Abstract
Glutamate transporters (EAAT) have been implicated in the drug addiction behavior. We determined whether EAAT type 3 (EAAT3) played a role in morphine addiction. Six- to eight-week old EAAT3 knockout (EAAT3−/−) mice and their wild-type littermates received 3 intraperitoneal injections of 10 mg/kg morphine, each on an alternative day, to induce conditioned place preference (CPP). Two days after the place preference returned to baseline, mice received 2.5 mg/kg morphine to induce reinstatement. Some mice received intraperitoneal injection of 4 mg/kg riluzole, an EAAT activator, 30 min before morphine or saline injection. Hippocampus, medial prefrontal cortex, nucleus accumbens and ventral tegmental area were harvested for Western analysis 24 h after the last dose of morphine was injected. Morphine induced CPP in wild-type and EAAT3−/− mice. Gender is not a statistically significant factor to influence this behavior. This conditioned behavior extinguished after morphine administration was stopped for 8 to 9 days in wild-type mice, while this extinction occurred 6 days after discontinuation of morphine injection in EAAT3−/− mice. A small dose of morphine similarly reinstated the conditioned behavior in the wild-type and EAAT3−/− mice. Riluzole abolished morphine-induced CPP during the initial place preference. Morphine increased EAAT3 expression in the plasma membrane of medial prefrontal cortex, nucleus accumbens and ventral tegmental area but did not affect EAAT3 expression in the hippocampus. These results suggest that EAAT3 delays the extinction of morphine-induced CPP. EAAT activation may prevent the formation of morphine-induced CPP.
Keywords: conditioned place preference, glutamate transporter, mice, morphine
Introduction
Opioids are a major class of analgesics used clinically. However, it was estimated that about 5.1 million Americans in 2010 abused opioids (topics in Brief, NIDA, 2011). Activation of various rewarding pathways, such as dopamine system in ventral tegmental area (VTA), contributes to the opioid addiction (Johnson and North, 1992). However, glutamatergic system may also be involved in opioid addiction (Del Pozo et al., 1996; Popik and Wrobel, 2002).
Glutamate, the major excitatory neurotransmitter, can be taken up by glutamate transporters (also called excitatory amino acid transporters, EAAT) into cells after it is released from presynaptic termini. In fact, glutamate uptake via EAAT is a major mechanism to regulate extracellular glutamate levels due to the lack of extracellular enzyme to metabolize glutamate (Danbolt, 2001). Consistent with this function, it has been shown that EAAT can regulate glutamate neurotransmission (Danbolt, 2001; Sepkuty et al., 2002).
Five types of EAAT have been known: EAAT1 – 5. EAAT1 and EAAT2 are mainly expressed in glia. EAAT3 and EAAT4 are mostly in neurons. EAAT5 exists in the retina. EAAT1 – 3 are expressed in many regions of the central nervous system; whereas EAAT4 is mainly in the cerebellum. Based on the amount of protein, EAAT2 and EAAT3 are considered the major glial and neuronal EAAT, respectively (Danbolt, 2001). Consistent with their functions of regulating glutamate neurotransmission, glial EAATs, especially EAAT2, have been indicated to play a role in drug addiction, such as cocaine addiction (Fujio et al., 2005; Fischer et al., 2013).
Morphine, a commonly used analgesic and addicted opioid, is known to regulate the expression of EAAT. For example, morphine can reduce EAAT1 and EAAT3 expression in the dorsal horn of spinal cord (Mao et al., 2002). Morphine withdrawal increases EAAT2 expression in the hippocampus (Xu et al., 2003). However, it is not known yet whether EAATs play a role in morphine addiction behavior.
