miller CC. There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pajak B, Kania E, Orzechowski A. Killing me softly: connotations to unfolded protein response and oxidative stress in Alzheimer’s disease. Oxid Med Cell Longev. 2016;2016:1805304. doi: 10.1155/2016/1805304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paul-Pletzer K, Yamamoto T, Ikemoto N, Jimenez L, Morimoto H, Williams P, Ma J, Parness J. Probing a putative dantrolene-binding site on the cardiac ryanodine receptor. Biochem J. 2005;387:905–909. doi: 10.1042/BJ20041336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Payne AJ, Kaja S, Koulen P. Regulation of ryanodine receptor-mediated calcium signaling by presenilins. Receptors Clin Investig. 2015;2:e449. doi: 10.14800/rci.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett. 2012;516:274–279. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perez MF, Ford KA, Goussakov I, Stutzmann GE, Hu XT. Repeated cocaine exposure decreases dopamine D2-like receptor modulation of Ca2+ homeostasis in rat nucleus accumbens neurons. Synapse. 2011;65:168–180. doi: 10.1002/syn.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pierrot N, Ghisdal P, Caumont AS, Octave JN. Intraneuronal amyloid-beta1–42 production triggered by sustained increase of cytosolic calcium concentration induces neuronal death. J Neurochem. 2004;88:1140–1150. doi: 10.1046/j.1471-4159.2003.02227.x. [DOI] [PubMed] [Google Scholar]
- 119.Platt N, Speak AO, Colaco A, Gray J, Smith DA, Williams IM, Wallom KL, Platt FM. Immune dysfunction in Niemann-Pick disease type C. J Neurochem. 2016;136:74–80. doi: 10.1111/jnc.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Popugaeva E, Bezprozvanny I. Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front Mol Neurosci. 2013;6:29. doi: 10.3389/fnmol.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Popugaeva E, Bezprozvanny I. Can the calcium hypothesis explain synaptic loss in Alzheimer’s disease? Neurodegener. Dis. 2014;13:139–141. doi: 10.1159/000354778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Popugaeva E, Supnet C, Bezprozvanny I. Presenilins, deranged calcium homeostasis, synaptic loss and dysfunction in Alzheimer’s disease. Messenger. 2012;1:53–62. [Google Scholar]
- 123.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qin Z, Zhou X, Gomez-Smith M, Pandey NR, Lee KF, Lagace DC, Beique JC, Chen HH. LIM domain only 4 (LMO4) regulates calcium-induced calcium release and synaptic plasticity in the hippocampus. J Neurosci. 2012;32:4271–4283. doi: 10.1523/JNEUROSCI.6271-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Querfurth HW, Jiang J, Geiger JD, Selkoe DJ. Caffeine stimulates amyloid beta-peptide release from beta-amyloid precursor protein-transfected HEK293 cells. J Neurochem. 1997;69:1580–1591. doi: 10.1046/j.1471-4159.1997.69041580.x. [DOI] [PubMed] [Google Scholar]
- 126.Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 127.Ris L, Capron B, Sclavons C, Liegeois JF, Seutin V, Godaux E. Metaplastic effect of apamin on LTP and paired-pulse facilitation. Learn Mem. 2007;14:390–399. doi: 10.1101/lm.571007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robinson JL, Molina-Porcel L, Corrada MM, Raible K, Lee EB, Lee VM, Kawas CH, Trojanowski JQ. Perforant path synaptic loss correlates with cognitive impairment and Alzheimer’s disease in the oldest-old. Brain. 2014;137:2578–2587. doi: 10.1093/brain/awu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rossi B, Maton G, Collin T. Calcium-permeable presynaptic AMPA receptors in cerebellar molecular layer interneurones. J Physiol. 2008;586:5129–5145. doi: 10.1113/jphysiol.2008.159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 132.Sajikumar S, Li Q, Abraham WC, Xiao ZC. Priming of short-term potentiation and synaptic tagging/capture mechanisms by ryanodine receptor activation in rat hippocampal CA1. Learn Mem. 2009;16:178–186. doi: 10.1101/lm.1255909. [DOI] [PubMed] [Google Scholar]
- 133.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol. 2015;8:206–222. doi: 10.2174/1874467208666150507105105. [DOI] [PubMed] [Google Scholar]
- 134.Saura CA, Parra-Damas A, Enriquez-Barreto L. Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Front Cell Neurosci. 2015;9:318. doi: 10.3389/fncel.2015.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scimemi A, Diamond JS. The number and organization of Ca2+ channels in the active zone shapes neurotransmitter release from Schaffer collateral synapses. J Neurosci. 2012;32:18157–18176. doi: 10.1523/JNEUROSCI.3827-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharp AH, Mcpherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shilling D, Mak DO, Kang DE, Foskett JK. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem. 2012;287:10933–10944. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shilling D, Muller M, Takano H, Mak DO, Abel T, Coulter DA, Foskett JK. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J Neurosci. 2014;34:6910–6923. doi: 10.1523/JNEUROSCI.5441-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stevens LM, Brown RE. Reference and working memory deficits in the 3xTg-AD mouse between 2 and 15-months of age: a cross-sectional study. Behav Brain Res. 2015;278:496–505. doi: 10.1016/j.bbr.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 141.Stiller I, Lizak B, Banhegyi G. Physiological functions of presenilins; beyond gamma-secretase. Curr Pharm Biotechnol. 2014;15:1019–1025. doi: 10.2174/1389201015666141122204139. [DOI] [PubMed] [Google Scholar]
- 142.Stutzmann GE. The pathogenesis of Alzheimers disease: Is it a lifelong “calciumopathy”? Neuroscientist. 2007;13:546–559. doi: 10.1177/1073858407299730. [DOI] [PubMed] [Google Scholar]