Studies from our and other groups have shown that EAAT3, the major neuronal EAAT, participates in the learning and memory processes (Aoyama et al., 2006; Lee et al., 2012; Cao et al., 2014; Wang et al., 2014). Drug addiction is a pathological form of learning and memory related to rewards and cures that predict rewards (Hyman, 2005; Rosen et al., 2015). Thus, we hypothesize that EAAT3 plays a role in morphine addiction that can be considered as pathological learning and memory towards morphine use. To test this hypothesis, we exposed wild-type and EAAT3 knockout (EAAT3−/−) mice to morphine and measured their conditioned place preference (CPP). The conditioning, extinction and reinstatement behaviors of these mice were studied. In addition, we performed these tests in male and female mice to test whether there is a gender difference in these behaviors because inconsistent findings regarding gender difference of opioid addiction have been reported (Lee and Ho, 2013).
Materials and methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications number 80-23) revised in 2011.
Animals
Six- to eight-week old EAAT3−/− mice and their wild-type littermates were used in this study. The EAAT3 knockout mice were descendants of mice used in our previous studies (Lee et al., 2010, Li and Zuo, 2011). They were initially back-crossed with wild-type CD-1 mice for more than 10 generations to produce a strain of EAAT3 knockout mice before they were used in our studies. These CD-1 wild-type mice were from Charles River Laboratories (Wilmington, MA). It has been confirmed that the EAAT3 knock-out mice did not express EAAT3 in our previous study (Li and Zuo, 2011).
The animals were housed 5 animals per cage in standard plastic cages in room temperature (21 ± 1°C) and humidity-controlled environment with an automatic 12 h light/dark cycle (light on 6:00 am, off 6:00 pm). Food and water were available ad libitum. Each experimental group consisted of 7 – 13 mice and each mouse was used only for one study.
Apparatus for testing conditioned place preference
The box for testing CPP (LE 893, automated place preference system, Panlab/HARVARD Apparatus, Barcelona, Spain) consisted of three Perspex compartments. The larger compartments on either side are the same size (19 cm width×19 cm length × 25 cm height) with one in white color and the other in black color. These two compartments were connected by a central grey corridor (6.5 cm width×9 cm length ×25 cm height). One guillotine door was at each end of the corridor. The door color was the same as that of the compartments the door led to. The compartments can be differentiated by both visual and tactile cues: the color of the walls and the texture of the floors (smooth or rough). These distinct cues served as conditioned stimuli (CS). The use of distinct colors and tactile floor cues allowed mice to be in direct contact with CS to experience its conditioned effect during preference experiment. Animal position is detected by transducers installed below the cage platform. Only the grey and black compartments had transducers underneath the floor. When the system did not detect the animal in the grey or black compartment, it was assumed that the animal was in the white compartment. The transducer system was connected to a PC-based software PPCW in V2.0, which provided a raw data table containing permanence time in each compartment. The behavioral test room had dim lighting with a 10 W bulb positioned about 1.5 m above the apparatus. The apparatus was kept clean.
Drugs
Morphine sulfate (10 mg/ml, Hospira Lake Forest, IL) was diluted in normal saline to the concentration of 1 mg/ml. It was given intraperitoneally at 10 mg/kg. This dose was chosen based on previous studies (Nakagawa et al., 2001, Nakagawa et al., 2005). Control group received normal saline at 10 ml/kg.
Riluzole hydrochloride (2-Amino-6-trifluoromethoxybenzothiazole hydrochloride; TOCRIS Bioscience, Bristol, UK), a glutamate transporter activator (Frizzo et al., 2004, Fumagalli et al., 2008), was first dissolved in DMSO (Fish Scientific, NJ) to 100 mM (27 mg/ml), and then diluted in saline to 0.4 mg/ml with gentle warming. The dose of riluzole was 4 mg/kg injected intraperitoneally 30 min before each morphine or saline injection. The same concentration of DMSO (1.6%) was used as vehicle for injection in control group.
Experimental design
Total 114 male and female mice weighing 18 – 26 g were used in this experiment. The experiments were carried out between 9:00 am and 5:00 pm.
The experimental procedure consisted of four phases: 1). Habituation (preconditioning) (3 days, day −3 to day −1), 2) Conditioning with drugs or vehicle (6 days, day 0 to day 5), 3) Place preference and its extinction (6 – 9 days), and 4) Reinstatement (1 day) (Fig. 1). Place preference was tested in the first, third and fourth phases.
Fig. 1.
Diagram of experiments.
During the preconditioning phase, mice were taken to the testing room, weighed and handled by the experimenter for at least 5 min including caressing the back, grasping them by the tail and turning them upside down to imitate the position needed for intraperitoneal injection to reduce their stress in response to experimental manipulation. Mice were carefully placed into the central grey compartment and the guillotine was removed to give mice free access to all three compartments for 15 min. The white compartment was paired with smooth floor and the black compartment was paired with rough floor.
Baseline placement preference of each mouse was assessed on the 3rd day. Mice showing strong unconditioned aversion (less than 33% of the session time) or preference (more than 67% session time) for the white or black compartments were discarded. Twelve of the initial 126 mice were excluded for this reason in these experiments. Although mice tended to stay in the black compartment in a dim room, there was no significant difference in the permanence time in each of the three compartments.
During the subsequent time, morphine injection (10 mg/kg) was paired with the white compartment and smooth floor. Vehicle injection was paired with black compartment and rough floor. Mice were kept in the respective compartments for 30 min immediately after they received intraperitoneal injection of the drug or vehicle with the corresponding guillotine doors closed.
Mice in the groups receiving morphine had the drug injection on day 0, 2 and 4 and vehicle injection on day 1, 3 and 5. Mice in the groups receiving vehicle treatment had vehicle injection paired with the black compartment and rough floor and on alternate days paired with the white compartment and smooth floor.
Mice did not receive any injections after day 6. They were placed in the central grey compartment and all guillotine doors were removed, allowing the mice free access to the three compartments for 15 min.
Two days after CPP was extinguished, reinstatement was assessed. The mice received a small dose (2.5 mg/kg) of morphine and CPP was then assessed.
The following sets of experiments were performed.
Experiment 1: Morphine-induced CPP in EAAT3 knockout and wild-type mice.
Morphine sulfate at 10 mg/kg was administered through intraperitoneal injection to EAAT3 knockout mice (male n = 13, female n = 12) or wild-type mice (male n = 8, female n = 10). Saline was administered in the same way to EAAT3 knockout mice (male n = 10, female n = 12) or wild-type mice (male n = 8, female n = 10). Two days after the expression of CPP was extinguished, mice in the morphine groups received 2.5 mg/kg morphine and CPP was tested again (Fig. 1).
Experiment 2: Effects of riluzole on morphine-induced CPP in mice.
To determine the effects of riluzole and the vehicle DMSO on morphine-induced CPP, wild-type mice (n = 7 or 8 per group) received DMSO or riluzole injection 30 min before the administration of 10 mg/kg morphine or 10 ml/kg saline on the conditioning phase. All four groups of mice in this experiment received DMSO. Mice were subjected to CPP in the same way as for experiment 1 (Fig. 1).
Experiment 3: Effect of morphine on the expression of EAATs in the mouse brain.
Wild-type mice received 10 mg/kg morphine (n = 8) or saline (n= 8) injection as described for the conditioning phase of experiment 1 but were not subjected to place preference testing. They were sacrificed by deep isoflurane anesthesia and perfused with normal saline at 24 h after the last injection. Their hippocampus, medial prefrontal cortex (mPFC), nucleus accumbens and VTA were harvested for Western blotting (Fig. 1). We chose to use these four regions because hippocampus is involved in learning and memory and mPFC, nucleus accumbens and VTA are important brain regions for drug addiction (Wise, 1989; Shah and Treit, 2004; Jasinska et al., 2014; Rosen et al., 2015).