- 143.Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca2+ handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stutzmann GE, Smith I, Caccamo A, Oddo S, LaFerla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s Disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev. 2016;68:20–48. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun S, Zhang H, Liu J, Popugaeva E, Xu NJ, Feske S, White CL, III, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82:79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Supnet C, Bezprozvanny I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J Alzheimer’s Dis. 2010;20(Suppl 2):S487–S498. doi: 10.3233/JAD-2010-100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Supnet C, Bezprozvanny I. Presenilins function in ER calcium leak and Alzheimer’s disease pathogenesis. Cell Calcium. 2011;50:303–309. doi: 10.1016/j.ceca.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1–42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J Biol Chem. 2006;281:38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 150.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 151.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thireau J, Pasquie JL, Martel E, Le Guennec JY, Richard S. New drugs vs. old concepts: a fresh look at antiarrhythmics. Pharmacol Ther. 2011;132:125–145. doi: 10.1016/j.pharmthera.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 153.Ullah G, Demuro A, Parker I, Pearson JE. Analyzing and Modeling the Kinetics of Amyloid Beta Pores Associated with Alzheimer’s Disease Pathology. PLoS One. 2015;10:e0137357. doi: 10.1371/journal.pone.0137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA. Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J Neurosci. 2004;24:9612–9622. doi: 10.1523/JNEUROSCI.5583-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Petegem F. Ryanodine receptors: allosteric ion channel giants. J Mol Biol. 2015;427:31–53. doi: 10.1016/j.jmb.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 156.Verkhratsky A, Parpura V. Store-operated calcium entry in neuroglia. Neurosci Bull. 2014;30:125–133. doi: 10.1007/s12264-013-1343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Walter S, Atzmon G, Demerath EW, Garcia ME, Kaplan RC, Kumari M, Lunetta KL, Milaneschi Y, Tanaka T, Tranah GJ, Völker U, Yu L, Arnold A, Benjamin EJ, Biffar R, Buchman AS, Boerwinkle E, Couper D, De Jager PL, Evans DA, Harris TB, Hoffmann W, Hofman A, Karasik D, Kiel DP, Kocher T, Kuningas M, Launer LJ, Lohman KK, Lutsey PL, Mackenbach J, Marciante K, Psaty BM, Reiman EM, Rotter JI, Seshadri S, Shardell MD, Smith AV, van Duijn C, Walston J, Zillikens MC, Bandinelli S, Baumeister SE, Bennett DA, Ferrucci L, Gudnason V, Kivimaki M, Liu Y, Murabito JM, Newman AB, Tiemeier H, Franceschini N. A genome-wide association study of aging. Neurobiol Aging. 2011;32:2109. doi: 10.1016/j.neurobiolaging.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang J, Lu R, Yang J, Li H, He Z, Jing N, Wang X, Wang Y. TRPC6 specifically interacts with APP to inhibit its cleavage by gamma-secretase and reduce Abeta production. Nat Commun. 2015;6:8876. doi: 10.1038/ncomms9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang R, Zhong X, Meng X, Koop A, Tian X, Jones PP, Fruen BR, Wagenknecht T, Liu Z, Chen SRW. Localization of the dantrolene-binding sequence near the FK506-binding protein-binding site in the three-dimensional structure of the ryanodine receptor. J Biol Chem. 2011;286:12202–12212. doi: 10.1074/jbc.M110.194316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y, Wu J, Rowan MJ, Anwyl R. Ryanodine produces a low frequency stimulation-induced NMDA receptor-independent long-term potentiation in the rat dentate gyrus in vitro. J Physiol. 1996;495:755–767. doi: 10.1113/jphysiol.1996.sp021631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ward A, Chaffman MO, Sorkin EM. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs. 1986;32:130–168. doi: 10.2165/00003495-198632020-00003. [DOI] [PubMed] [Google Scholar]
- 162.Waring JF, Anderson DJ, Kroeger PE, Li J, Chen SP, Hooker BA, Gopalakrishnan M, Briggs CA. Gene expression changes related to synaptic deficits in the Tg2576 mouse model of Alzheimer’s disease. J Drug Metab Toxicol. 2012;3:115. [Google Scholar]
- 163.Wu J, Ryskamp DA, Liang X, Egorova P, Zakharova O, Hung G, Bezprozvanny I. Enhanced store-operated calcium entry leads to striatal synaptic loss in a Huntington’s disease mouse model. J Neurosci. 2016;36:125–141. doi: 10.1523/JNEUROSCI.1038-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, Bezprozvanny I. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s disease. J Neurosci. 2015;35:13275–13286. doi: 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E, Bezprozvanny I. Calcium signaling, excitability and synaptic plasticity defects in mouse model of Alzheimer’s disease. J Alzheimer’s Dis. 2015;45:561–580. doi: 10.3233/JAD-142427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J Neurosci. 2010;30:8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang X, Garbett K, Veeraraghavalu K, Wilburn B, Gilmore R, Mirnics K, Sisodia SS. A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J Neurosci. 2012;32:8633–8648. doi: 10.1523/JNEUROSCI.0556-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem. 2001;276:13810–13816. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 170.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci. 2008;11:741–743. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- 171.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.