Western blotting
Hippocampus, mPFC, nucleus accumbens and VTA were homogenized in lysis buffer (80 mM HEPES, 200 mM mannitol and 41 mM KOH, pH 7.4) containing 200 μM phenylmethylsulfonyl fluoride, 0.5 mM EDTA and protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO) to extract membrane protein as we described previously (Li and Zuo, 2011). The tissue lysates were then incubated on ice for 30 min and centrifuged at 1,000 g for 10 min at 4°C. The supernatants were ultra-centrifuged at 100,000 g for 1 h at 4°C. The pellet was re-suspended in 200 μl lysis buffer thoroughly with sonication (1 s burst, 1 s interval, for 5 circles). Protein concentration was determined by Bradford assay. Protein at 30 μg per lane was separated on 7.5% polyacrylamide gels (BIO-RAD Laboratories, Hercules, CA) and transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA) overnight at 4°C. The membranes were blocked with Protein-Free T20 Blocking Buffer (Thermo Scientific, Logan, UT) and incubated overnight at 4°C with the following primary antibodies: rabbit polyclonal anti-EAAT3 antibody (1:250, #12179, Cell Signaling Technology, Danvers, MA) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:5000, G9545, Sigma Aldrich, St. Louis, MI). Membranes were then washed and incubated with the secondary antibody: goat anti-rabbit IgG-horseradish peroxidase antibody (1:5000, sc-2004, Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 h. Protein bands were visualized with the enhanced chemiluminescence method. The densities of EAAT1 - 3 protein bands were normalized to those of GAPDH from the same sample.
Statistical analysis
Results are presented as means ± S.E.M. (for the data with n ≥ 8) or in range (for the data with n = 4). The data from the sessions of measuring extinction of CPP within the same group were tested by one-way repeated measures analysis of variance followed by Tukey's post-hoc testing after confirmation of normal distribution of the data or by one-way repeated measures analysis of variance on ranks followed by Tukey's post-hoc testing if the data were not normally distributed. The data that did not pass the normality test (for example, data of wild-type mice receiving morphine in figure 2) in these time-course experiments were presented as means ± S.E.M. in figures because the data of other groups in the same set of experiments passed the normality test and it is difficult to present the data clearly if median and all data points of each group were reported in the figures. The between group data from the sessions of measuring extinction of place preference were tested by two-way repeated measures analysis of variance followed by Tukey's post-hoc testing. To test the effects of genders and gene types on reinstatement, two-way analysis of variance was used. Since Tukey's procedure has corrected for family-wise error rate, additional correction for multiple comparisons was not performed in the above analyses. The comparison of Western blotting and reinstatement results between control mice and the corresponding morphine-treated mice was performed by t-test after confirmation of normal distribution of the data (all data were normally distributed). A P ≤ 0.05 was accepted as significant. All statistical analyses were performed with the SigmaStat (Systat Software, Inc., Point Richmond, CA, USA).
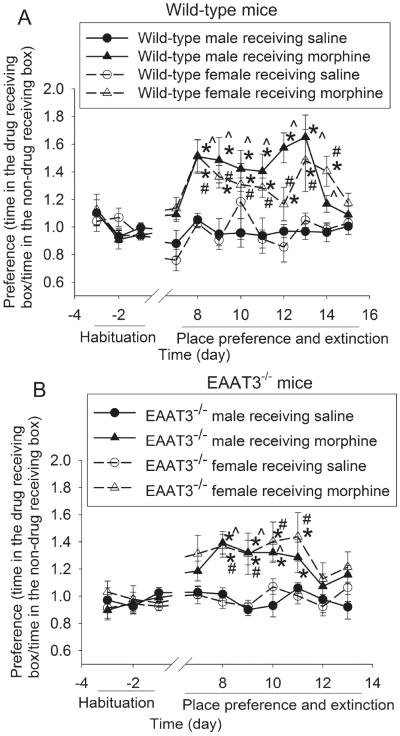
Fig. 2.
Morphine-induced CPP. Baseline place preference was tested on day −3 to −1. Mice received 10 mg/kg morphine or saline on day 0, 2 and 4. All mice received saline on day 1, 3 and 5 and did not receive any injections on the following days when they were tested for place preference extinction. A: wild-type mice. B: EAAT3−/− mice. Results are mean ± S.E.M. (n = 8 − 13). * P < 0.05 compared with the corresponding data on day −1. ^ P < 0.05 compared with corresponding data of male mice receiving saline. # P < 0.05 compared with corresponding data of female mice receiving saline.
Results
Male and female mice developed significant place preference after morphine injection. In supporting this point, they stayed more in the box where they received morphine than in the box where they received saline after the conditioning phase; whereas saline injection did not induce this place preference. Gender was not a significant factor to affect this conditioned activity for both wild-type and EAAT3−/− mice [main effect of gender: F(1,16) = 1.836, P = 0.194 for wild-type mice; F(1, 23) = 0.313, P = 0.581 for EAAT3−/− mice]. EAAT3 knockout did not affect the induction of this conditioned behavior [F(1,41) = 1.797, P = 0.187]. However, wild-type mice took 8 to 9 days for the CPP to extinct; whereas the extinction occurred 6 days after morphine application was stopped in the EAAT3−/− mice (Fig. 2), suggesting that EAAT3 is important for the maintenance of CPP.
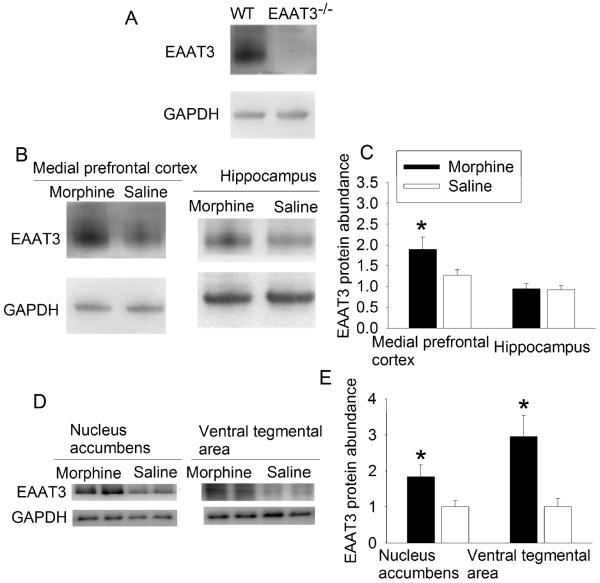
To further implicate the role of EAAT3 in morphine-induced place preference, the effects of morphine on EAAT3 expression was determined. Interestingly, morphine increased EAAT3 protein expression in the plasma membrane of mPFC, nucleus accumbens and VTA but not in the hippocampus of wild-type mice at 24 h after the last dose of morphine. Consistent with our previous studies (Lee et al., 2010; Li and Zuo, 2011), EAAT3−/− mice did not express EAAT3 in the mPFC (Fig. 3). Similar to the situation that gender did not affect morphine-induced CPP, EAAT3 abundance in the plasma membrane did not differ between male and female mice in the nucleus accumbens (ranged from 0.9 to 2.7 of control and from 1.3 to 3.7 of control for male and female mice, respectively, n = 4, P > 0.05) and VTA (ranged from 2.5 to 4.3 of control and from 0.9 to 5.9 of control for male and female mice, respectively, n = 4, P > 0.05) after morphine application. Although the initial experiment had 8 animals for each experimental condition, 4 males and 4 females were included for each experimental condition. Thus, we had a sample size of 4 to determine whether there was a gender difference in EAAT3 expression after morphine application. These EAAT3 expression change patterns are consistent with the findings that EAAT3 plays a role in morphine-induced CPP that may not have a gender difference.
Fig. 3.
Morphine-induced increase of EAAT3 expression. A: medial prefrontal cortex was collected from wild-type (WT) and EAAT3−/− mice for Western analysis. B to E: Animals received 3 doses of 10 mg/kg morphine, each on alternative days. Brain tissues were harvested at 24 h after the last dose of morphine. Representative images of Western blots are presented in panel B and D. Quantitative results are in panel C and E (mean ± S.E.M., n = 8). * P < 0.05 compared with mice receiving saline.
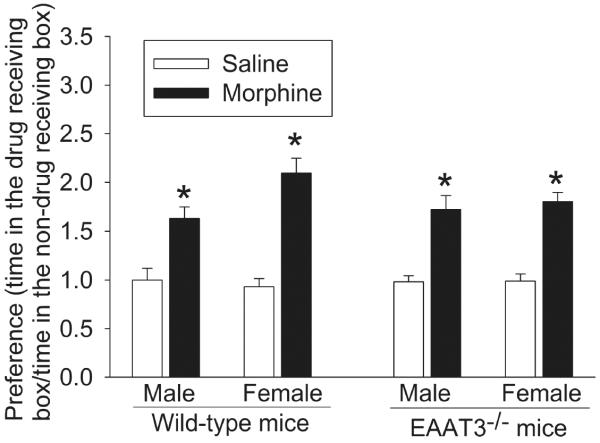
Next, we studied the reinstatement of CPP, an important biological component of drug addiction. A small dose of morphine given after the extinction of the CPP reinstated the CPP in both wild-type and EAAT3−/− mice (Fig. 4). EAAT3 knockout did not affect this reinstatement [F(1,79) = 0.401, P = 0.529]. Of note, the data presented in figure 4 and figure 2 were from the same mice. These results suggest that EAAT3 may not play a role in the reinstatement of morphine-induced CPP.
Fig. 4.
Reinstatement of morphine-induced CPP. Two days after the extinction of morphine-induced CPP, mice whose CPP data are shown in figure 2 received 2.5 mg/kg morphine or saline. Results are mean ± S.E.M. (n = 8 − 13). * P < 0.05 compared with mice receiving saline.
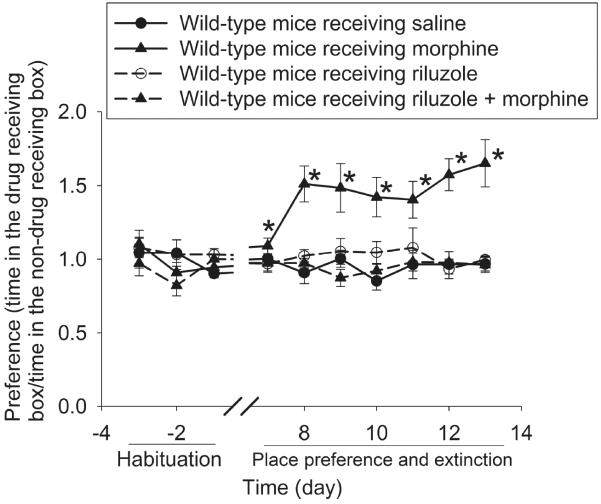
Our study with using EAAT3 knockout mice determined the effects of EAAT3 inhibition/deletion on morphine-induced CPP. To further study the role of EAATs in this conditioned behavior, we used riluzole that can activate EAAT. An activator specifically for EAAT3 might have been more desirable than a general activator to implicate the role of EAAT3. However, such an activator has not been developed yet. We reproduced the results of morphine-induced CPP in this third experiment. Interestingly, whereas riluzole did not induce place preference; it abolished morphine-induced CPP (Fig. 5). These results suggest that inhibition of EAAT activation is important for the initiation of morphine-induced CPP. This finding is consistent with the results that morphine induced CPP in EAAT3−/− mice.
Fig. 5.
Riluzole abolished morphine-induced CPP. Baseline place preference was tested on day −3 to −1. Mice received 10 mg/kg morphine or saline on day 0, 2 and 4. All mice received saline on day 1, 3 and 5. Some mice received riluzole 30 min before each injection of morphine or saline. Results are mean ± S.E.M. (n = 7 − 8). * P < 0.05 compared with the corresponding data on day −1.
Discussion
Our results clearly showed that morphine induced CPP in both male and female mice. Gender does not appear to affect this preference and the morphine-induced increase of EAAT3 expression in the cell plasma membrane. Consistent with our study, both male and female patients can develop addiction to morphine. There is no consistent effect of gender on this addiction (Lee and Ho, 2013).
Glutamatergic neurotransmission has been implicated in the morphine addiction (Del Pozo et al., 1996; Popik and Wrobel, 2002). Although EAATs that can regulate glutamatergic neurotransmission have been shown to participate in the development of addiction to drugs, such as cocaine (Fujio et al., 2005, Fischer et al., 2013), it is not clear whether EAATs play a role in morphine addiction. Our recent studies have shown that EAAT3, the major neuronal EAAT, may be an important component in the biochemical processes for learning and memory (Lee et al., 2012, Cao et al., 2014, Wang et al., 2014). Thus, EAAT3 may regulate morphine addiction. We showed here that induction of morphine-induced CPP was not affected by EAAT3 knockout. Its reinstatement was not affected by EAAT3 knockout either. However, the extinction of morphine-induced CPP was quicker in the EAAT3 knockout mice than in wild-type mice. Also, the amount of EAAT3 in the cell plasma membrane, the function site of EAATs, was increased in multiple addiction-related brain regions at 24 h after the last dose of morphine. These results suggest that EAAT3 plays an important role in the extinction of morphine-induced CPP. The increase of EAAT3 may contribute to delaying this behavior.
Although EAAT3 may not be involved in the induction of morphine-induced CPP, our results suggest that other EAATs may play a role in the behavior because riluzole, an EAAT activator (Frizzo et al., 2004, Fumagalli et al., 2008), abolished morphine-induced CPP. Consistent with this suggestion, a few studies have shown that morphine reduces EAAT1 and EAAT2 expression in the central nervous system (Ozawa et al., 2001; Mao et al., 2002).
Our results showed that EAAT3 expression in the plasma membrane was increased in the mPFC, nucleus accumbens and VTA but not in the hippocampus at 24 h after the last dose of morphine. This result suggests region-specific regulation of EAAT3 by morphine. However, future studies are needed to identify the mechanisms for this regulation. Nevertheless, mPFC, nucleus accumbens and VTA are important brain regions for drug addiction (Shah and Treit, 2004; Jasinska et al., 2014; Rosen et al., 2015; Sadeghzadeh et al., 2016); whereas hippocampus is a critical brain region for learning and memory. These results may not mean that learning and memory are not involved in morphine addiction but suggest that EAAT3 among many factors in the hippocampus may not be important for morphine addiction.
VTA and its projections to the nucleus accumbens have been shown to be the primary neural structures for the acute rewarding behaviors of opioids (Wise, 1989; Rosen et al., 2015). PFC is implicated in opioid-related learning and memory and receives inputs from VTA (Daglish et al., 2001; Rosen et al., 2015). Dopamine and glutamate neurotransmission in these brain regions plays an important role in these learning and memory functions (D'Souza, 2015; Rosen et al., 2015). Our finding that EAAT3 may participate in delaying extinction of morphine-induced CPP suggests that glutamate neurotransmission in the related structures contributes to the extinction of morphine addiction.
Our findings may have significant implications. There is no effective intervention for morphine addiction. Our study suggests that riluzole may have a great therapeutic potential for preventing morphine addiction. This finding may be easy for clinical translation because riluzole has already been used in clinic for neurodegenerative diseases (Cheah et al., 2010). Our data also suggest that EAAT3 may regulate the extinction of morphine-induced CPP. This finding is in agreement with our previous results that EAAT3 may regulate learning and memory (Lee et al., 2012; Cao et al., 2014; Wang et al., 2014) and add additional evidence for its role in learning and memory. In addition, our results suggest that EAAT3 can be a target for developing interventions to speed up the recovery from morphine addiction.
Our study has limitations. We used riluzole to determine the possible role of EAATs in morphine-induced CPP. However, riluzole is a general EAAT activator. Thus, it is not clear which types of EAATs may play a role in the morphine-induced CPP. In addition, riluzole has other effects, such as inhibition of glutamate release (Wang et al., 2004). These effects may be relevant to the effects because glutamate receptor plasticity has been shown to play a role in morphine sensitization (Xia et al., 2011). It is not clear whether these effects may contribute to its inhibition of morphine-induced CPP. Nevertheless, our major goal of this study was to determine the role of EAAT3 in the morphine-induced CPP. Currently, specific inhibitor or activator of EAAT3 has not been identified yet. It is not possible to perform a study using a specific EAAT3 inhibitor or activator to support our findings from EAAT3 knockout mice.
In summary, our results showed that EAAT3 may regulate the extinction of morphine-induced CPP but may not affect the induction and reinstatement of this behavior. Riluzole abolishes morphine-induced CPP and, therefore, presents a great therapeutic potential for preventing morphine addiction.
Highlights.
Morphine induces conditioned place preference in wild-type and glutamate transporter type 3 knockout mice
Glutamate transporter type 3 knockout shortens the extinction of morphine-induced conditioned place preference
Riluzole abolished morphine-induced conditioned place preference.
Acknowledgments
This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of interest: The authors have no conflict of interest.
Author contributions: ZZ conceived the idea of the study. ZZ, LW, JB and JL designed the study. LW, JB and JL carried out the experiments. LW, JB, JL and ZZ analyzed the data. LW drafted the methods and materials section. ZZ wrote the manuscript. All authors had final approval of the submitted manuscript.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Cao MN, Zhou YB, Gao AH, Cao JY, Gao LX, Sheng L, Xu L, Su MB, Cao XC, Han MM, Wang MK, Li J. Curcusone D, a novel ubiquitin-proteasome pathway inhibitor via ROS-induced DUB inhibition, is synergistic with bortezomib against multiple myeloma cell growth. Biochim Biophys Acta. 2014;1840:2004–2013. doi: 10.1016/j.bbagen.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17:1942–1199. doi: 10.2174/092986710791163939. [DOI] [PubMed] [Google Scholar]
- D'Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Del Pozo E, Barrios M, Baeyens JM. The NMDA receptor antagonist dizocilpine (MK-801) stereoselectively inhibits morphine-induced place preference conditioning in mice. Psychopharmacology (Berl) 1996;125:209–213. doi: 10.1007/BF02247330. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci. 2013;33:9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzo ME, Dall'Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24:123–128. doi: 10.1023/b:cemn.0000012717.37839.07. [DOI] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Chen BT, Bonci A, Stein EA. Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies. Addict Biol. 2014;20:215–226. doi: 10.1111/adb.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Ho IK. Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol Pain. 2013;9:45. doi: 10.1186/1744-8069-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park S, Zuo Z. Effects of isoflurane on learning and memory functions of wild-type and glutamate transporter type 3 knockout mice. J Pharm Pharmacol. 2012;64:302–307. doi: 10.1111/j.2042-7158.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neurosci. 2010;171:788–793. doi: 10.1016/j.neuroscience.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001;905:254–258. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Rosen LG, Sun N, Rushlow W, Laviolette SR. Molecular and neuronal plasticity mechanisms in the amygdala-prefrontal cortical circuit: implications for opiate addiction memory formation. Front Neurosci. 2015;9:399. doi: 10.3389/fnins.2015.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghzadeh F, Namvar P, Naghavi FS, Haghparast A. Differential effects of intra-accumbal orexin-1 and -2 receptor antagonists on the expression and extinction of morphine-induced conditioned place preference in rats. Pharmacol Biochem Behav. 2016;142:8–14. doi: 10.1016/j.pbb.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AA, Treit D. Infusions of midazolam into the medial prefrontal cortex produce anxiolytic effects in the elevated plus-maze and shock-probe burying tests. Brain Res. 2004;996:31–40. doi: 10.1016/j.brainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Wang KY, Wang WC. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) Neurosci. 2004;125:191–201. doi: 10.1016/j.neuroscience.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Wang Z, Park SH, Zhao H, Peng S, Zuo Z. A critical role of glutamate transporter type 3 in the learning and memory of mice. Neurobiol Learn Mem. 2014;114:70–80. doi: 10.1016/j.nlm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Xia Y, Portugal GS, Fakira AK, Melyan Z, Neve R, Lee HT, Russo SJ, Liu J, Moron JA. Hippocampal GluA1-containing AMPA receptors mediate context-dependent sensitization to morphine. J Neurosci. 2011;31:16279–16291. doi: 10.1523/JNEUROSCI.3835-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci. 2003;23:4775–4784. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]